Introduction
Despite the fact that the incidence of gastric
cancer (GC) has decreased substantially over the past few decades,
it remains the fourth most common cancer and the second leading
cause of cancer-related mortality worldwide (1). Generally, surgical resection with
adjuvant chemotherapy is used to treat GC (2); however, the overall survival (OS)
remains poor and no standard treatment has been determined.
Discovering new molecular biological prognostic factors could
provide a more accurate prediction of clinical outcome and help in
the management of patients with GC.
Excision repair cross-complementing group 1 (ERCC1)
protein, serving as a rate-limiting enzyme, is a key component in
the nucleotide excision repair (NER) pathway (3). The NER pathway functions to remove
bulky adducts that are introduced by platinum-containing drugs,
such as cisplatin and oxaliplatin (4,5).
Platinum-based chemotherapy for GC is one of the most widely used
types of anticancer treatment (6,7).
Previously, numerous studies have investigated the association
between ERCC1 expression and survival in GC (8–10).
In brief, they indicate that ERCC1 is not only a prognostic marker
for survival but also a predictor of the response to platinum
compounds; however the previous studies yielded inconsistent
results and no robust evidence. Considering the potential value of
ERCC1, a meta-analysis has been conducted to provide evidence-based
results on the prognostic and predictive utility of ERCC1 in
GC.
Materials and methods
Search strategy and inclusion
criteria
A comprehensive search of Medline and Embase
databases was conducted for all relevant literature published in
the English language up to April 1, 2014. The medical subject
headings for the search were ‘ERCC1’ and ‘gastric cancer’. In order
to find additional studies, the references cited in the papers
found in the database search were also manually searched. The
eligible studies included in this meta-analysis met the following
criteria: i) Patients with GC; ii) evaluation of ERCC1 expression
and OS; and iii) presented the data for OS or data that allowed the
OS to be calculated. When the patient population was duplicated,
only the most recent or most complete study was included.
Data extraction
Two authors independently reviewed all the
potentially relevant studies and extracted the data required using
standard forms. The following information was collected from the
eligible studies: Surname of first author; year of publication;
country; sample size; ERCC1 expression assessment method; cutoff
values for high/positive vs. low/negative ERCC1 expression;
stage; neoadjuvant or adjuvant chemotherapy; the hazard ratios
(HRs) and their 95% confidence intervals (CIs). If HR was not
directly reported, the HR estimate and its variance were
reconstructed based on published methodology (11). Disagreements were resolved through
discussion among the authors.
Quality assessment
The study quality was evaluated by two investigators
using the scale reported previously (Table I) (12,13).
Briefly, this scale contained seven elements: i) The inclusion and
exclusion criteria for patients; ii) the study design; iii)
characteristics of the patients; iv) ERCC1 expression ascertainment
method; v) the study endpoint; vi) follow-up time; and vii) time
lost to follow-up. A high quality element was awarded one point and
a maximum of two points was awarded for the method used to measure
ERCC1 expression; hence, the maximum score was eight points. Each
score provided by a different reader was compared and a consensus
was achieved.
 | Table ICriteria for quality assessment by De
Graeff (12). |
Table I
Criteria for quality assessment by De
Graeff (12).
| Score |
|---|
|
|
|---|
| Criteria | Sub-criteria | Criteria |
|---|
| 1. Is the population
under study defined with in and exclusion criteria? | | 1 |
| 2. Were patient data
prospectively collected? | | 1 |
| 3. Are the main
prognostic patient and tumor characteristics presented?a | | 1 |
| 4. Is the method used
for determination of protein expression specified? | | 2 |
| Criteria for
immunohistochemistry: |
| Is the
immunohistochemical staining protocol specified?b | 1 | |
| Were stainings
evaluated by >1 observer? | 1 | |
| Criteria for
RT-PCR: |
| Is the RNA
isolation method and cDNA synthesis specified? | 1 | |
| Is the PCR protocol
specified?c | 1 | |
| 5. Is the study
endpoint defined? | | 1 |
| 6. Is the time of
follow up specified? | | 1 |
| 7. Is loss during
analysis or follow up described? | | 1 |
| Total | | 8 |
Statistical analysis
The individual HRs corresponding to their 95% CIs
were pooled into a summary HR to evaluate the association between
ERCC1 level and OS. The significance of the summary HR was measured
by a Z-test; P≤0.05 was considered to indicate statistical
significance. Fixed-effects models were used with the assumption of
homogeneity of studies in the first stage. The assumption was
examined by assessing the heterogeneity across studies using
χ2 test and I2. If the heterogeneity was
significant between studies (Pheterogeneity<0.1 and
I2>50%), a random-effects model was performed in the
second stage. Additionally, random-effects models were applied in
cases where there was qualitative evidence of methodological
heterogeneity within studies (e.g., different methods of measuring
ERCC1 expression). To explore the possible heterogeneity among
different studies, the following key characteristics were examined
in a meta-regression model: Study location; ERCC1 expression
ascertainment method; sample size; HR estimation method; and
quality score. Sensitivity analysis was carried out by sequential
omission of each study. Publication bias was evaluated with funnel
plots, Begg’s test and Egger’s test (14). The analyses were performed with
STATA software (version 12.0; Stata, College Station, TX, USA).
