Introduction
For the effective treatment of localized renal
carcinoma, surgical resection is considered the primary choice.
Partial or radical nephrectomy may be used, depending on the tumor
and the specific patient characteristics (1). Nephron-sparing partial nephrectomy is
used when the tumor is small (<4 cm in diameter) or when the
patient has other medical concerns, such as diabetes or
hypertension (2). Radical
nephrectomy is most often used when there is a large tumor present
in only one kidney and the other kidney is fully functional. Open
surgery is the traditional method for nephrectomy; however, with
the development of technology and improvement in surgical skills,
laparoscopic or robotic techniques are increasingly being used.
According to the 2009 American Urological Association management
guidelines, in patients with a T1 renal mass, complete surgical
excision by partial nephrectomy is the standard treatment option
and is strongly recommended (3).
As compared with open partial nephrectomy, laparoscopic partial
nephrectomy is technically challenging and associated with longer
warm renal ischemia time, more major intraoperative complications
and more postoperative urological complications (4,5).
However, laparoscopic partial nephrectomy is minimally invasive,
and achieves similar intermediate-term cancer cure and renal
functional outcome, with the additional advantages of decreased
postoperative narcotic use, earlier hospital discharge and a more
rapid convalescence (5,6). With the development of laparoscopy
and the widespread use of imaging technology, laparoscopic partial
nephrectomy has become the standard for treating T1-stage renal
carcinoma (6). Laparoscopic
partial nephrectomy can be performed transperitoneally or
retroperitoneally. Retroperitoneoscopic partial nephrectomy (RPN)
has the advantages of minimal abdominal interference and
convenience in processing renal pedicle vessels but it also bears
drawbacks, including a narrow operational space, a lack of
