Introduction
The incidence of pancreatitis is markedly increased
with the improvement of living conditions, particularly acute
pancreatitis (AP). AP is a common clinical disease, and one of the
main causes of it is hypertriglyceridemia (1). The prognosis of the majority of cases
of AP is good; however, some types, particularly severe AP, may
lead to multiple organ failure, resulting in serious adverse
consequences for the patient (2).
Currently, the cause and mechanisms of AP are not completely clear,
and there is no specific drug used to treat AP. The predominant
treatment method is comprehensive therapy (3). It is believed that AP may be closely
associated with obesity, trypsin activation, inflammatory mediator
activation, pancreatic blood circulation disorder, pancreatic cell
apoptosis and other factors (4,5). AP
includes acute edematous pancreatitis (AEP) and acute necrotizing
pancreatitis (ANP). AEP has moderate clinical symptoms, and often
doesn’t require treatment; whereas ANP has severe clinical
symptoms. ANP easily causes complications and sequelae, and may be
life-threatening (6).
Obesity is considered an independent risk factor for
the development of severe AP (7,8);
however, the underlying mechanism remains unknown. Resistin is a
newly identified peptide hormone, secreted specifically by
adipocytes (9). Resistin can cause
obesity and hypertriglyceridemia, due to its association with
insulin resistance (10). Previous
studies have revealed that resistin is also an important cytokine
in inflammatory reactions, and the regulation of other cytokines
(11,12). Resistin levels are increased in the
pancreatic tissues of patients with AP, and the increased
expression has been shown to correlate with the severity of AP
(13–15). Thus, resistin may play an important
role in the pathogenesis of AP. The aim of the present study was to
investigate the association between resistin expression and AP, in
order to provide a novel target for the treatment of
pancreatitis.
Materials and methods
Animal grouping and modeling
A total of 40 healthy male Sprague-Dawley rats
(eight weeks old, weighing 200–250 g), provided by the Experimental
Animal Center of Nanjing Medical University (Nanjing, China), were
housed in a specific-pathogen-free room at a constant temperature
of 22–28°C and a relative humidity of 40–70%. The rats were divided
at random into four groups (n=10 for each group), which included
the control, sham-operated, AEP and ANP groups. The rats were
fasted for 12 h, but had free access to water. Caerulein (40 μg/kg;
Sigma-Aldrich, Steinheim, Germany) was administered to the rats in
the AEP group twice by intraperitoneal injection, with an interval
of 1 h. At 12 h after the first injection, a successful model of
AEP was established. Rats in the ANP group were treated with
arginine using the same method (2 g/kg; Sigma-Aldrich). The ANP rat
model was established successfully at 24 h after the first
injection. Rats in the sham-operated group were treated
identically, but with an equivalent dose of physiological saline.
Rats in the control group were fasted and received no further
treatment. Following the successful establishment of the models,
all the rats were anesthetized with 3% pentobarbital sodium (30
mg/kg) by intraperitoneal injection, and weighed. This study was
conducted in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. The animal use protocol was reviewed and
approved by the Institutional Animal Care and Use Committee of
Nanjing Medical University.
Blood and specimen collection
The abdominal cavity of each rat was opened for
blood sample collection from the inferior vena cava and removal of
the pancreas. Serum was isolated from the blood samples by
centrifugation at 1,409 × g for 10 min at 4°C, and was stored at
−80°C. The pancreases were weighed and fixed in formalin for
histopathological examination.
Measurement of serum amylase
According to a reported method (16), serum amylase levels were measured
using an Hitachi-7150 automatic biochemistry analyzer (Hitachi
Corp., Tokyo, Japan) according to the manufacturer’s
instructions.
Pancreatic histopathology scoring
The pancreases were fixed with 10% neutral buffered
formalin for 18–24 h, they were then dehydrated and embedded in
paraffin. The samples were cut into 4-μm sections, deparaffinized
and stained with hematoxylin-eosin. The sections were observed
under a light microscope (Olympus, Tokyo, Japan) and scored
histopathologically, according to the Schmid scoring criteria
(17).
