Introduction
Human papillomavirus (HPV) infection is closely
associated with cervical cancer. Numerous studies from around the
world have reported that >90% of cervical cancer and
precancerous lesions contain HPV DNA (1,2).
Among these cases, high-risk HPV type 16 infection is the most
important factor in tumorigenesis (3), and subsequently the first choice
target for vaccine development. HPV type 16 E6 and E7 proteins are
continuously expressed in cervical cancer and precancerous lesions,
and are a completely exogenous viral antigen. Therefore, these
proteins are an ideal target antigen for a HPV treatment vaccine
(4).
A replication-defective recombinant adenovirus has
the advantage of having a wide range of hosts, without integrating
into the host cells. Furthermore, large genes (up to 37 kb) may be
inserted into adenovirus vectors, and the vector can be used to
express multiple genes at the same time at high expression levels.
The expressed antigen is modified and presented in the host cells,
which subsequently produces strong humoral and cell immunity. All
these characteristics make the adenovirus a suitable vector for
tumor vaccines.
Research into a HPV type 16 vaccine using an
adenovirus as the vector has entered the development stage
(5). Furthermore, in China, the
early stage construction and the first-stage immunity evaluation of
a HPV type 16 E6/E7 recombinant adenovirus vector vaccine,
developed by the Houwen Tian research group from the Virus
Institute at the Chinese Center for Disease Control and Prevention
(Beijing, China), have been completed (6). According to the technology agreement,
the present research group constructed the master seed bank (MSB)
and working seed bank (WSB) required for expressing the HPV type 16
E6/E7 vaccine. In accordance with the management procedure
requirements for toxic bacteria species used for biological
production from the State Food and Drug Administration, as
previously described (7,8), the replication ability of the
recombinant adenovirus vector vaccine seed libraries (MSB and WSB),
the genetic stability of the inserted target genes and the
expression of the target proteins and their biological effects were
studied. The present study reports these results.
Materials and methods
Materials
A recombinant adenovirus rAd5HPV16SmE7E6 strain was
provided by the Viral Diseases Prevention and Control Institute at
the Chinese Center for Disease Control and Prevention, according to
a technology transfer agreement (4).
HEK293 cells were purchased from the American Type
Culture Collection (ATCC; CRL-1573; Manassas, VA, USA).
HEK293-EcRShh (adv5) cells were verified by the Chinese Academy of
Food and Drug Testing and were passaged to establish the cell
library.
Major reagents
Dulbecco’s modified Eagle’s medium (DMEM) was
purchased from Gibco Life Technologies (Beijing, China) and viral
genomic DNA/RNA extraction kits were purchased from Tiangen Biotech
(Beijing) Co., Ltd. (DP-315; Beijing, China). A Trans2K Plus II DNA
Marker was purchased from Beijing Quanshijin Biology Technology
Co., Ltd. (Beijing, China). A primary antibody against HPV type 16
E7 (sc-6981) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), while a secondary horseradish peroxidase
(HRP)-conjugated goat-anti-mouse IgG antibody was purchased from
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China).
Polymerase chain reaction (PCR) primers were synthesized by
Shanghai ShengGong Biological Engineering Technology Co., Ltd.
(Shanghai, China). Female C57BL/6 mice of specific-pathogen free
grade (age, 6–8 weeks) were purchased from Shanghai Xipuer-Bikai
Experimental Animal Co., Ltd. (Shanghai, China) and the TC-1 tumor
cell line was purchased from the ATCC (CRL-2785).
Cell passage of the recombinant
adenovirus expressing HPV E6/E7 and infectious viral titer
measurements
A recombinant adenovirus expressing HPV type 16
E6/E7 proteins was passaged continuously in HEK293 cells to
passages 14 and 15. The infected cells were subsequently harvested,
frozen and thawed three times. The harvested liquid was serially
diluted, and dilutions ranging between 10−3 and
10−10 were used to infect the seeded cells in 96-well
plates. After 48 h of incubation, the cell morphology was observed,
and the infected cells appeared and were counted under a microscope
(Olympus CKX41; Olympus, Tokyo, Japan) to calculate the infectious
titers.
Verification of the inserted target
genes
For PCR, 1 μl samples were used. In total, five
samples were analyzed, including the negative control (adenovirus
without the E6/E7 genes, subsequently referred to as the empty
adenovirus), the positive control (rAd5HPV16SmE6E7), the two
experimental samples (Ad-HPV16E6E7 MSB and WSB) and a blank control
(reagents without real samples).
