Introduction
High mobility group box 1 (HMGB1) is a ubiquitous
and abundant nuclear protein. HMGB1 can be passively released into
the extracellular space in response to necrotic signals or actively
secreted in response to inflammatory signals (1,2).
Previous studies have shown that HMGB1 functions as a
proinflammatory cytokine in certain cardiovascular diseases, and is
associated with the severity of coronary artery disease (3,4).
Statins (3-hydroxy 3-methylglutaryl coenzyme A
reductase inhibitors) are a class of cholesterol-lowering drug that
are widely used in the prevention and treatment of cardiovascular
diseases (5). Previous studies
have shown that preconditioning with statins may attenuate
myocardial injury in rats following acute myocardial infarction
(AMI) (6,7).
In cases of acute myocardial infarction, rapid
reperfusion by percutaneous coronary intervention or thrombolytic
therapy is important to salvage myocardial tissue from necrosis and
reduce the size of infarct tissue. Paradoxically, reperfusion is
frequently associated with an exacerbation of tissue injury,
enlargement of the infarct size, and a marked inflammatory
response, known as ischemia/reperfusion injury (8). Ischemia/reperfusion injury is defined
as the necrosis of those cells that remained viable following a
period of ischemia.
Postconditioning is defined as the rapid sequential
intermittent interruption of blood flow applied during early
moments of reperfusion. Postconditioning has been demonstrated to
attenuate organ injury, including the heart (9), spinal cord (10), brain (11), kidneys (12), liver (13), muscle (14), lungs (15) and intestines (16) in previous studies. Drug
postconditioning involves the administration of a protective
substance immediately prior to reperfusion and may be performed as
a post-ischemic intervention to reduce myocardial tissue damage
during the study of ischemia/reperfusion injury. There are a number
of controversial issues regarding the efficacy of postconditioning
in various animal models with comorbidities; however, the majority
of preclinical studies have indicated that postconditioning is an
effective intervention for reducing organ necrosis and apoptosis.
Ischemia postconditioning may be able to reduce
ischemia/reperfusion injury in cases of myocardial infarction by
inhibiting the inflammatory response and reducing the levels of
tumour necrosis factor (TNF)-α and interleukin-6 (17).
Simvastatin is a type of HMG-CoA reductase
inhibitors. As simvastatin is inexpensive, it has been widely used
in the treatment of coronary heart disease and associated
conditions in China.
However, whether postconditioning with simvastatin
is able to alleviate myocardial injury is yet to be fully
understood. Thus, the present study investigated the effect of
simvastatin postconditioning on myocardial ischemia reperfusion
injury, and the mechanism underlying the protective effect.
Materials and methods
Animal model
The study was approved by the Institutional Review
Board of Liaocheng People’s Hospital of Shandong Province
(Liaocheng, China). In total, 30 male Sprague-Dawley rats (weight,
250–300 g) were obtained from the Experimental Laboratory of
Shandong University of Traditional Chinese Medicine (Jinan, China).
The rats were housed in a quiet environment with a humidity of
60±5% and a temperature of 22±2°C. A 12-h light-dark cycle (light
beginning at 8am) was applied, with all operations performed during
the light phase of the cycle. The rats were divided at random into
three groups that each received a different treatment. The sham
operation group (sham; n=10) rats underwent surgery without the
ligation of the left anterior descending artery (LAD). AMI group
(n=10) rats were subjected to LAD occlusion for 30 min, followed by
reperfusion for 180 min. The simvastatin group (sim; n=10) rats
were subjected to LAD occlusion for 30 min, followed by reperfusion
for 180 min. Furthermore, 20 mg/kg simvastatin was dissolved in
saline and injected intravenously at <5 min prior to reperfusion
(6). Rats in the sham and AMI
groups received equal volumes of normal saline at the same time
points.
A rat model of AMI was established in accordance
with previously reported methods (7,18).
The rats were anesthetized with an intraperitoneal injection of 60
mg/kg sodium pentobarbital and the trachea was cannulated for
artificial ventilation with room air at a rate of 55 breaths/min.
The inspiratory/expiratory ratio was set to 1:1 and the tidal
volume was adjusted to 2–3 ml/100 g body weight. The body
temperature of the rats was maintained at 37±0.5°C with a heating
pad. Lead II of an electrocardiogram (ECG) was monitored with
stainless needle electrodes that were attached to the rat limbs.