Results
Search results, study characteristics and
quality assessment
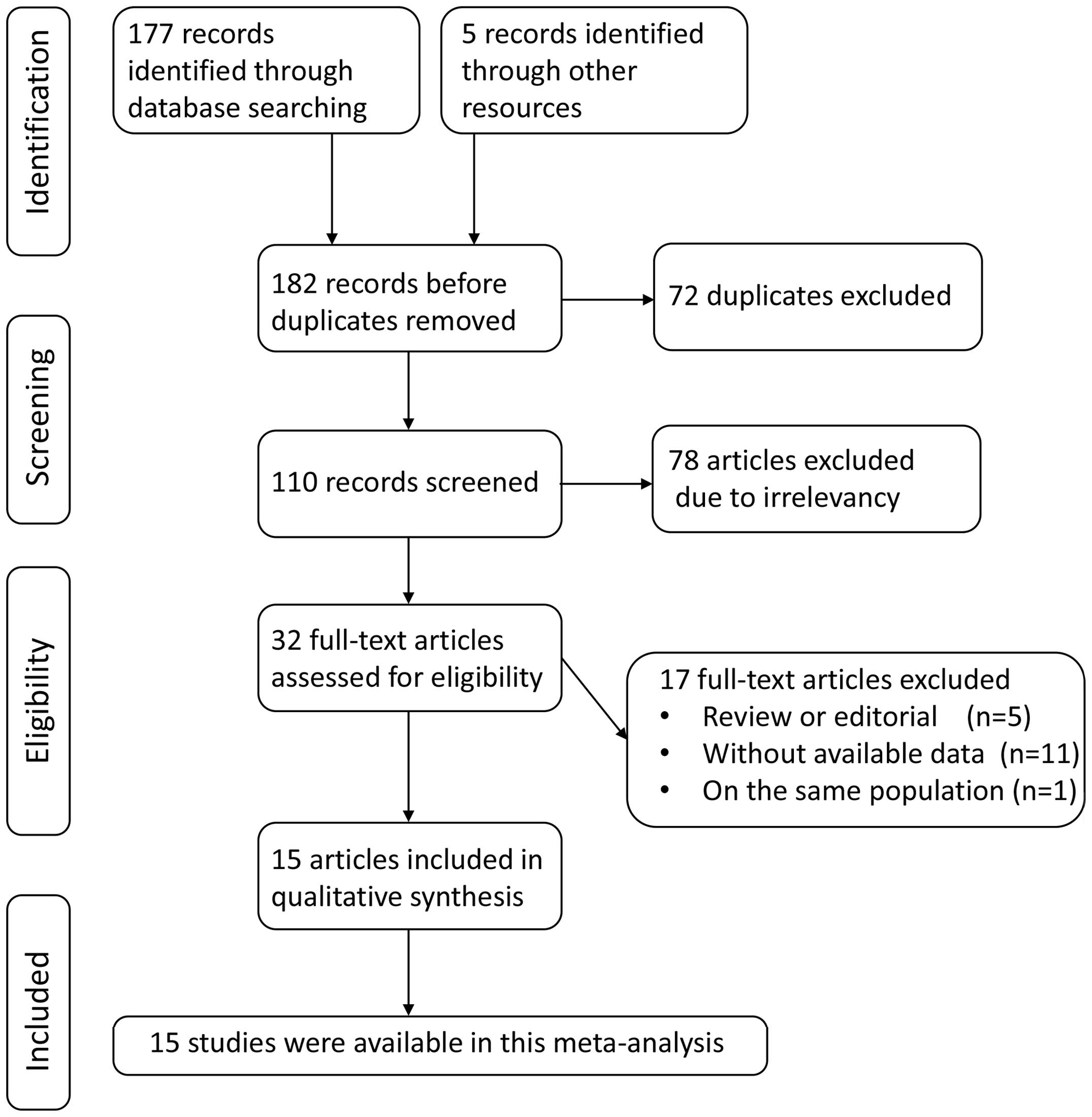
According to the search strategy, a total of 182
articles that mentioned ERCC1 expression and GC were identified.
Following the removal of duplicates, 110 abstracts were screened
based on the inclusion criteria. Among them, 32 articles remained
for detailed evaluation. Seventeen of those 32 articles were
subsequently excluded for the following reasons: Review or
editorial (n=5); without available data (n=11); or on the same
population (n=1). Finally, 15 studies were selected for this
meta-analysis (8–10,15–26)
(Fig. 1).
The main characteristics of the 15 studies are
summarized in Table II. The
sample size ranged from 41 to 322. Eleven studies were based in
Asia, three in Europe and one in North America. The expression of
ERCC1 was evaluated by immunohistochemistry (IHC) in ten studies,
and reverse transcription polymerase chain reaction (RT-PCR) in
five studies. Different cutoff values of ERCC1 expression
evaluation were used. IHC was mainly divided by staining intensity
and the percentage of cells stained, whereas ERCC1 mRNA levels were
categorized according to median values and maximal χ2
method. In addition, the patients were receiving chemotherapy; most
of them were using platinum-based regimens. The quality scores of
included studies are summarized in Table II and ranged from 5 to 8.
 | Table IIMain characteristics of all studies
included in this meta-analysis. |
Table II
Main characteristics of all studies
included in this meta-analysis.
| First author
(ref) | Year | Location | No. of
patients | Method | Cutoff | Stage | Chemotherapy | Survival
analysis | Quality score |
|---|
|
|---|
| High/Positive | Total |
|---|
| Baek (15) | 2006 | Korea | 28 | 44 | ICH | Percentage of cells
stained 10% | Advanced | Cisplatin,
5-FU | Estimated | 5 |
| Kwon (22) | 2007 | Korea | 45 | 64 | ICH | Both staining
intensity and percentage of cells stained ≥2 | Advanced | Oxaliplatin,
5-FU | Multivariate | 7 |
| Wei (9) | 2008 | China | 15 | 76 | RT-PCR | Maximal
χ2 method 0.47 | III–IV | Oxaliplatin,
5-FU | Multivariate | 7 |
| Matsubara (10) | 2008 | Japan | 36 | 139 | RT-PCR | Maximal
χ2 method 1.42×10−3 | Advanced | Cisplatin, S-1 | Univariate | 7 |
| Huang (19) | 2008 | China | 31 | 62 | RT-PCR | Median 0.672 | I–IV | Oxaliplatin,
5-FU | Multivariate | 7 |
| Kim (20) | 2009 | Korea | 86 | 153 | ICH | Staining <17.5
vs. ≥17.5 | III–IV | Cisplatin,
5-FU | Univariate | 5 |
| Bamias (16) | 2010 | Greece | 46 | 66 | ICH | Staining 0–1 vs.
2–6 | I–IV |
Cisplatin/carboplatin, docetaxel | Univariate | 8 |
| Ozkan (24) | 2010 | Turkey | 13 | 41 | ICH | Percentage of cells
stained 10% | Advanced | Cisplatin,
5-FU | Estimated | 6 |
| Fareed (18) | 2010 | UK | 19 | 57 | ICH | Staining 0 vs.