The target gene, HPV16 E6/E7, was ~800 bp in length,
and the amplification primers were as follows: mCMVup,
CAGTCTTCGGTCTGACCACCG; and pE6dn, GGC CGAATTCATCACAGCTGGGTCTCTCTTC.
If a sample showed a band of ~800 bp, the target gene was
considered to have successfully been inserted. The PCR
amplification program was as follows: Predenaturation at 94°C for
50 sec; followed by 35 cycles of denaturation at 94°C for 30 sec,
annealing at 51°C for 30 sec and extension at 72°C for 1 min; and a
final extension at 72°C for 10 min. Following PCR, a 5-μl sample of
the PCR product was analyzed on 1.0% agarose gel electrophoresis to
verify the product size.
Western blot analysis detection of the
Ad-HPV16E6E7 target protein
HEK293 cells were grown in 25-cm2 cell
culture flasks until 90% confluence was reached. The empty
adenovirus, Ad-HPV16E6E7 MSB and WSB were used to infect the cells.
The viruses were incubated at 37°C for 1–2 h, and the DMEM were
added. The cells were incubated at 37°C in 5% CO2 for
24–48 h until all the cells were infected. The supernatant was
discarded, and the cells were washed with phosphate-buffered saline
(PBS) twice and then harvested with scrapers. Next, 50 μl 2X SDS
loading buffer was added to each sample and the samples were boiled
at 100°C for 3 min. Samples of 20 μl were loaded onto the gel,
analyzed by 10% SDS-PAGE and transferred to a nitrocellulose
membrane. An anti-HPV type 16 E7 mouse monoclonal antibody
(1:1,000) was used as the primary antibody, while a HRP-conjugated
goat anti mouse IgG (1:2,000) was used as the secondary antibody.
The immunoblots were developed using an ECL chemiluminescence
detection system (Thermo Fisher Scientific, Waltham, MA, USA) and
visualized using a Kodak BIOMAX Light Film (Sigma-Aldrich, St.
Louis, MO, USA).
Immunofluorescence detection of the
Ad-HPV16E6E7 target protein
When the HEK293 cells reached 80–90% confluence, the
cells were infected with the empty virus, Ad-HPV16E6E7 MSB or WSB.
The cells were incubated at 37°C for 1–2 h, and the media were
added. The cells were incubated further at 37°C for 36–48 h, and
subsequently digested with trypsin and centrifuged at 1,000 rpm for
5 min (centrifugal radius of 6.5 cm). The supernatants were
discarded and the pellets were washed with PBS three times. The
cells were put on slides, blocked with blocking solution (methyl
acetone; 1:1) for 10 min and dried. The antibodies were diluted
with PBS, and a 5–10-μl sample of the HPV type 16 E7 monoclonal
primary antibody was incubated with the samples at room temperature
for 2 h. Moisture was maintained to prevent antibody evaporation.
The samples were gently washed with K+-free PBS three
times, each time for 2–3 min. An immunofluorescence-labeled
secondary antibody (aMo IgG; cat. no. ZF-0312; 1:100; ZSGB-Bio,
Beijing, China) was added and incubated for 2 h at room temperature
(25°C). The sample was stained with Evans blue (cat. no. ab120869;
Abcam, Sangon Biotec Co., Ltd., Shanghai, China) for 10 min and
then observed under a fluorescence microscope (Leica DM13000B;
Leica Microsystems GmbH, Wetzlar, Germany).
Mouse tumor growth inhibition assay
In total, 50 C57BL/6 mice were used in the study.
Each mouse received 1×104 TC-1 tumor cells by an
injection into the groin to induce tumor growth. After 24 h
(labeled as day 0), the mice were divided randomly into the PBS
control group, the empty adenovirus control group
(2.85×109 IU/ml), the MSB group (6.31×109
IU/ml), the WSB group (3.00×109 IU/ml) and the S090304B
recombinant adenovirus group (3.00×107 IU/ml). There
were 10 mice in each group. All the mice received intramuscular
administration, and the injection volume was 100 μl. Tumor growth
was observed at day 0, 7, 14, 21, 28, 35 and 42. S090304B was the
test product developed by the Viral Diseases Institute at Zhejiang
Academy of Medical Sciences (Hangzhou, China). The aim of this
experiment was to analyze the effect of the vaccine on mouse TC-1
tumor cell growth inhibition. The present study was approved by the
ethics committee of Zhejiang Academy of Medical Sciences (Hangzhou,
China).