The ECG was recorded and analyzed using an ECG-6511 data
acquisition system (Guangdian Medical Devices Co., Ltd., Shanghai,
China).
An incision was made by a left thoracotomy through
the fourth intercostal space. Following a pericardiotomy, a 5-0
silk ligature attached to a small needle was placed around the left
coronary artery close to its origin, and complete occlusion of the
coronary artery was verified by ST segment elevation on the body
surface ECG. In the sham operation group, the needle and ligature
were placed at the origin of the left coronary artery; however,
complete ligation was not conducted. Prior to ligation of the LAD,
200 IU/kg heparin was intravenously administered.
Biochemical analysis
Following reperfusion for 180 min, blood samples
were obtained from the right femoral vein. After leaving to settle
for 30 min, the blood samples were centrifuged at 1,400 × g for 10
min. The serum samples were stored at −80°C for subsequent
analysis. Serum levels of TNF-α and cardiac troponin (c-TnI) were
measured using previously reported methods (7).
Following reperfusion for 180 min, the rat hearts
were removed and washed with normal saline. A 0.5-g sample of
ischemic heart tissue was obtained and ground to powder at 0–4°C.
Next, the myocardial homogenate was centrifuged at 2,000 × g for 30
min. The supernatant was collected and stored at −80°C until
required for the MDA concentration and SOD activity assays. An MDA
assay kit and SOD assay kit (Nanjing Jiancheng Bioengineering, Co.
Ltd., Nanjing, China) were used to measure the MDA concentration
and SOD activity, following the manufacturer’s instructions.
Assessment of infarct size
Infarct size was assessed by
2,3,5-triphenyltetrazolium chloride (TTC) staining, using a
previously reported method (7).
Following reperfusion for 180 min, the LAD was reoccluded and 1 ml
Evans blue dye (2.0%) was injected via the femoral vein. The entire
heart was excised, rinsed of excess Evans blue dye and the right
ventricle and right and left atria were removed. The remaining left
ventricle was frozen at −80°C. The frozen left ventricle was sliced
horizontally from the apex to the base, yielding five slices. The
slices were incubated in 1% TTC for 15 min at 37°C. The viable
myocardium was stained red and the infarcted myocardium was stained
white. The slices were photographed using a PowerShot N digital
camera (Canon, Inc., Tokyo, Japan). The borders of the infarct,
ischemic and nonischemic areas of the heart images were traced and
measured using Image-Pro Plus 3.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). Infarct sizes were expressed as a percentage
of the risk area volume (infarct size/risk area).
Western blot analysis of HMGB1 expression
levels
Myocardial HMGB1 protein expression levels were
measured using western blot analysis, following previously reported
methods (7). Myocardial proteins
were extracted using a lysis buffer containing 20 mM Tris (pH 7.5),
150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1mM
Na3VO4 and 1 μg/ml leupeptin.
Phenylmethylsulfonyl fluoride (1 mM) was added immediately prior to
use. Protein concentrations were determined using Pierce
bicinchoninic acid protein assay kit (#23225; Pierce Biotechnology
Inc., Rockford, IL, USA), and 50 μg protein was loaded onto and
separated by SDS-PAGE and subsequently transferred onto a
polyvinylidene fluoride membrane. After blocking for 30 min with 5%
skimmed milk, the membranes were incubated with a monoclonal rabbit
anti-HMGB1 primary antibody (1/30,000, #ab79823; Abcam, Cambridge,
UK) for 1 h, washed and incubated for 30 min with a goat
anti-rabbit IgG (H+L) horseradish peroxidase-conjugated secondary
antibody (1:1,000, #A0208; Beyotime Institute of Biotechnology,
Haimen, China). Bands were detected with with BeyoECL Plus enhanced
chemiluminescence reagent (#P0018; Beyotime Institute of
Biotechnology). β-Actin (Abcam) was detected as a loading
control.