ELISA
ELISA kits specific for serum resistin, C-reactive
protein (CRP), tumor necrosis factor-α (TNF-α) and interleukin
(IL)-1β were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). The tests were performed following the manufacturer’s
instructions.
Immunohistochemistry
The paraffin-embedded sections were roasted in an
oven at 59°C overnight, and were then immersed twice in xylene, for
15 min each time. The sections were successively immersed in 100%,
95%, 90% and 85% ethanol for 1 min, and then with
phosphate-buffered saline three times, for 5 min each time. The
endogenous peroxidase activity was blocked with 1%
H2O2 for 15 min, followed by antigen
retrieval in citrate buffer (pH 6.0). Following cooling to room
temperature, the sections were incubated overnight with a
polyclonal rabbit anti-rat resistin antibody (1:200 dilution; cat.
no. A00100-01; Beijing Aidibo Biological Technology Co. Ltd.,
Beijing, China) at 4°C, and then treated with a horseradish
peroxidase-conjugated mouse anti-rabbit immunoglobulin G secondary
antibody (1:200 dilution; cat. no. A0303; Shanghai Long Island
Antibody Diagnostic Reagents Co., Ltd., Shanghai, China) for 30 min
at room temperature. Reactivity was detected with a
diaminobenzidine kit (Shanghai Long Island Antibody Diagnostic
Reagents Co., Ltd.), and the nuclei were counterstained with
hematoxylin.
RNA extraction and quantitative
polymerase chain reaction (PCR)
Pancreatic tissues were dissolved in TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and the total RNA
was extracted and reverse transcribed using a PrimeScript RT
reagents kit (Invitrogen Life Technologies), according to the
manufacturer’s instructions. The cDNA concentration and purity were
subsequently measured using an ND-1000 ultraviolet
spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE,
USA).
Quantitative PCR was performed with an ABI 7500
Real-time PCR System (Applied Biosystems Life Technologies,
Carlsbad, CA, USA), using the SYBR Green PCR Master kit
(Sigma-Aldrich). The PCR conditions were set as follows: 95°C for 1
min; 95°C for 15 sec, 60°C for 15 sec, and 72°C for 45 sec (40
cycles); followed by 95°C for 15 sec, 60°C for 60 sec and 99°C for
15 sec. The following gene-specific primer pairs were used:
Resistin forward, 5′-TGCCAGTGCGGAAGC ATAGA-3′ and reverse,
5′-TCCAGACCCTACTCTCGTTT-3′; β-actin forward,
5′-ATACTCCTGCTTGCTGATCC-3′ and reverse, 5′-CCTGTACGCCAACACAGTGC-3′.
The PCR product sizes for resistin and β-actin were 196 and 201 bp,
respectively.
Statistical analysis
Statistical analysis was performed with the SPSS
v13.0 software program (SPSS, Inc., Chicago, IL, USA). Measurement
data are presented as the mean ± standard deviation and were
compared using the t test or Mann-Whitney U test. Numeration data
were analyzed using the χ2 test. Pearson’s correlation
analysis was used to analyze the associations among the levels of
serum resistin, CRP, TNF-α and IL-1β and the pancreatic
histopathological scores. For all the tests, two-sided P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum amylase, pancreas/body weight ratio
and histopathological changes
Serum amylase levels in the rats from the AEP and
ANP groups were significantly higher compared with those in the
normal control and sham-operated groups (P<0.01). Furthermore,
serum amylase levels in the rats from the ANP group were
significantly higher compared with the AEP group (P<0.05). Rats
in the AEP group exhibited a small amount of effusion in the
abdominal cavity and their pancreases were evidently swollen.