Statistical analysis
Tumor inhibition rates among the groups were
compared using the χ2 test, where P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the recombinant
adenoviruses expressing HPV E6/E7 in HEK293 cells
Recombinant adenoviruses expressing the HPV type 16
E6/E7 MSB and WSB were continuously passaged and used to infect
HEK293 cells. The infection time was stable and varied between 42
and 48 h. Cell morphology changed from attached polygonal spindle
shapes to round particles that gradually detached, showing evident
viral plaques. The infected cells exhibited stable morphological
changes, and the viruses were harvested. The MSB infectious titer
was 6.31×109 IU/ml, while the WSB infectious titer was
3.0×109 IU/ml, which met the seed library titer
requirement.
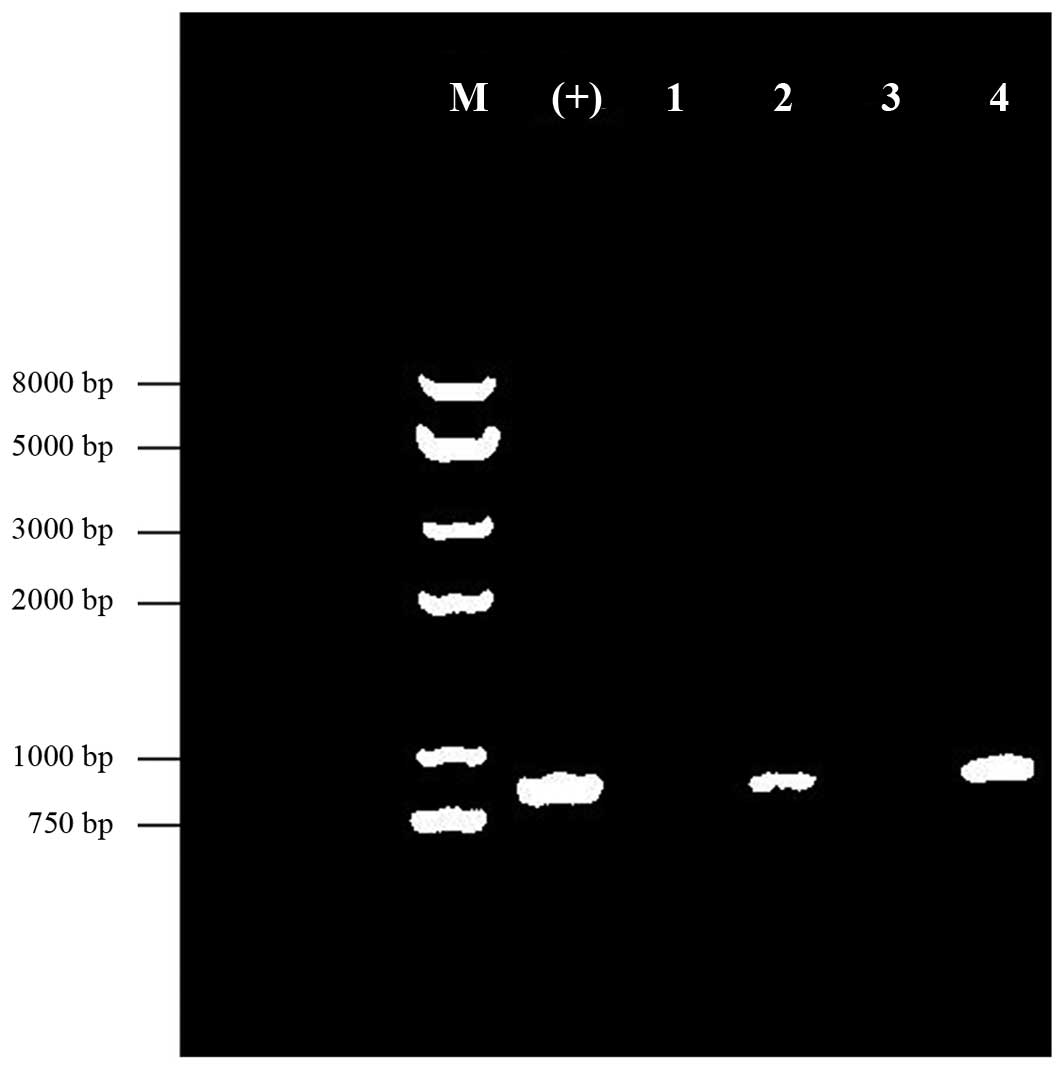
PCR analysis of the inserted target HPV
type 16 E6/E7 gene
The 771-bp, codon-optimized HPV type 16 E6/E7 gene
was inserted into the adenovirus. Agarose gel electrophoresis
analysis revealed that the negative control and blank control did
not yield any gene amplification bands. The amplification products
using the mCMVup and pE6dn primer set showed specific bands of ~800
bp in length. Thus, the results demonstrated specific amplification
of the bands (Fig. 2) in the MSB
and WSB samples.