Statistical analysis
Data are expressed as the mean ± standard deviation
or as percentages where appropriate. SAS 6.12 software (SAS
Institute, Inc., Cary, NC, USA) was used for statistical
processing. One-way analysis of variance was used to analyze the
mean values between groups, where P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum levels of c-TnI and TNF-α
Serum levels of c-TnI and TNF-α in the sim group
decreased when compared with the AMI group (P<0.05; Table I). In addition, serum levels of
c-TnI and TNF-α in the sham group were lower when compared with the
AMI and sim groups (P<0.05).
 | Table IValues of c-TnI, TNF-α, SOD, MDA and
IS. |
Table I
Values of c-TnI, TNF-α, SOD, MDA and
IS.
| Variables | Sham (n=10) | AMI (n=10) | Sim (n=10) |
|---|
| c-TnI (ng/ml) | 0.96±0.34 | 14.21±9.24a | 7.42±4.06ab |
| TNF-α (pg/ml) | 0.75±0.21 | 1.76±0.33a | 1.14±0.24ab |
| SOD (μU/l) | 146.52±14.37 | 95.76±12.45a | 112.05±13.42ab |
| MDA (μmol/l) | 1.81±0.83 | 5.11±1.17a | 3.43±1.41ab |
| IS (%) | - | 56.38±8.58 | 24.37±4.2b |
MDA levels and SOD activity
Following reperfusion for 180 min, the MDA levels in
the AMI group increased significantly, while the SOD activity
levels decreased significantly, when compared with the sham group
(P<0.05). The increase in MDA levels and reduction in SOD
activity were significantly inhibited by simvastatin treatment
(P<0.05; Table I).
Comparison of infarct size between the
groups
Infarct sizes in the sim group rats were
significantly decreased when compared with the AMI group rats
(P<0.05).
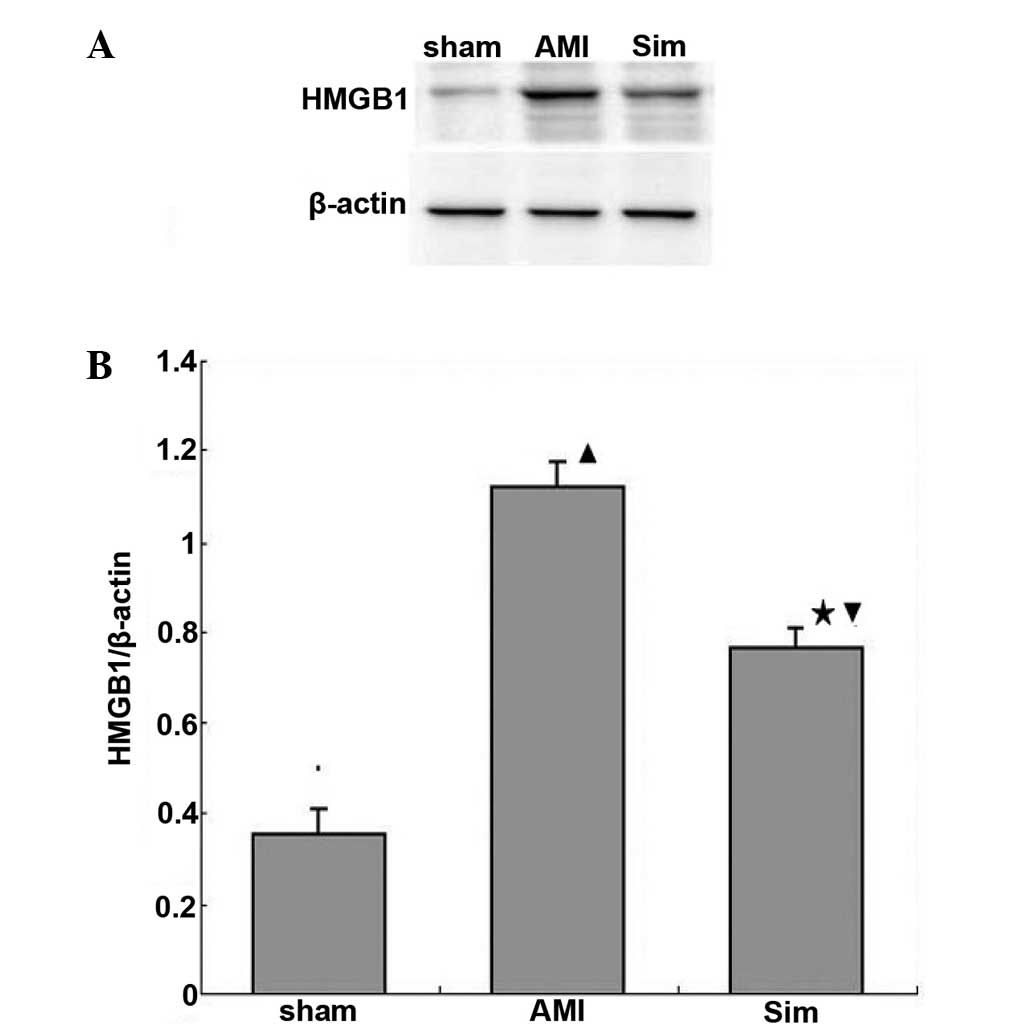
Protein expression levels of HMGB1
Expression levels of HMGB1 protein in the myocardium
decreased in the sim group when compared with the AMI group
(P<0.05; Fig. 1).