anatomical landmarks and a complex surgical procedure (7). The surgery is more difficult for
tumors located at the ventral side of the kidney or at the inferior
pole of the kidney, particularly at the renal hilum. Between April
2012 and June 2014, the renal-rotation technique was applied in the
RPN surgery of 38 patients, in which the kidney was rotated at a
certain angle following occlusion of the renal artery, prior to
tumor excision and kidney suturing.
Materials and methods
Patients
The present study enrolled 38 patients, including 22
males and 16 females (age range, 31–75 years; mean, 52 years). The
patients were confirmed as having renal carcinoma with no lymph
node or renal vessel involvement by Color Doppler ultrasound, renal
computed tomography (CT) or magnetic resonance imaging examination.
The renal tumors were all at the T1N0M0 stage according to the
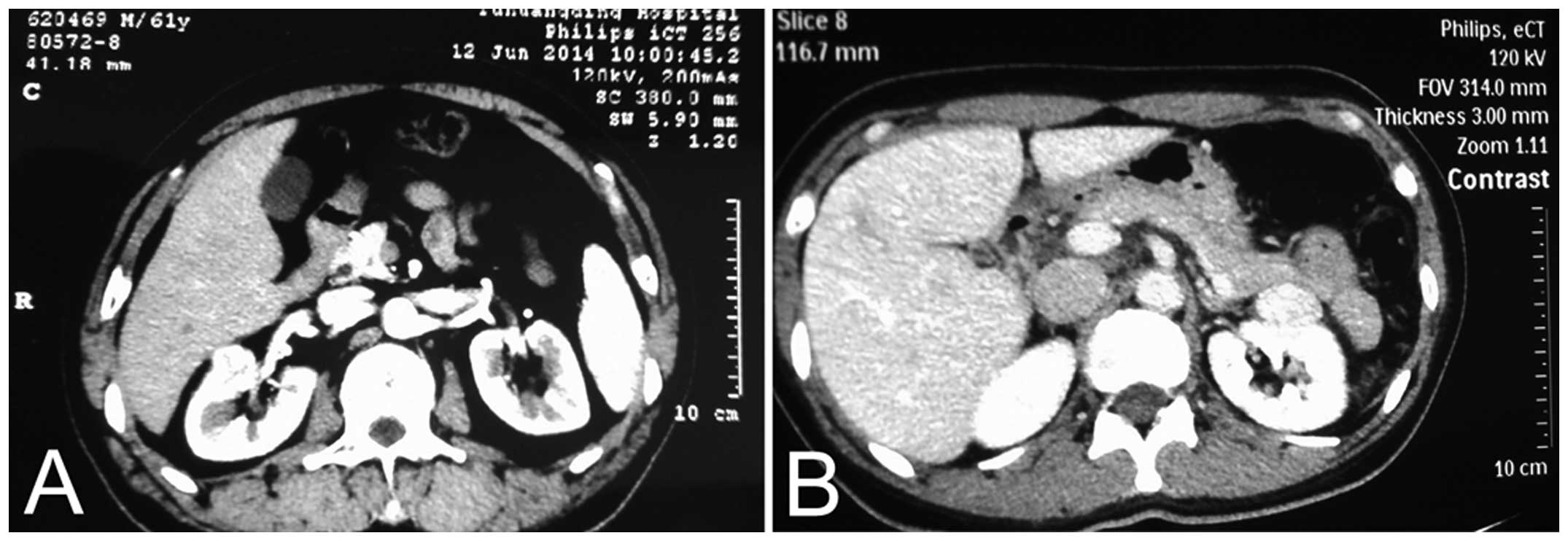
American Joint Committee on Cancer TNM staging (8). The tumors were located at the ventral
side of the kidney in 29 cases (including 22 at the renal hilum;
Fig. 1) and at the inferior pole
in nine cases. The diameter of the tumors ranged between 1.5 and
4.6 cm (mean, 2.8 cm). The tumors were confined to one kidney. No
suspicious nodules were identified by thoracic CT. Levels of serum
creatinine, blood urea nitrogen, blood sedimentation and alkaline
phosphatase were relatively normal and there were no surgical
contraindications. The present study was approved by the Medical
Ethics Committee of the China Yantai Yuhuangding Hospital (Yantai,
China). Written informed consent was obtained from either the
patient or the patient’s family prior to involvement in the study
and usage of the information for publication purposes.
Surgical procedure
Following tracheal intubation anesthesia, patients
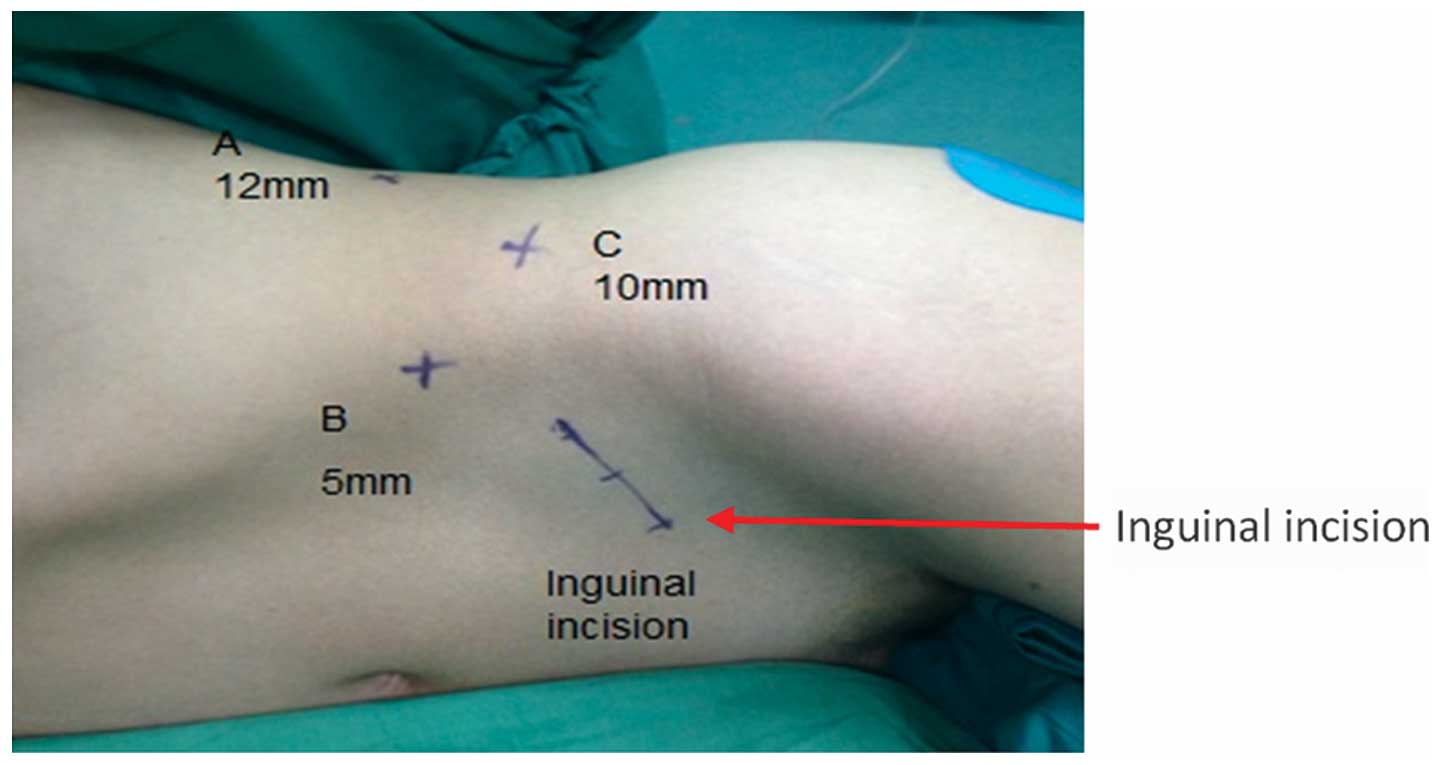
adopted a 90° lateral position with a raised waist bridge. The skin
was incised at the posterior axillary line 1 cm under the costal
margin (Fig. 2, point A). The
muscular layer and lumbar dorsal fascia were bluntly separated with