Comparatively, rats in the ANP group exhibited greater effusion in
the abdominal cavity and their pancreases were presented with
evident swelling, sporadic ecchymosis and regional saponification
spots. Pancreas weight in the AEP and ANP group rats increased
markedly, contributing to the increased pancreas/body weight
ratios. When compared with the normal control and sham-operated
groups, the pancreas/body weight ratios in the AEP and ANP groups
increased significantly (P<0.01), particularly in the ANP group,
which exhibited a statistically significant difference when
compared with the AEP group (P<0.05). Histopathologically,
pancreases in the AEP group presented with evident edema, enlarged
interlobular septum, inflammatory cell infiltration and acinus
edema. In addition to these changes, pancreases in the ANP group
also presented with regional necrosis, lobular structural damage
and intraparenchymal hemorrhage. Pancreases in the normal control
and sham-operated groups exhibited no significant histopathological
changes. Through scoring the histopathological changes according to
the Schmid scoring criteria, the ANP group score was shown to be
notably higher compared with the AEP group (P<0.05), while both
were markedly higher compared with the normal control and
sham-operated groups (P<0.01; Table
I).
 | Table ISerum amylase, ratio of pancreas/body
weight and histopathological scores in the four groups. |
Table I
Serum amylase, ratio of pancreas/body
weight and histopathological scores in the four groups.
| Groups | Cases (n) | Amylase (U/l) | Pancreas/body weight
ratio (g/kg) | Histopathological
scores |
|---|
| Normal control | 10 | 1230.3±58.58 | 4.24±0.37 | 0.54±0.05 |
| Sham-operated | 10 | 1442.2±182.76 | 4.34±0.42 | 1.1±0.21 |
| AEP | 10 |
4376.70±342.95a | 8.67±1.43a | 5.39±0.26a |
| ANP | 10 | 6750.2±321.81b | 9.33±1.76b | 7.81±0.28b |
Levels of serum resistin, CRP, TNF-α and
IL-1β
Serum resistin, CRP, TNF-α and IL-1β levels were all
elevated in the AEP and ANP groups, as compared with those in the
normal control and sham-operated groups (P<0.01). In addition,
the levels were significantly higher in the ANP group compared with
the AEP group (P<0.05; Table
II).
 | Table IISerum resistin, TNF-α, IL-1β and CRP
levels in the four groups. |
Table II
Serum resistin, TNF-α, IL-1β and CRP
levels in the four groups.
| Groups | Cases (n) | Resistin (ng/ml) | IL-1β (pg/ml) | TNF-α (pg/ml) | CRP (ng/ml) |
|---|
| Normal control | 10 | 4.79±0.7 | 106.03±29.38 | 103.41±18.95 | 2664.19±150.20 |
| Sham-operated | 10 | 5.13±0.74 | 108.74±31.03 | 106.44±21.31 | 2894.56±165.34 |
| AEP | 10 | 10.21±1.34a | 184.18±45.24a | 194.24±44.81a | 3585.85±63.03a |
| ANP | 10 | 15.14±0.84b | 349.31±94.54b | 315.59±37.04b |
4345.04±244.14b |
Correlation analysis
Pearson’s correlation analysis was performed to
analyze the correlations among the levels of serum resistin, CRP,
TNF-α and IL-1β and the pancreatic histopathological scores. The
serum resistin level was found to positively correlate with the
serum CRP (r=0.711, P<0.01), TNF-α (r=0.871, P<0.01) and
IL-1β levels (r=0.794, P<0.01). Furthermore, the serum resistin
level was shown to positively correlate with the pancreatic
histopathological scores (r=0.812, P<0.01), as were the serum
levels of CRP, TNF-α and IL-1β (r=0.796, 0.899 and 0.788,
respectively, P<0.01).