Discussion
The present study demonstrated that myocardial
expression of HMGB1 significantly increases in rats with AMI and
postconditioning with simvastatin reduces the infarct size,
exerting an anti-inflammatory effect and decreasing the myocardial
expression of HMGB1. These results indicated that simvastatin
postconditioning may alleviate myocardial injury following acute
myocardial ischemia by reducing the expression of HMGB1.
Reperfusion of the ischemic myocardium is important
for protecting myocardial tissue against necrosis following acute
myocardial ischemia. However, the premature opening of an occluded
coronary artery may result in myocardial ischemia/reperfusion
injury (19). Therefore, the
alleviation of myocardial ischemia/reperfusion injury is an
important approach for the management of acute myocardial
ischemia.
TNF-α strongly induces apoptosis and necrosis in
myocytes and is known to stimulate the remodeling process and
provoke myocardial dysfunction following AMI (20). C-TnI, which may be released in
infarct areas of the myocardium, is often elevated in cases of AMI.
Previous studies have revealed that TNF-α and c-TnI are
significantly elevated in AMI patients (4,7).
HMGB1 is actively secreted by macrophages and monocytes, or
released by necrotic cells into the extracellular milieu, where the
protein may trigger inflammation (21). A previous study showed that HMGB1
was able to stimulate the production of TNF-α (22).
In accordance with these previous studies, the
present study demonstrated that the ischemia-induced upregulation
of TNF-α was inhibited by postconditioning with simvastatin.
Furthermore, the myocardial expression of HMGB1 was decreased by
postconditioning with simvastatin. Therefore, it is feasible that
simvastatin, which is able to substantially reduce the expression
of proinflammatory factors, may exert a cardioprotective effect by
decreasing the myocardial expression of HMGB1.
During the process of ischemia/reperfusion injury, a
cascade of inflammatory responses are initiated, including
oxidative stress generation and cytokine production (23). Proinflammatory cytokines may
further promote inflammatory cell adhesion and infiltration into
the ischemic myocardium and enhance acute tissue injury (24). Previous studies have shown that
hypoxic hepatocytes release HMGB1 through an active process
facilitated by the production of radical oxygen species (ROS). ROS,
in turn, induce the release of HMGB1. Antioxidants are able to
reduce the release of HMGB1 and liver damage in liver
ischemia/reperfusion injury (25,26).
MDA levels are often used as an index of ROS, while SOD activity is
used as an indicator of lipid superoxide levels in the ischemic
myocardium.
In the present study, the MDA levels in the AMI
group were increased significantly and the SOD activity levels were
significantly decreased when compared with the sham group. The
increase in MDA levels and reduction in SOD activity were
significantly inhibited by simvastatin postconditioning.
Furthermore, the myocardial expression of HMGB1 and the infarct
size in the sim group were significantly reduced when compared with
the AMI group. These results indicated that simvastatin may exert a
cardioprotective effect by decreasing the myocardial expression of
HMGB1, which may be associated with the myocardial ischemia-induced
inhibition of ROS. This conclusion was consistent with the results
of a recent study (27).
The exact mechanism by which simvastatin protects
the heart against myocardial injury caused by acute myocardial
ischemia remains unclear. A prior study showed that when Toll-like
receptor 4 was mutated or deficient, the rate of cardiomyocyte
apoptosis and the infarct size decreased, with the expression of
HMGB1 and TNF-α notably inhibited following myocardial
ischemia/reperfusion (28).