1–3 | I–IV | Cisplatin,
5-FU/Xeloda | Estimated | 6 |
| Kim (21) | 2011 | Korea | 94 | 149 | ICH | Both staining
intensity and percentage of cells stained ≥2 | II–IV | Cisplatin,
5-FU | Estimated | 6 |
| Squires (26) | 2013 | USA | 16 | 73 | ICH | Staining 0–2 vs.
3–4 | I–III | 5-FU,
radiation | Multivariate | 6 |
| De Dosso (17) | 2013 | Switzerland | 34 | 67 | ICH | Staining 0–1 vs.
2–3 | II–III | Cisplatin,
5-FU | Univariate | 5 |
| Yamada (8) | 2013 | Japan | 160 | 322 | RT-PCR | Median | Advanced | Cisplatin +
irinotecan, 5-FU, S-1 | Univariate | 8 |
| Liu (23) | 2013 | China | 23 | 52 | RT-PCR | Median 7.32 | I–IV |
Oxaliplatin/cisplatin | Multivariate | 7 |
| Qi (25) | 2013 | China | 21 | 60 | ICH | Median value of
multiplying staining intensity by percentage of cells stained | Advanced | Oxaliplatin,
5-FU | Estimated | 5 |
Quantitative synthesis
As shown in Table
II, 10 of the 15 studies reported that high/positive ERCC1
expression in patients with GC was associated with poor survival,
three studies indicated no association between ERCC1 expression and
survival, and two studies exhibited an inverse association.
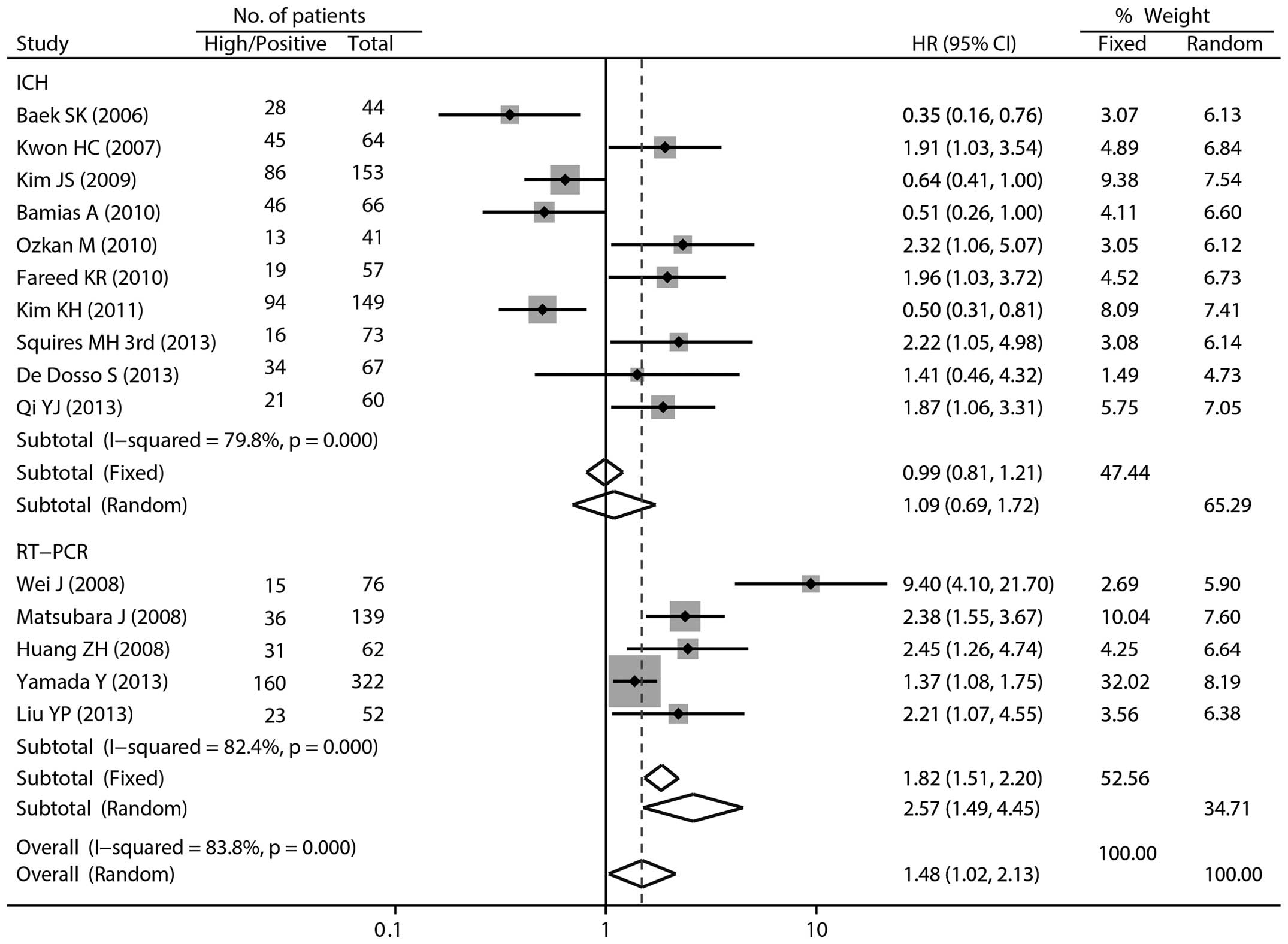
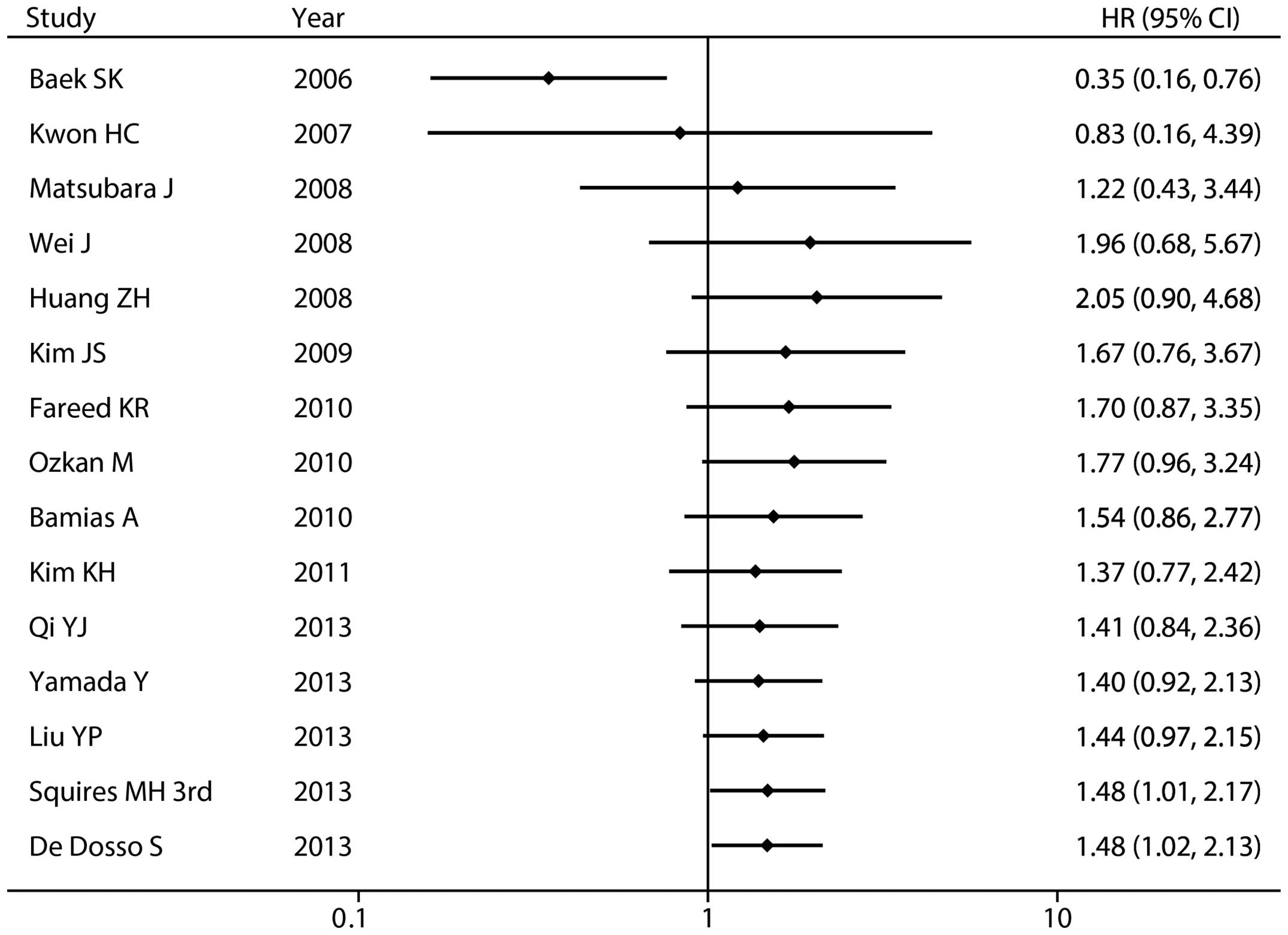
Overall, the pooled HR for the 15 studies was 1.48 (95% CI
1.02–2.10; P=0.036; random-effects model) with significant
heterogeneity (Pheterogeneity<0.001,
I2=83.8%), suggesting that high/positive ERCC1
expression was an indicator of poor survival in patients with