long vascular forceps. The inside of the frame was examined with an
index finger to confirm the entrance of the retroperitoneum and
separation of the posterior peritoneum prior to imbedding a
home-made latex balloon, inflated at 500–800 ml, for 3 min. Under
guidance of the index finger, a puncture was made 2 cm above the
axillary midline iliac crest (Fig.
2, point B) and 1 cm below the axillary front costal margin
(Fig. 2, point C). Trocars of 12,
5 and 10 mm were inserted at points A, B and C, respectively.
Subsequent to the closure of the incisions by suturing, a 0° or 30°
laparoscope was fixed in trocar B and the main surgical equipment
was inserted in trocar A. CO2 pneumoperitoneum was
established and the pressure was maintained at 12–15 mmHg.
Subsequent to entering the retroperitoneum, the
fatty tissue along the peritoneum and Gerota’s fascia was cleared
from top to bottom and from front to back using a harmonic scalpel
(Ethicon Endo-Surgery LLC, Guaynabo, Puerto Rico, US), so that the
peritoneal reflection and Gerota’s fascia could be clearly
identified. Gerota’s fascia was then incised near the peritoneal
reflection, from over the upper pole of the kidney to 2–3 cm below
the lower pole of the kidney, and as much as possible of the
perirenal fascia and fatty tissue that obstructed the surgical
field was removed forming an ‘arch window’ field for the
convenience of the surgery.
Dissociation along the psoas major fascia exposed
the renal pedicle, allowing separation of the renal artery. If
accompanied by lumbar veins riding across the renal artery, the
lumbar veins were disconnected using Hem-o-lok ligation clips
(Teleflex Medical, Durham, NC, USA) prior to the complete
dissociation of the renal artery. The perirenal adipose layer was
then incised longitudinally along the outer renal edge and the
kidney was dissociated completely from the fat layer. The renal
week fat layer was removed, only retaining the renal hilum blood
vessels and the renal collecting system fatty tissue.