Furthermore, it has been reported that the cardioprotective effects
of simvastatin against infarction are associated with a reduction
in myocardial edema. Simvastatin exerts this effect on edema by
suppressing the expression of aquaporin-1, -4, -8 and -9 in a
partially protein kinase A-dependent manner (29). However, the underlying mechanisms
of the infarct size-limiting and cardioprotective effects of
simvastatin are not yet fully understood.
The present study was consistent with previous
studies in finding that postconditioning with simvastatin may
attenuate myocardial injury induced by acute myocardial ischemia.
In particular, the present study showed that this effect may be
associated with the downregulation of HMGB1 expression in the
myocardium.
In conclusion, postconditioning with simvastatin
inhibited the myocardial expression of HMGB1, which may be
associated with its cardioprotective effects.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Shandong Province (no. ZR2013HL017) and the
Natural Science Foundation of Liaocheng City (no. 2012NS13).
References
|
1
|
Lakhan SE, Kirchgessner A and Hofer M:
Inflammatory mechanisms in ischemic stroke: therapeutic approaches.
J Transl Med. 7:972009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oozawa S, Mori S, Kanke T, Takahashi H,
Liu K, Tomono Y, Asanuma M, Miyazaki I, Nishibori M and Sano S:
Effects of HMGB1 on ischemia-reperfusion injury in the rat heart.
Circ J. 72:1178–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrassy M, Volz HC, Riedle N, Gitsioudis
G, Seidel C, Laohachewin D, Zankl AR, Kaya Z, Bierhaus A,
Giannitsis E, et al: HMGB1 as a predictor of infarct transmurality
and functional recovery in patients with myocardial infarction. J
Intern Med. 270:245–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao HC, Zhao AP, Han QF, Wu L, Yao DK and
Wang LX: Correlation between serum high-mobility group box-1 levels
and high-sensitivity C-reactive protein and troponin I in patients
with coronary artery disease. Exp Ther Med. 6:121–124.
2013.PubMed/NCBI
|
|
5
|
Wang CY, Liu PY and Liao JK: Pleiotropic
effects of statin therapy: molecular mechanisms and clinical
results. Trends Mol Med. 14:37–44. 2008. View Article : Google Scholar
|
|
6
|
Ke D, Fang J, Fan L, Chen Z and Chen L:
Regulatory T cells contribute to rosuvastatin-induced
cardioprotection against ischemia-reperfusion injury. Coron Artery
Dis. 24:334–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao HC, Han QF, Wang LH, Wu L, Liu T, Fu
ZL, Tian KL and Zhang M: Simvastatin attenuates myocardial injury
by inhibiting the expression of high mobility group box 1 in
myocardium of rats following acute myocardial infarction. Exp Clin
Cardiol. 20:2342. 2014.
|
|
8
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion - from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan Q, Yang XC, Liu Y, Wang LF, Liu SH, Ge
YG, Chen ML, Wang W, Zhang LK, Irwin MG and Xia Z: Postconditioning
attenuates myocardial injury by reducing nitro-oxidative stress in
vivo in rats and in humans. Clin Sci (Lond). 120:251–261. 2011.
|
|
10
|
Jiang XJ, Ai CY, Shi EY, Nakajima Y and Ma
H: Neuroprotection against spinal cord ischemia-reperfusion injury
induced by different ischemic postconditioning methods: Roles of
phosphatidylinositol 3-kinase-Akt and extracellular
signal-regulated kinase. Anesthesiology. 111:1197–1205. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong Y, Rogers MR and Qin X: Effective
neuroprotection by ischemic postconditioning is associated with a
decreased expression of RGMa and inflammation mediators in ischemic
rats. Neurochem Res. 38:815–825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu
H and Chen Z: Ischemic postconditioning inhibits apoptosis after
renal ischemia/reperfusion injury in rat. Transpl Int. 21:364–371.