GC.
When stratifying by study location, no association
between ERCC1 expression and OS was observed in the Asian region
(HR 1.54; 95% CI 0.99–2.38) or the non-Asian region (HR 1.31; 95%
CI 0.63–2.75; Table III).
Focusing the analysis on ERCC1 expression ascertainment methods,
the pooled HR was 1.09 (95% CI 0.69–1.72) with an I2 of
79.8% for the ICH group, and 2.57 (95% CI 1.49–4.45) with an
I2 of 82.4% for the RT-PCR group, respectively (Fig. 2). The significant correlation was
also present in the subgroup analysis by sample size (<100), HR
estimated (directly obtained) and quality score (7–8)
(Table III).
 | Table IIIStratified analysis of excision
repair cross-complementation group 1 expression with overall
survival. |
Table III
Stratified analysis of excision
repair cross-complementation group 1 expression with overall
survival.
| Variables | na | Pooled HR (95%
CI) | Heterogeneity
test | Meta-regression
P-value |
|---|
|
|
|---|
| Fixed | Random | Q | P-valueb | I2
(%) |
|---|
| Location | | | | | | | 0.759 |
| Asian | 11 | 1.38 (1.19,
1.59) | 1.54 (0.99,
2.38) | 75.37 | <0.001 | 86.7 | |
| Non-Asian | 4 | 1.28 (0.88,
1.86) | 1.31 (0.63,
2.75) | 10.81 | 0.013 | 72.3 | |
| Method | | | | | | | 0.044 |
| IHC | 10 | 0.99 (0.82,
1.21) | 1.09 (0.69,
1.72) | 44.58 | <0.001 | 79.8 | |
| RT-RCR | 5 | 1.82 (1.51,
2.20) | 2.57 (1.49,
4.45) | 22.78 | <0.001 | 82.4 | |
| Sample size | | | | | | | 0.262 |