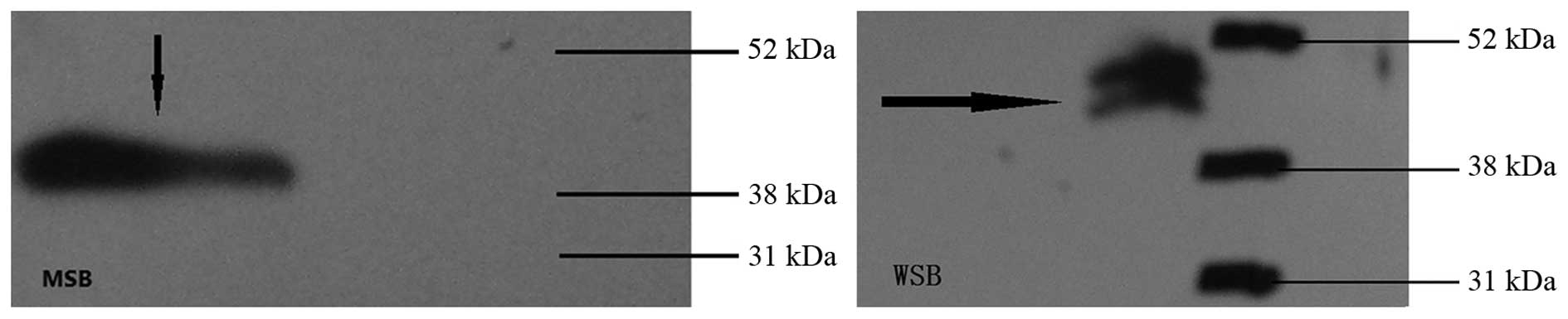
Western blot analysis of HPV type 16
E6/E7 expression
Results from western blot analysis are shown in
Fig. 3. The MSB and WSB showed
specific bands at a molecular weight of 40,000 Da, indicating that
the MSB and WSB expressed the HPV E6/E7 fusion proteins. However,
this size was larger than the predicted protein size of 35,000 Da,
which may be due to protein phosphorylation. This band was observed
due to the specific binding between the monoclonal E7 antibody and
the protein of interest. Thus, the band was not detected in the
empty adenovirus sample.
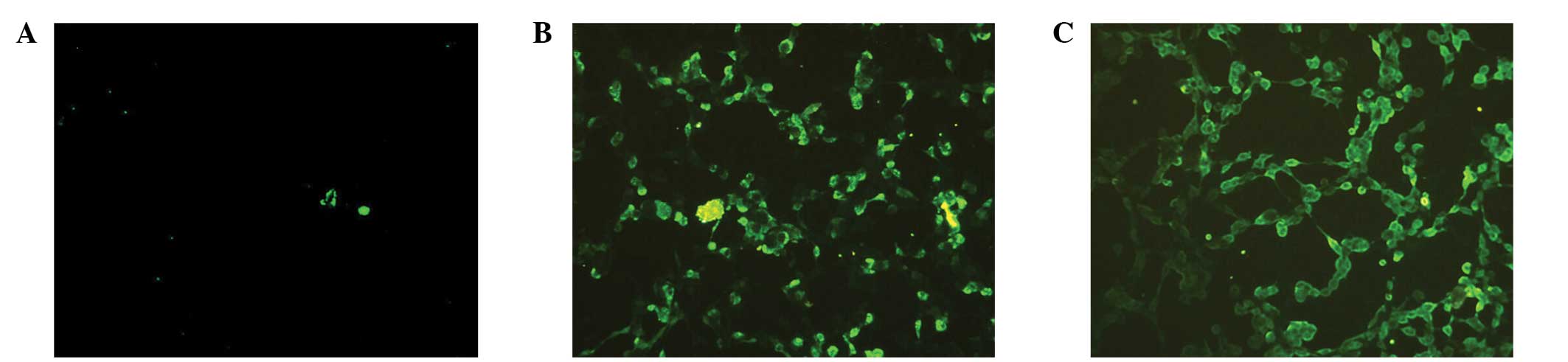
Immunofluorescence analysis of HPV type
16 E6/E7 expression
Immunofluorescence analysis is shown in Fig. 4. The MSB and WSB showed specific
binding, as detected by immunofluorescence. No recombinant HPV
E6/E7 gene or specific immune reaction was observed in the empty
adenovirus sample; therefore, there was no immunofluorescence
activity indicating specific binding.