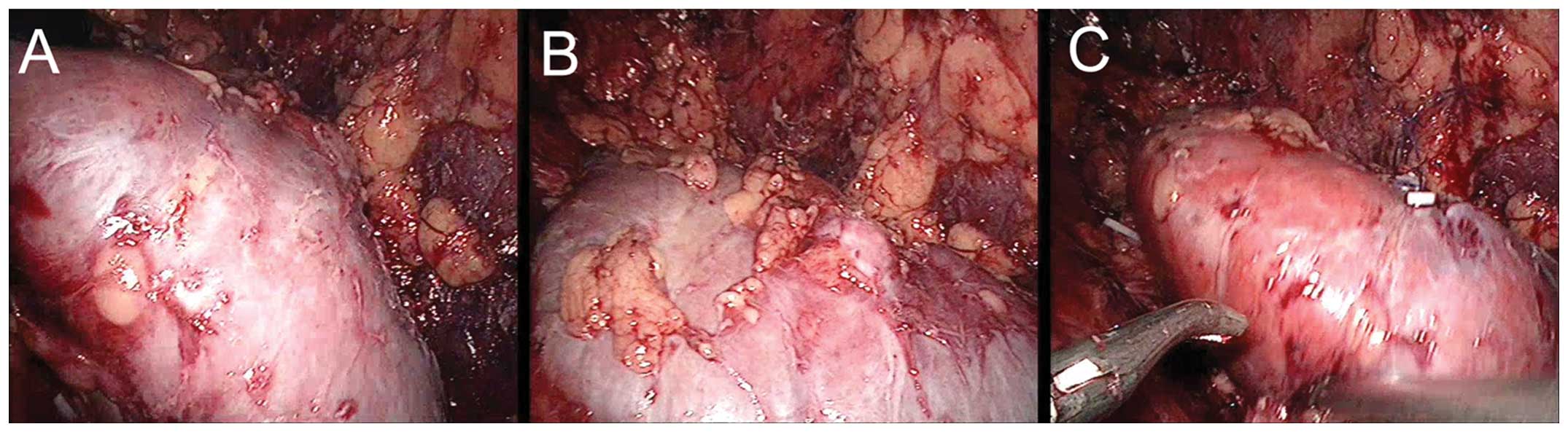
The direction of kidney rotation was decided by the
size and location of the tumor. For tumors located at the ventral
upper pole or at the renal hilum, the kidney was rotated internally
for 45–90° toward the inferior pole. Following tumor removal and
suturing, the kidney was rotated back to its original position
(Fig. 3). If the tumor was located
at the ventral inferior pole, the kidney was rotated internally for
45–90° toward the upper pole. During surgery the renal blood
vessels were closely monitored to avoid vascular tears. The
rotation angle could be adjusted at any time during the surgery for
better exposure of the tumor.
Following complete exposure of the tumor, a bulldog
clamp was used and the cross-clamp time was recorded. The tumor and
surrounding tissues were excised 0.5–1.0 cm from the tumor margin.
Wounds were then sutured using a no. 1 14×14 cm 1/2 circle
bidirectional barbed absorption line (Quill™ line; Angiotech
Pharmaceuticals, Inc., Reading PA, USA) (9). If renal pelvis and calyx fissure
existed, the needle was inserted from the surface of the kidney,
continuously closing the fissure without tying a knot, and then
pierced out to the surface, continuously suturing the kidney wound.
Each stitch was tightened and either tied with a knot, closed with
Hem-o-lok or cut off the line at both ends. The blood vessel clamps
were then loosened to check for evident bleeding. The tumor body
was taken out from expanded point A and sent for pathological
examination. A retroperitoneal drainage tube was put in place, the
trocars were removed and the wound was closed up.