| <100 | 11 | 1.72 (1.39,
2.14) | 1.74 (1.08,
2.80) | 47.53 | <0.001 | 79.0 | |
| ≥100 | 4 | 1.16 (0.97,
1.39) | 1.02 (0.55,
1.91) | 31.14 | <0.001 | 90.4 | |
| HR estimated | | | | | | | 0.304 |
| Directly
obtained | 10 | 1.50 (1.28,
1.76) | 1.73 (1.13,
2.64) | 52.25 | <0.001 | 82.8 | |
| Indirectly
obtained | 5 | 1.02 (0.77,
1.34) | 1.08 (0.51,
2.29) | 28.27 | <0.001 | 85.9 | |
| Quality score | | | | | | | 0.177 |
| 5–6 | 8 | 0.98 (0.78,
1.22) | 1.12 (0.66,
1.89) | 36.49 | <0.001 | 80.8 | |
| 7–8 | 7 | 1.68 (1.41,
2.00) | 1.99 (1.22,
3.26) | 35.66 | <0.001 | 83.2 | |
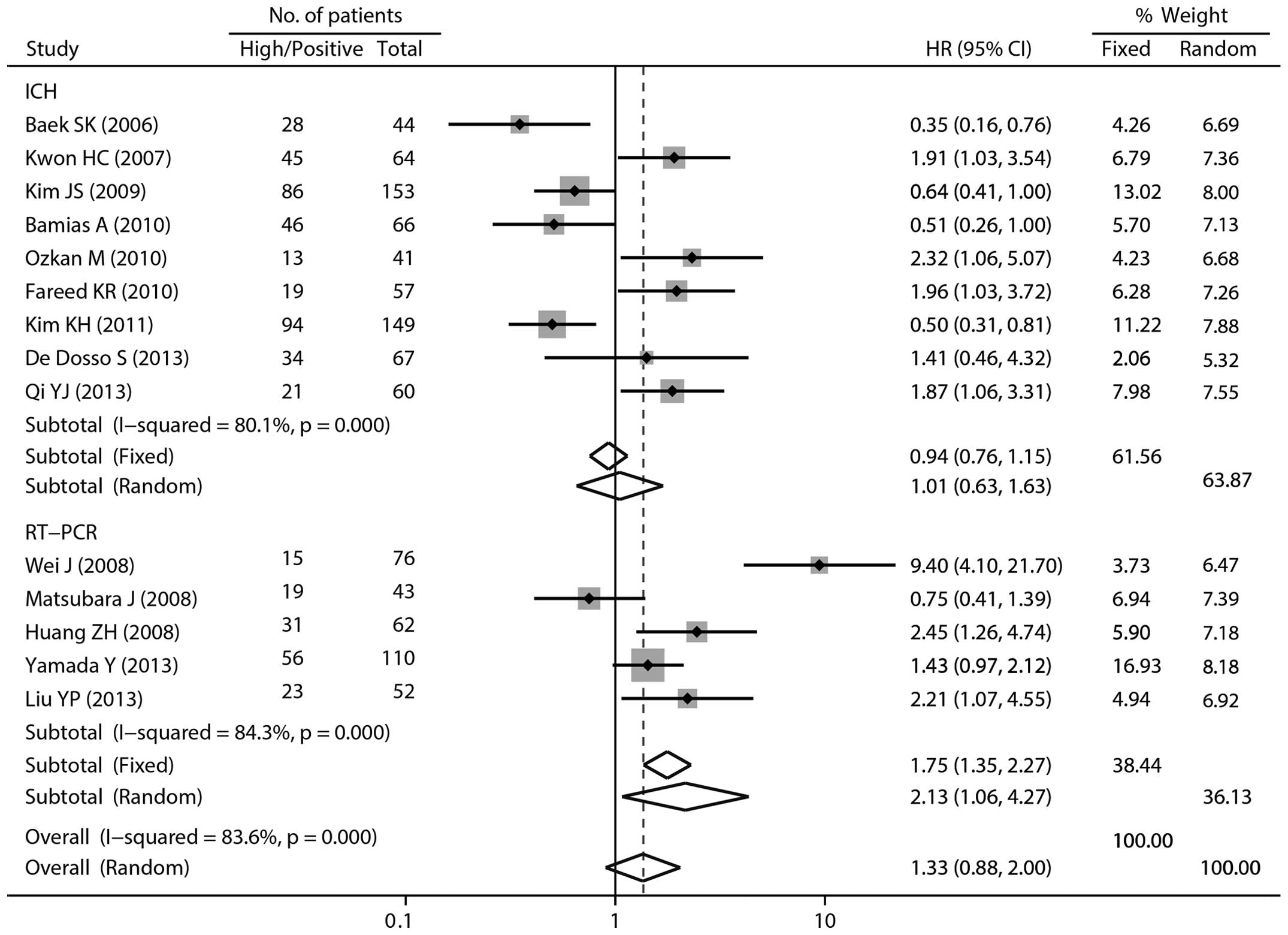
Of the 15 studies, there were 14 reports regarding
the OS of patients receiving platinum-based chemotherapy.
Meta-analysis of these studies also provided evidence of a trend
toward poor survival with high ERCC1 expression (HR 1.33; 95% CI
0.88–2.00; I2=83.6%; random-effects model), although
this was not considered statistically significant. In subgroup
analysis by ERCC1 expression measurement method, a significant
association was observed in the RT-PCR group (HR 2.13; 95% CI
1.06–4.27) with marked heterogeneity (I2=84.3%, Fig. 3).
Meta-regression
In univariate meta-regression analysis, only the
method used to measure ERCC1 expression (P=0.044) was found to be a
significant source of heterogeneity (Table III); however, the estimated
between-study variance (τ2) was reduced from 0.410 to
0.403, which could only explain 1.7% of the τ2.