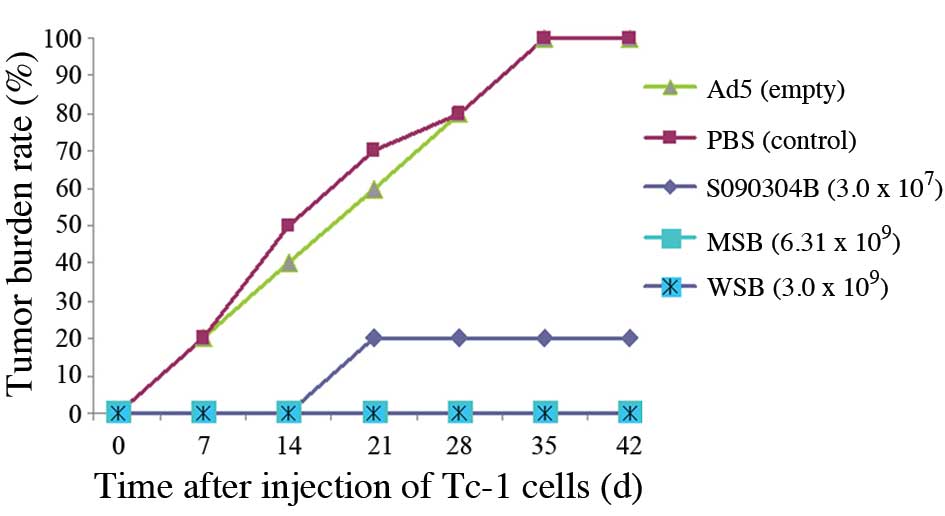
Mouse tumor cell growth inhibition
analysis
Inhibitory effects on mouse tumor cell growth are
shown in Fig. 5. Tumor formation
was slow in the mice injected with TC-1 tumor cells in the PBS and
empty adenovirus control groups. Tumors formed in two mice on day 7
(2/10), in five mice on day 14 (5/10) and in all 10 mice on day 35
(10/10), which was a different result from that of previous
experiments in which the control group showed 100% tumor formation
in 14–21 days. The number of tumors formed in the mice in the MSB
and WSB groups on day 42 was zero; thus, the tumor inhibition rate
was 100%. The percentage of tumor formation in the mice in the
S090304B group was 20%, while the percentage of tumor formation in
the mice from the control group was 100%. The differences between
the MSB, WSB and S09034B groups when compared with the control
group were statistically significant (χ2=20.00, 20.00
and 13.33, respectively; P<0.01). In the S090304B group, two
mice started to form tumors on day 21, and the tumor diameters had
reached 1.0–1.5 cm by day 42. Therefore, these tumors were smaller
compared with those of the mice in the control group on day 42,
which had diameters ranging between 2.5 and 3.5 cm.
Discussion
As vectors, adenoviruses have played an important
role in various fields, including gene therapy, live vector vaccine
development and in vitro gene transfection. Adenovirus
vectors have been used in prostate cancer treatment (9). In addition, the recombinant human
adenovirus 5 (H101) has been approved as a clinical drug for head
and neck cancer treatment in China (10). Their application has produced
substantial evidence for the safety and efficacy of adenoviruses
used as vectors. Previous research formed the basis for the
long-term aim of the present study, which was to treat cervical
intraepithelial neoplasia or precancerous lesions using recombinant
adenoviruses expressing HPV type 16 E6/E7 (6).
The requirement of a seed library for recombinant
adenoviruses used as biological products has been clearly defined
(8). As a gene recombination
product, the adenovirus vaccine MSB and WSB must assure the
stability of the inserted target genes and their expression levels
(11), as well as the biological
effects of the corresponding proteins. The present study used these
indicators to assess adenovirus vaccine seed libraries. The
recombinant adenovirus HPV type 16 E6/E7 MSB and WSB were found to
have high titers, correct target gene band sizes, stable target
protein expression and suitable biological effects in a mouse TC-1
tumor cell growth inhibition assay. These results indicated that
MSB and WSB were genetically stable and were able to meet the
vaccine seed requirements.