2008. View Article : Google Scholar
|
|
13
|
Knudsen AR, Kannerup AS, Dich R,
Funch-Jensen P, Grønbaek H, Kruhøffer M and Mortensen FV: Ischemic
pre- and postconditioning has pronounced effects on gene expression
profiles in the rat liver after ischemia/reperfusion. Am J Physiol
Gastrointest Liver Physiol. 303:G482–G489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang H, Yu F, Tong Z, Yuan B and Wang C:
Effect of ischemia postconditioning on skeletal muscle oxidative
injury, mTOR, Bax, Bcl-2 proteins expression, and HIF-1α/β-actin
mRNA, IL-6/β-actin mRNA and caveolin-3/β-actin mRNA expression in
ischemia-reperfusion rabbits. Mol Biol Rep. 40:507–514. 2013.
View Article : Google Scholar
|
|
15
|
Cao QF, Qu MJ, Yang WQ, Wang DP, Zhang MH
and Di SB: Ischemia postconditioning preventing lung
ischemia-reperfusion injury. Gene. 554:120–124. 2015. View Article : Google Scholar
|
|
16
|
Leng YF, Zhang Y, Zhang Y, Xue X, Wang T
and Kang YQ: Ischemic post-conditioning attenuates the intestinal
injury induced by limb ischemia/reperfusion in rats. Braz J Med
Biol Res. 44:411–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao ZQ: Postconditioning in reperfusion
injury: A status report. Cardiovasc Drugs Ther. 24:265–279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao HC, Liu T, Meng XY, Han QF, Zhang M
and Wang LX: Effect of basic fibroblast growth factor on the
myocardial expression of hypoxia-inducible factor-1α and vascular
endothelial growth factor following acute myocardial infarction.
Heart Lung Circ. 22:946–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braunwald E and Kloner RA: Myocardial
reperfusion: a double-edged sword? J Clin Invest. 76:1713–1719.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skyschally A, Gres P, Hoffmann S, Haude M,
Erbel R, Schulz R and Heusch G: Bidirectional role of tumor
necrosis factor-alpha in coronary microembolization: progressive
contractile dysfunction versus delayed protection against
infarction. Circ Res. 100:140–146. 2007. View Article : Google Scholar
|
|
21
|
Bonaldi T, Talamo F, Scaffidi P, Ferrera
D, Porto A, Bachi A, Rubartelli A, Agresti A and Bianchi ME:
Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect
it towards secretion. EMBO J. 22:5551–5560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren D, Sun R and Wang S: Role of inducible
nitric oxide synthase expressed by alveolar macrophages in high
mobility group box 1-induced acute lung injury. Inflamm Res.
55:207–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prasad A, Stone GW, Holmes DR and Gersh B:
Reperfusion injury, microvascular dysfunction, and
cardioprotection: the ‘dark side’ of reperfusion. Circulation.
120:2105–2112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitano K, Usui S, Ootsuji H, Takashima S,
Kobayashi D, Murai H, Furusho H, Nomura A, Kaneko S and Takamura M:
Rho-kinase activation in leukocytes plays a pivotal role in
myocardial ischemia/reperfusion injury. PLoS One. 9:e922422014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsung A, Klune JR, Zhang X, Jeyabalan G,
Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR and Billiar TR:
HMGB1 release induced by liver ischemia involves Toll-like receptor
4 dependent reactive oxygen species production and calcium-mediated
signaling. J Exp Med. 204:2913–2923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu X, Zhou X, He B, Xu C, Wu L, Cui B, Wen
H, Lu Z and Jiang H: Minocycline protects against myocardial
ischemia and reperfusion injury by inhibiting high mobility group
box 1 protein in rats. Eur J Pharmacol. 638:84–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du X, Hu X and Wei J: Postconditioning
with rosuvastatin reduces myocardial ischemia-reperfusion injury by
inhibiting high mobility group box 1 protein expression. Exp Ther
Med. 7:117–120. 2014.
|
|
28
|
Ding HS, Yang J, Chen P, Yang J, Bo SQ,
Ding JW and Yu QQ: The HMGB1-TLR4 axis contributes to myocardial
ischemia/reperfusion injury via regulation of cardiomyocyte
apoptosis. Gene. 527:389–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li XD, Yang YJ, Geng YJ, et al: The
cardioprotection of simvastatin in reperfused swine hearts relates
to the inhibition of myocardial edema by modulating aquaporins via
the PKA pathway. Int J Cardiol. 167:2657–2666. 2013. View Article : Google Scholar
|