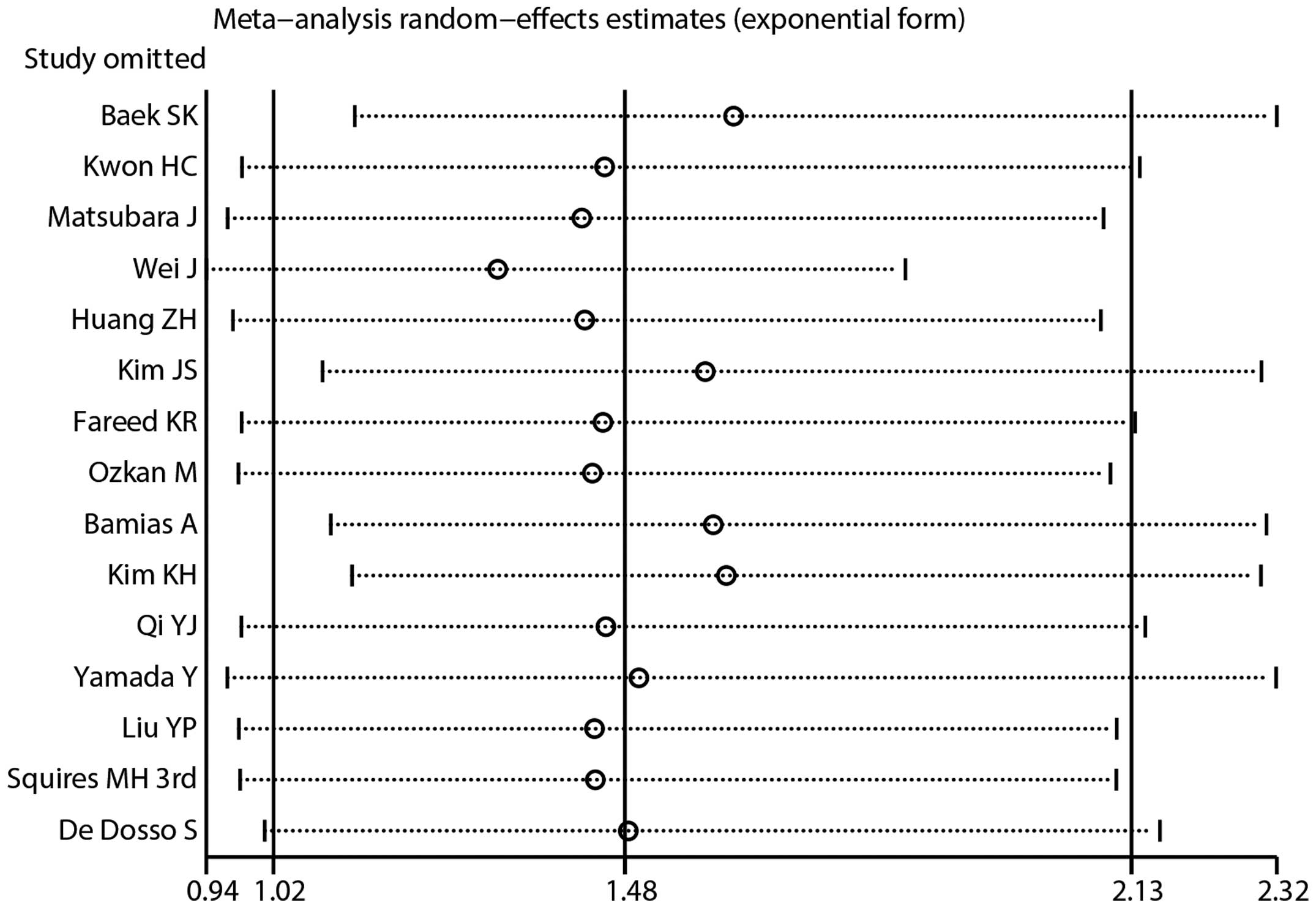
Sensitivity analysis and cumulative
analysis
Sensitivity analysis was performed to assess the
influence of individual studies on the pooled HR. Similar HRs and
95% CIs were generated by omitting any single study using a
random-effects model, and indicated that the results were
relatively stable (Fig. 4). A
cumulative meta-analysis of the 15 studies was conducted according
to the publication date. As displayed in Fig. 5, the phenomenon that high ERCC1
expression was associated with a poor prognosis was first observed
in the study reported by Squires et al (26) in 2013 (HR 1.48; 95% CI 1.01–2.17).
Following that, only one study was added cumulatively, resulting in
an overall effect estimate of 1.48 (95% CI 1.02–2.13).
Publication bias
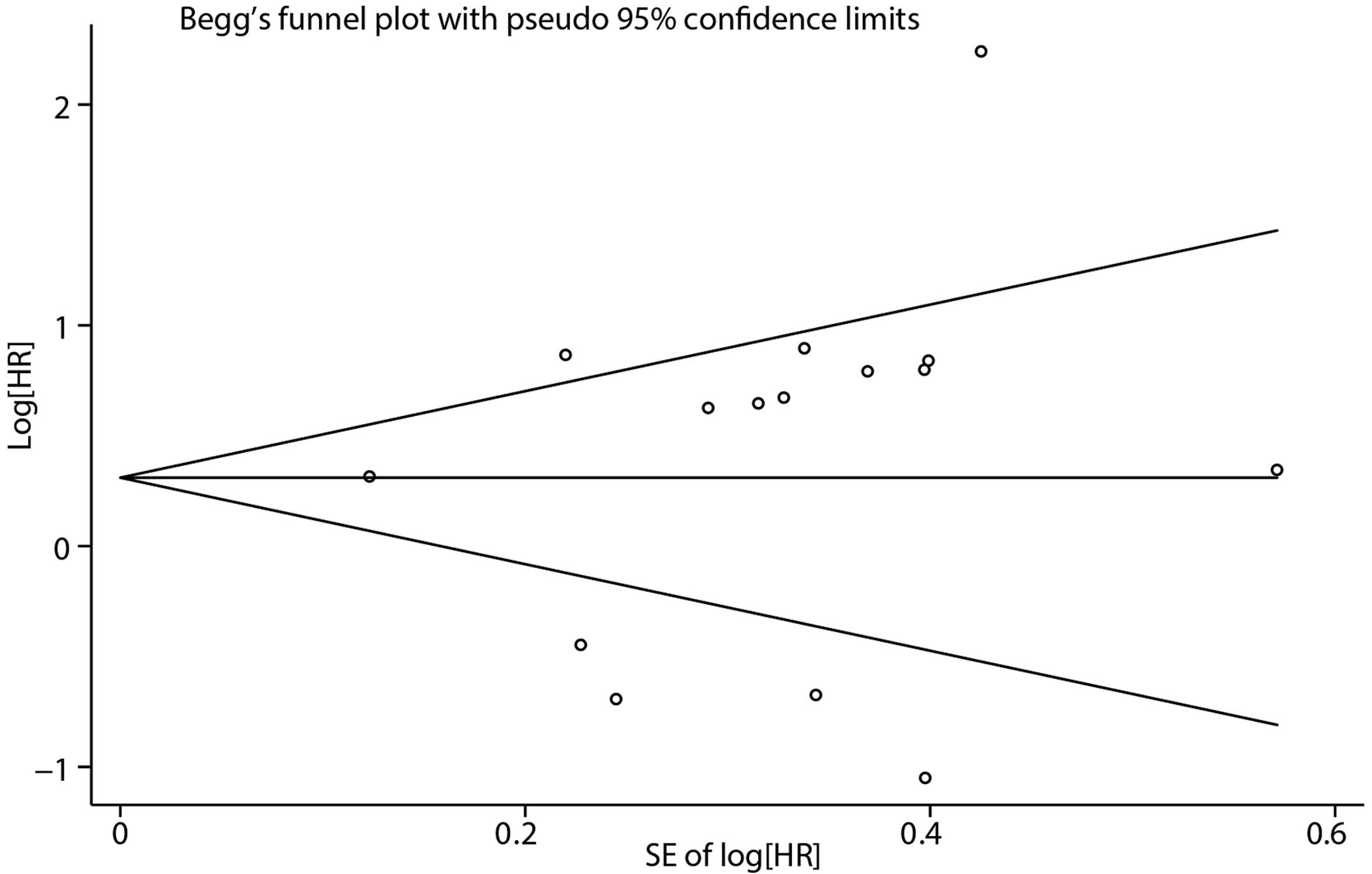
The shape of the funnel plot did not exhibit any
evident asymmetry (Fig. 6). The
Begg’s and Egger’s tests indicated no evidence of publication bias
(P=0.276 for Begg’s test; P=0.559 for Egger’s test).