The mouse TC-1 tumor inhibition assay demonstrated
that when the titer of recombinant adenovirus was 109
IU/ml, the tumor formation rate was zero. However, when the titer
of the S090304B recombinant adenovirus was 107 IU/ml,
the tumor formation rate was 20%, indicating that the titer of the
adenovirus vector vaccine may affect tumor formation.
Theoretically, the production of a recombinant adenovirus vaccine
with a 109 IU/ml titer may be highly effective in
clinical application. However, obtaining a recombinant adenovirus
with such a high titer is impossible in cell culture, which makes
it difficult to use it as a product. Nevertheless, the recombinant
adenovirus can meet the requirement of a seed library.
However, the results of the present study are
limited, since only the stability of the seed library was
investigated. According to the requirements of recombinant
adenovirus vaccine research, further study is required to
investigate the genetic stability of the WSB passaged products,
including the vaccine products and the products resulting from six
passages of adenovirus vaccine products, to assure the safety and
efficacy for human vaccines.
An adenovirus vector vaccine has a number of
advantages; however, further improvements are required. The major
disadvantage of this type of vaccine is that the original
host-background neutralizing antibodies produced following
adenovirus infections can reduce the efficacy of the vaccine. In
addition, the antiviral antibody produced after the immunization
can inhibit reimmunization; therefore, the effects of immunization
may be improved through using combined immunization (12). The present study revealed that the
Ad-HPV16 E6E7 vaccine seed bank (MSB; WSB) was genetically stable
and meets the requirements for vaccine development.
Acknowledgements
The study was supported by grants from the Zhejiang
Provincial Medical Biological Engineering Vaccine Research Key
Laboratory (no. 2008F3022) and Hangzhou City Major Innovation
Projects (no. 20112313A37).
References
|
1
|
zur Hausen H: Papillomavirus infections -
a major cause of human cancers. Biochem Biophys Acta. 1288:F55–F78.
1996.
|
|
2
|
Bosch FX, Lorincz A, Munoz N, Meijer CJ
and Shan KV: The causal relation between human papillomavirus and
cervical cancer. J Clin Pathol. 55:244–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roden RB, Ling M and Wu TC: Variation to
prevent and treat cervical cancer. Hum Pathol. 35:971–982. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murakami M, Ugai H, Wang M, Belousova N,
Dent P, Fisher PB, Glasgow JN, Everts M and Curiel DT: An
adenoviral vector expressing human adenovirus 5 and 3 fiber
proteins for targeting heterogeneous cell populations. Virology.
407:196–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren J, Zhao L, Tian HW, Gao J, Feng J,
Pang Z, Wu XB, Tan WJ and Ruan L: Immunogenicity and antitumor
efficacy of the recombinant adenovirus expressing E7 and E6 fusion
proteins of HPV type 16 in mice. Zhonghua Min Guo Wei Sheng Wu Ji
Mian Yi Xue Za Zhi. 32:276–280. 2012.(In Chinese).
|
|
7
|
China Food and Drug Administration. Human
gene therapy research and drug preparation quality control
technology guidelines. http://www.sda.gov.cn/WS01/CL0237/15708.htmluri.
Accessed May 13, 2013
|
|
8
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the People’s Republic of China 2010 (English
edition). China Medical Science Press; Beijing: pp. 3–9. 2011
|
|
9
|
de Vrij J, Willemsen RA, Lindholm L and
Hoeben RC; GIANT Consortium. Bangma CH, et al: Adenovirus-derived
vectors for prostate cancer gene therapy. Hum Gene Ther.
21:795–805. 2010. View Article : Google Scholar
|
|
10
|
Jiang Y, Yin N, Bai L and Yuan XR: Effect
observation of 9 cases patients with nasopharyngeal carcinoma
locally injected with recombinant adenovirus 5. Bulletin of the
Academy of Military Medical Science. 34:2152010.
|
|
11
|
Wang J: Biological Engineering Drugs
Research Development and Quality Control. Science Press; Beijing:
pp. 606–612. 2002
|
|
12
|
Smyth LJ, Van Poelgeest MI, Davidson EJ,
et al: Immunological responses in women with human papillomavirus
type 16 (HPV-16)-associated anogenital intraepithelial neoplasia
induced by heterologous prime-boost HPV-16 oncogene vaccination.
Clin Cancer Res. 10:2954–2961. 2004. View Article : Google Scholar : PubMed/NCBI
|