Location of resistin
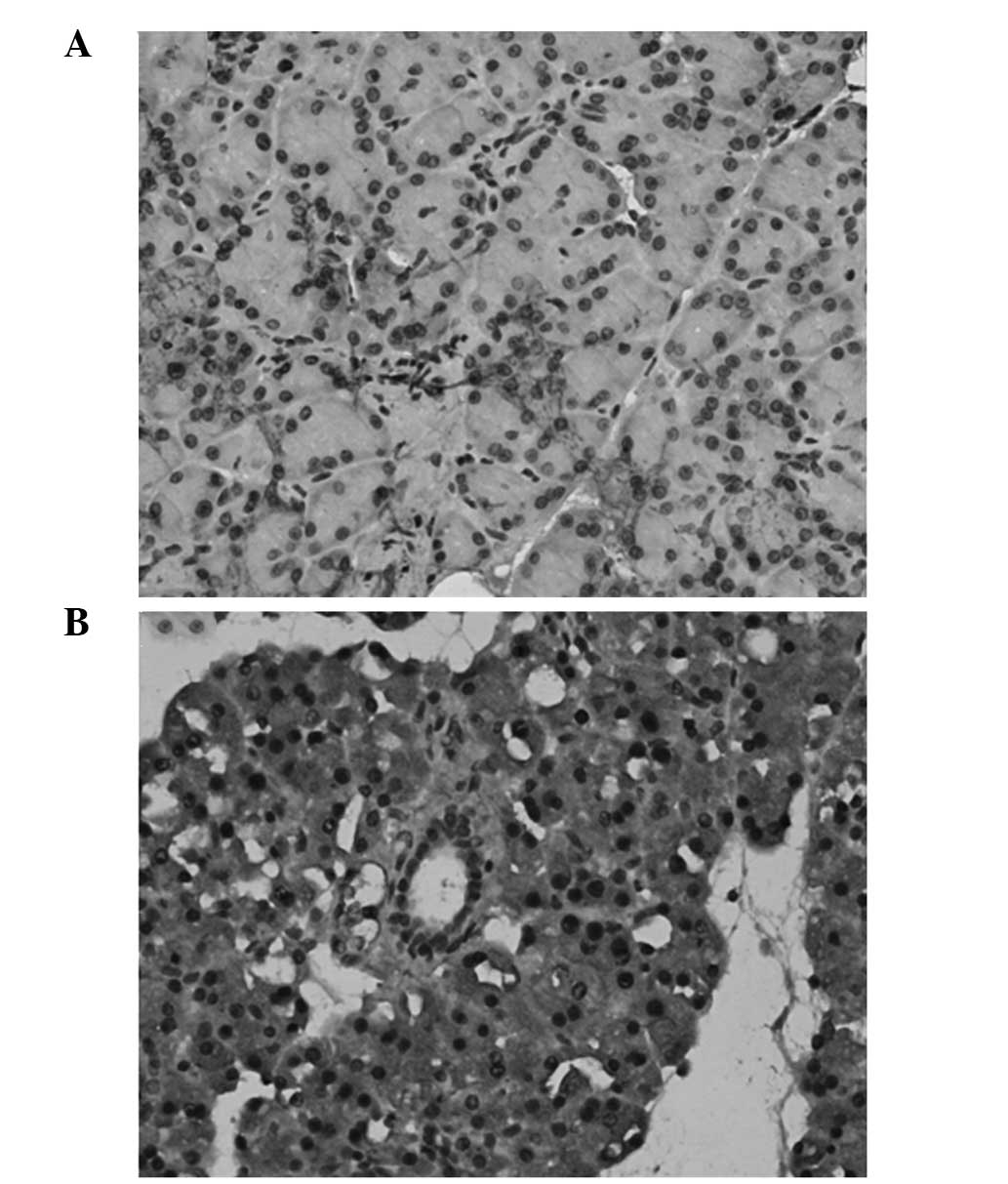
The location of resistin in the pancreatic tissues
was detected by immunohistochemical analysis. Resistin was shown to
be primarily located in the cytoplasm of the pancreatic acinar
cells and strongly expressed in the islet cells. The expression of
resistin was increased markedly in the pancreatic tissues of the
rats in the AEP and ANP groups (Fig.
1).