Discussion
The identification of molecular biomarkers with
prognostic value for GC is clinically useful. In this
meta-analysis, the effects of ERCC1 expression on the OS for GC
were evaluated. The results indicate that high/positive ERCC1
expression is a significant poor prognostic factor for GC with
chemotherapy regardless of the treatment regimen, compared with
low/negative ERCC1 expression. Low mRNA levels of ERCC1 may be
associated with a significant favorable OS benefit for
platinum-based chemotherapy.
Numerous studies have reported that high/positive
ERCC1 expression is associated with the prognosis of other types of
cancer, including non-small cell lung (27), bladder (28), colorectal (29) and breast cancer (30). In addition, polymorphisms of ERCC1
have been found to affect OS in the platinum-based treatment of
patients with GC (31). Overall,
aberrant expression of ERCC1 appears to be associated with cancer
risk. The biological role of ERCC1 may partly explain its poor
prognosis. Cytotoxicity from platinum drugs leads to the formation
of platinum DNA adducts, whereas ERCC1 acts to remove these bulky
adducts and repair DNA double-strand damage. Furthermore, a high
level of ERCC1 has been demonstrated to confer resistance to
platinum agents and reconstitutes the ability of the cell to remove
cisplatin from cellular DNA in an animal model (32). The aberrant methylation of DNA
repair genes including ERCC1 has also been reported to affect the
sensitivity to chemotherapeutic agents (33,34).
The current results indicate that for the patients who received
platinum-based chemotherapy, the risk of mortality increased with a
high/positive expression of ERCC1 compared with the risk with
low/negative ERCC1 expression.
To evaluate the effectiveness of different
assessment methods, HRs were pooled from the IHC- or RT-PCR-based
methods separately. In the present meta-analysis, RT-PCR appeared
to be better than IHC in predicting OS for GC. ERCC1 expression
using the IHC method was categorized by a visual grading system
based on the staining intensity and percentage of cells stained,
resulting in objectivity in certain circumstances. The ERCC1 mRNA
levels were assessed with RT-PCR, which is a sensitive and
quantitative method. This may one of the reasons why RT-PCR is more
effective than IHC; however, the total sample size of the RT-PCR
group was smaller than that of the IHC group (343 vs. 701).
However, ERCC1 plays its role at the protein level. As is
well-known, numerous factors can impact mRNA transcription.
Notably, subgroup analyses demonstrated that the decreased survival
associated with high ERCC1 expression was pronounced among
high-quality-score studies. There is an urgent requirement for
large-scale clinical studies to confirm these findings.
To the best of our knowledge, this was the first
meta-analysis to evaluate the association between ERCC1 expression
and the survival of patients with GC. There are, however, the
following limitations to consider. Firstly, heterogeneity was
significant in this meta-analysis. Although meta-regression
analysis was used to clarify the source of heterogeneity, it was
not successful. Additionally, sensitivity analysis did not help to
find the source of heterogeneity. Secondly, where there were no
directly obtained HRs in the studies, the estimated HRs were
calculated from the data provided or extrapolated from the survival
curves. The estimated HRs may be less reliable than those obtained
directly from the papers. Thirdly, the cutoff values among these
studies were different: Even in the IHC or RT-PCR subgroups the
cutoff values were not unified. Studies with the same cutoff are,
therefore, warranted to generate a more definitive conclusion.
Fourthly, the cumulative meta-analysis presented significant
associations until 2013, suggesting that this finding was not very
robust with time. Finally, though no publication bias was detected
in the present study, it could not be neglected. Since negative
studies are often not published, and if these studies are
published, they are often reported in a simplified way, this leads
to difficulty in retrieving these data.
In conclusion, high/positive ERCC1 expression may be
a poor prognostic factor for patients with GC. Due to the conferred
resistance to platinum drugs, patients with high/positive ERCC1
expression (particularly with high ERCC1 mRNA levels) do not seem
to benefit from platinum-based chemotherapy. Large scale and
well-designed prospective studies are required to confirm the
present findings.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mari E, Floriani I, Tinazzi A, et al:
Efficacy of adjuvant chemotherapy after curative resection for
gastric cancer: a meta-analysis of published randomised trials. A
study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi
dell’Apparato Digerente). Ann Oncol. 11:837–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: the role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sijbers AM, de Laat WL, Ariza RR, et al:
Xeroderma pigmentosum group F caused by a defect in a
structure-specific DNA repair endonuclease. Cell. 86:811–822. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friboulet L, Olaussen KA, Pignon JP, et
al: ERCC1 isoform expression and DNA repair in non-small-cell lung
cancer. N Engl J Med. 368:1101–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen WW, Wang F and Xu RH: Platinum-based
versus non-platinum-based chemotherapy as first line treatment of
inoperable, advanced gastric adenocarcinoma: a meta-analysis. PLoS
One. 8:e689742013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richards D, McCollum D, Wilfong L, et al:
Phase II trial of docetaxel and oxaliplatin in patients with
advanced gastric cancer and/or adenocarcinoma of the
gastroesophageal junction. Ann Oncol. 19:104–108. 2008. View Article : Google Scholar
|
|
8
|
Yamada Y, Boku N, Nishina T, et al: Impact
of excision repair cross-complementing gene 1 (ERCC1) on the
outcomes of patients with advanced gastric cancer: correlative
study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol.