Resistin mRNA expression
The mRNA expression levels of resistin in the
pancreatic tissues of the rats in the ANP and AEP groups were
significantly higher compared with the control and sham-operated
groups (P<0.01). In addition, mRNA expression was significantly
higher in the ANP group compared with the AEP group (P<0.05;
Table III).
 | Table IIIResistin mRNA expression in the
pancreatic tissues of the four groups. |
Table III
Resistin mRNA expression in the
pancreatic tissues of the four groups.
| Groups | Cases (n) | mRNA expression of
resistina |
|---|
| Normal control | 10 | 0.83±0.05 |
| Sham-operated | 10 | 0.88±0.08 |
| AEP | 10 | 2.04±0.19b |
| ANP | 10 | 3.29±0.30c |
Discussion
AP is a common clinical disease. Although the
etiology and pathogenesis are not yet fully understood, the
pathogenesis of AP is associated with a disordered blood
circulation and cell apoptosis in the pancreas (18,19).
The present study revealed that serum resistin
levels, as well as resistin mRNA and protein expression, in the
pancreatic tissues increased significantly in the AEP and ANP
groups, as compared with those in the normal control rats
(P<0.01). These results indicated that resistin in the
pancreatic tissues had been activated and was associated with the
degree of inflammation. Moreover, the mRNA and protein expression
levels of resistin increased more notably in the ANP group compared
with the AEP group, indicating that resistin is activated in the
pathogenesis of AP and is involved in pancreatic tissue damage.
In the pathogenesis of AP, particularly in ANP,
patients may develop multiple organ dysfunction syndrome, the
mechanism of which is hypothesized to be associated with systemic
inflammatory response syndrome (SIRS). SIRS is considered to be an
inflammatory cascade effect involving cytokines, immune cells and
the complement system. Various pathogenic factors damage the
pancreas glandular cells and activate immature trypsin, which may
further damage the autologous pancreatic tissues in an autocrine
manner and activate monocyte-macrophage cells. Cytokines and
chemokines released from monocyte-macrophage cells recruit
inflammatory cells into the pancreas and other organs, including
the lungs, kidneys and liver. Consequently, the inflammatory cells
release more cytokines, oxygen free radicals and nitric oxide
synthase, which may cause a cascade effect and lead to a vicious
circle (20). TNF-α and IL-1β are
two of the first cytokines to increase in the pathogenesis of AP,
which can initiate a variety of cytokines to promote the formation
of pancreatic tissue edema, hemorrhage, necrosis and systemic
toxemia. In addition, these cytokines may act on vascular
endothelial cells to induce systemic symptoms, such as shock, and
increase the deterioration of pancreatitis (21). Moreover, IL-1β is an important
factor in sustained pancreatic necrosis and systemic deterioration.
IL-1β induces the release of TNF-α and initiates a feedback loop
that plays an important role in the onset and development of
inflammation (22). Thus, TNF-α
and IL-1β are hypothesized to be associated with the progression of
AP, and inhibiting their activity may reduce the severity of AP and
improve the survival rate in rats (23,24).
Norman et al (22) confirmed that the mRNA and protein
expression levels of TNF-α and IL-1β in the pancreatic tissues
increased significantly in the early stages of AP; however, the
increase was delayed in other remote organs. In the damaged organs,
TNF-α and IL-1β levels increased significantly and were associated
with the degree of organ damage (25).
The present study also demonstrated that serum TNF-α
and IL-1β levels were significantly elevated in the pathogenesis of
AEP and ANP, particularly in ANP. In addition, the levels were
found to correlate with the severity of pancreatitis. Furthermore,
serum resistin levels were shown to positively correlate with the
serum TNF-α and IL-1β levels, indicating that resistin activation
stimulated the release of TNF-α and IL-1β. The underlying mechanism
may involve the activation of nuclear factor-κB by resistin, which
subsequently stimulates the release of TNF-α and IL-1β, thereby
increasing the inflammation of the pancreas. Thus, overproduction
of obesity-related circulating resistin and associated low-grade
inflammation may result in mild injury to pancreatic acini,
increasing the risk and severity of AP (26,27).
CRP is an acute phase protein that is synthesized in
the liver and can react to the pneumococcal polysaccharide C. The
functions of CRP include binding nucleic acids and phosphatidyl
choline, activating the complement system and promoting
phagocytosis and immunoregulation. In addition, CRP is a very
sensitive indicator for acute inflammation, particularly in AP.
Thus, CRP has been used to analyze the severity of AP and evaluate
the prognosis of patients with AP (28).
In the present study, the levels of CRP were shown
to positively correlate with the pathological scores of AP. In
addition, the serum resistin levels were found to correlate with
the CRP levels and with the histopathological scores, indicating
that resistin may also be used as an indicator for evaluating the
severity of AP and the prognosis of patients with AP. Inflammatory
cytokines, including IL-6, TNF-α and leptin, are known to be
important inducers of CRP (29).
With regard to the increased expression of resistin observed in AP,
and the associations with CRP and the severity of AP, it was
hypothesized that resistin may also be a CRP inducer.
In conclusion, resistin was demonstrated to be
activated in the pathogenesis of AP and involved in the injury
process of pancreatic tissues. The expression of resistin was found
to be associated with the severity of the damage in the pancreatic
tissues. Therefore, resistin may be used as an indicator to assess
the severity and prognosis of AP, and the hormone may prove a prime
target for future therapeutic interventions.
Acknowledgements
This study was supported by the Social Developmental
fund of Changzhou, China (grant no. CS2008212).
Abbreviations:
|
AEP
|
acute edematous pancreatitis
|
|
ANP
|
acute necrotizing pancreatitis
|
|
AP
|
acute pancreatitis
|
|
SIRS
|
systemic inflammatory response
syndrome
|
|
CRP
|
C-reactive protein
|
|
IL
|
interleukin
|
|
TNF-α
|
tumor necrosis factor-α
|
|
PCR
|
polymerase chain reaction
|
References
|
1
|
Castro FS, Nascimento AM, Coutinho IA,
Alcazar FR and Mugayar Filho J: Plasmapheresis as a therapeutic
approach for hypertriglyceridemia-induced acute pancreatitis. Rev
Bras Ter Intensiva. 24:302–307. 2012. View Article : Google Scholar
|
|
2
|
Thandassery RB, Yadav TD, Dutta U,
Appasani S, Singh K and Kochhar R: Dynamic nature of organ failure
in severe acute pancreatitis: the impact of persistent and
deteriorating organ failure. HPB (Oxford). 15:523–528. 2013.
View Article : Google Scholar
|
|
3
|
Geoffroy PA, Etain B, Henry C and
Bellivier F: Combination therapy for manic phases: a critical
review of a common practice. CNS Neurosci Ther. 18:957–964. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woo SM, Noh MH, Kim BG, et al: Comparison
of serum procalcitonin with Ranson, APACHE-II, Glasgow and
Balthazar CT severity index scores in predicting severity of acute
pancreatitis. Korean J Gastroenterol. 58:31–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda K, Yokoe M, Takada T, et al:
Assessment of severity of acute pancreatitis according to new
prognostic factors and CT grading. J Hepatobiliary Pancreat Sci.
17:37–44. 2010. View Article : Google Scholar
|
|
6
|
Wu BU and Banks PA: Clinical management of
patients with acute pancreatitis. Gastroenterology. 144:1272–1281.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sempere L, Martinez J, de Madaria E, et
al: Obesity and fat distribution imply a greater systemic
inflammatory response and a worse prognosis in acute pancreatitis.
Pancreatology. 8:257–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papachristou GI, Clermont G, Sharma A,
Yadav D and Whitcomb DC: Risk and markers of severe acute
pancreatitis. Gastroenterol Clin North Am. 36:277–296. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coelho M, Oliveira T and Fernandes R:
Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci.