24:2560–2565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei J, Zou Z, Qian X, et al: ERCC1 mRNA
levels and survival of advanced gastric cancer patients treated
with a modified FOLFOX regimen. Br J Cancer. 98:1398–1402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsubara J, Nishina T, Yamada Y, et al:
Impacts of excision repair cross-complementing gene 1 (ERCC1),
dihydropyrimidine dehydrogenase, and epidermal growth factor
receptor on the outcomes of patients with advanced gastric cancer.
Br J Cancer. 98:832–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Graeff P, Crijns AP, de Jong S, et al:
Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial
ovarian cancer: a meta-analysis. Br J Cancer. 101:149–159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: a systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek SK, Kim SY, Lee JJ, Kim YW, Yoon HJ
and Cho KS: Increased ERCC expression correlates with improved
outcome of patients treated with cisplatin as an adjuvant therapy
for curatively resected gastric cancer. Cancer Res Treat. 38:19–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bamias A, Karina M, Papakostas P, et al: A
randomized phase III study of adjuvant platinum/docetaxel
chemotherapy with or without radiation therapy in patients with
gastric cancer. Cancer Chemother Pharmacol. 65:1009–1021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Dosso S, Zanellato E, Nucifora M, et
al: ERCC1 predicts outcome in patients with gastric cancer treated
with adjuvant cisplatin-based chemotherapy. Cancer Chemother
Pharmacol. 72:159–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fareed KR, Al-Attar A, Soomro IN, et al:
Tumour regression and ERCC1 nuclear protein expression predict
clinical outcome in patients with gastro-oesophageal cancer treated
with neoadjuvant chemotherapy. Br J Cancer. 102:1600–1607. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang ZH, Hua D, Du X, et al: ERCC1
polymorphism, expression and clinical outcome of oxaliplatin-based
adjuvant chemotherapy in gastric cancer. World J Gastroenterol.
14:6401–6407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JS, Kim MA, Kim TM, et al: Biomarker
analysis in stage III–IV (M0) gastric cancer patients who received
curative surgery followed by adjuvant 5-fluorouracil and cisplatin
chemotherapy: epidermal growth factor receptor (EGFR) associated
with favourable survival. Br J Cancer. 100:732–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KH, Kwon HC, Oh SY, et al:
Clinicopathologic significance of ERCC1, thymidylate synthase and
glutathione S-transferase P1 expression for advanced gastric cancer
patients receiving adjuvant 5-FU and cisplatin chemotherapy.
Biomarkers. 16:74–82. 2011. View Article : Google Scholar
|
|
22
|
Kwon HC, Roh MS, Oh SY, et al: Prognostic
value of expression of ERCC1, thymidylate synthase, and glutathione
S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in
advanced gastric cancer. Ann Oncol. 18:504–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu YP, Ling Y, Qi QF, et al: The effects
of ERCC1 expression levels on the chemosensitivity of gastric
cancer cells to platinum agents and survival in gastric cancer
patients treated with oxaliplatin-based adjuvant chemotherapy.
Oncol Lett. 5:935–942. 2013.PubMed/NCBI
|
|
24
|
Ozkan M, Akbudak IH, Deniz K, et al:
Prognostic value of excision repair cross-complementing gene 1
expression for cisplatin-based chemotherapy in advanced gastric
cancer. Asian Pac J Cancer Prev. 11:181–185. 2010.PubMed/NCBI
|
|
25
|
Qi YJ, Cui S, Yang YZ, et al: Excision
repair cross-complementation group 1 codon 118 polymorphism, micro
ribonucleic acid and protein expression, clinical outcome of the
advanced gastric cancer response to first-line FOLFOX-4 in
Qinghai-Tibetan plateau population. J Cancer Res Ther. 9:410–415.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Squires MH III, Fisher SB, Fisher KE, et
al: Differential expression and prognostic value of ERCC1 and
thymidylate synthase in resected gastric adenocarcinoma. Cancer.
119:3242–3250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Zhang J, Wang R, Luo X and Chen H:
The platinum-based treatments for advanced non-small cell lung
cancer, is low/negative ERCC1 expression better than high/positive
ERCC1 expression? A meta-analysis. Lung Cancer. 70:63–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Wu J, Chen Y, et al: ERCC1
expression levels predict the outcome of platinum-based
chemotherapies in advanced bladder cancer: a meta-analysis.
Anticancer Drugs. 25:106–114. 2014. View Article : Google Scholar
|
|
29
|
Huang MY, Tsai HL, Lin CH, et al:
Predictive value of ERCC1, ERCC2, and XRCC1 overexpression for
stage III colorectal cancer patients receiving FOLFOX-4 adjuvant
chemotherapy. J Surg Oncol. 108:457–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gerhard R, Carvalho A, Carneiro V, et al:
Clinicopathological significance of ERCC1 expression in breast
cancer. Pathol Res Pract. 209:331–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin M, Yan J, Martinez-Balibrea E, et al:
ERCC1 and ERCC2 polymorphisms predict clinical outcomes of
oxaliplatin-based chemotherapies in gastric and colorectal cancer:
a systemic review and meta-analysis. Clin Cancer Res. 17:1632–1640.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parker RJ, Eastman A, Bostick-Bruton F and
Reed E: Acquired cisplatin resistance in human ovarian cancer cells
is associated with enhanced repair of cisplatin-DNA lesions and
reduced drug accumulation. J Clin Invest. 87:772–777. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Satoh A, Toyota M, Itoh F, et al:
Epigenetic inactivation of CHFR and sensitivity to microtubule
inhibitors in gastric cancer. Cancer Res. 63:8606–8613.
2003.PubMed/NCBI
|
|
34
|
Chen HY, Shao CJ, Chen FR, Kwan AL and
Chen ZP: Role of ERCC1 promoter hypermethylation in drug resistance
to cisplatin in human gliomas. Int J Cancer. 126:1944–1954.
2010.
|