9:191–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steppan CM, Bailey ST, Bhat S, et al: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu CL, Lin YJ, Ho CT and Yen GC: The
inhibitory effect of pterostilbene on inflammatory responses during
the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J
Agric Food Chem. 61:602–610. 2013. View Article : Google Scholar
|
|
12
|
Minn AH, Patterson NB, Pack S, et al:
Resistin is expressed in pancreatic islets. Biochem Biophys Res
Commun. 310:641–645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daniel P, Leśniowski B, Mokrowiecka A,
Jasińska A, Pietruczuk M and Małecka-Panas E: Circulating levels of
visfatin, resistin and pro-inflammatory cytokine interleukin-8 in
acute pancreatitis. Pancreatology. 10:477–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leśniowski B, Kumor A, Jasińska A, Daniel
P, Pietruczuk M and Małecka-Panas E: Resistin - a new laboratory
marker useful in diagnosis of acute pancreatitis? Pol Merkur
Lekarski. 22:385–387. 2007.(In Polish).
|
|
15
|
Schäffler A, Landfried K, Völk M, Fürst A,
Büchler C, Schölmerich J and Herfarth H: Potential of
adipocytokines in predicting peripancreatic necrosis and severity
in acute pancreatitis: pilot study. J Gastroenterol Hepatol.
22:326–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stefanutti C, Labbadia G and Morozzi C:
Severe hypertriglyceridemia-related acute pancreatitis. Ther Apher
Dial. 17:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cruz-Santamaría DM, Taxonera C and Giner
M: Update on pathogenesis and clinical management of acute
pancreatitis. World J Gastrointest Pathophysiol. 3:60–70. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charbonney E and Nathens AB: Severe acute
pancreatitis: a review. Surg Infect (Larchmt). 9:573–578. 2008.
View Article : Google Scholar
|
|
20
|
Mitchell RMS, Byrne MF and Baillie J:
Pancreatitis. Lancet. 361:1447–1455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gómez-Cambronero LG, Sabater L, Pereda J,
Cassinello N, Camps B, Viña J and Sastre J: Role of cytokines and
oxidative stress in the pathophysiology of acute pancreatitis:
therapeutical implications. Curr Drug Targets Inflamm Allergy.
1:393–403. 2002. View Article : Google Scholar
|
|
22
|
Norman JG, Fink GW, Messina J, Carter G
and Franz MG: Timing of tumor necrosis factor antagonism is
critical in determining outcome in murine lethal acute
pancreatitis. Surgery. 120:515–521. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hughes CB, Grewal HP, Gaber LW, Kotb M,
El-din AB, Mann L and Gaber AO: Anti-TNFalpha therapy improves
survival and ameliorates the pathophysiologic sequelae in acute
pancreatitis in the rat. Am J Surg. 171:274–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka N, Murata A, Uda K, et al:
Interleukin-1 receptor antagonist modifies the changes in vital
organs induced by acute necrotizing pancreatitis in a rat
experimental model. Crit Care Med. 23:901–908. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Norman JG, Fink GW, Denham W, et al:
Tissue-specific cytokine production during experimental acute
pancreatitis. A probable mechanism for distant organ dysfunction.
Dig Dis Sci. 42:1783–1788. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calabrò P, Cirillo P, Limongelli G, et al:
Tissue factor is induced by resistin in human coronary artery
endothelial cells by the NF-κB-dependent pathway. J Vasc Res.
48:59–66. 2011. View Article : Google Scholar
|
|
27
|
Jiang CY, Wang W, Tang JX and Yuan ZR: The
adipocytokine resistin stimulates production of proinflammatory
cytokines TNF-α and IL-6 in pancreatic acinar cells via NF-κB
activation. J Endocrinol Invest. 36:986–992. 2013.PubMed/NCBI
|
|
28
|
Mayer JM, Raraty M, Slavin J, et al: Serum
amyloid A is a better early predictor of severity than C-reactive
protein in acute pancreatitis. Br J Surg. 89:163–171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. J Pathol. 190:117–125. 2000. View Article : Google Scholar : PubMed/NCBI
|