Data collection
The surgery duration, intraoperative blood loss,
renal artery occlusion (ischemic) time, suture time, duration of
hospital stay and post-operative follow-ups were recorded.
Results
In the present study, the 38 cases of RPN were
completed successfully without conversion to open surgery. In no
case did intraoperative complications occur, such as injury of
large vessels or adjacent viscera. Three cases had postoperative
complications, including one case of hematuria and two cases of
subcutaneous emphysema. The patient with hematuria recovered
following bed rest. The surgery duration was between 45 and 116 min
(mean, 59 min). Intraoperative blood loss was between 10 and 120 ml
(mean, 40 ml) and without intraoperative blood transfusion. Renal
artery occlusion time was between 15 and 38 min (mean, 21 min).
Pathological examination reported 29 cases of renal clear cell
carcinoma and one case of papillary carcinoma, all with a negative
margin, and eight cases of renal angiomyolipoma. Patients were
followed for between one and 26 months subsequent to surgery (mean,
17 months), and there were no tumor recurrences or distant
metastases. Renal functions remained normal.
Discussion
Laparoscopic partial nephrectomy can be performed by
two routes: the transperitoneal route or the retroperitoneal route.
Following a comparison between retroperitoneal and transperitoneal
laparoscopic partial nephrectomy procedures, Marszalek et al
(10) suggested that the
retroperitoneal approach had more advantages regarding surgery
duration, blood loss and surgical complications; however, this
approach also has drawbacks, such as a narrow operational space and
a lack of anatomical landmarks. If the tumor is located at the
ventral side or at the inferior pole, RPN is problematic due to the
entry route of the laparoscope, which is also the bottleneck of the
majority of RPN procedures.
During our long-term clinical practice and technical
exploration, we found that the renal-rotation technique could solve
the problem associated with RPN by providing sufficient operational
space. Tumors located at the ventral side, inferior pole or even at
the renal hilum could be removed using this technique. This
technique is clearly advantageous in several aspects.
Firstly, the renal-rotation technique could markedly
reduce the surgical complexity and increase the success rate of
RPN. The most difficult aspect of RPN lies in the exposure of the
ventral side renal tumor and subsequent suturing. For tumors
located in the renal hilum, RPN would be even more problematic.
Using the rotation technique the ventral side and even the hilum of
the kidney can be clearly exposed, thus countering the disadvantage
of the entry route. This is likely to increase the success rate of
the surgery, boost the self-confidence of the surgeon and shorten
the training time for young endoscopic urology physicians.
Another challenge of RPN is insufficient exposure of
the surgical field. A limited operational space in the initial
stage makes the procedure difficult, and may even cause the surgery
to fail. As renal rotation requires the complete dissociation of
the kidney and renal week fatty tissue, the surgical field is,
therefore, relatively large and anatomical landmarks are obvious.
This could also significantly reduce damage to the inferior vena
cava, duodenum, liver and other organs caused by an obstructed
surgical field, and reduce the incidence of complications, such as
rupture of the peritoneum.
Finally, another aim for RPN is to reduce the renal
warm ischemia time. The suturing of kidney sections is an important
step affecting the warm ischemia time, and is the most effective
method to maintain renal stability and prevent urine leakage.
Suturing is relatively time-consuming, however, and manually
challenging. Good suture practice could reduce the incidence of
complications and shorten suture time (11). Renal parenchymal bleeding is mainly
controlled by blocking the renal pedicle blood vessels; however,
prolonged room temperature renal ischemia would lead to renal warm
ischemia and kidney function damage. Kijvikai et al
(12) used ‘cold ischemia’ to
occlude the renal pedicle. Although it clearly prolonged the
occlusion time, the surgical procedure was complicated. Thompson
et al (13) considered the
optimal blocking time to be within 25 min following investigations
into 362 cases. In the present study the renal artery blocking time
was 20–30 min (average, 23 min). A follow-up of renal function
post-operatively in all patients showed no obvious abnormality. The
use of renal-rotation techniques could sufficiently expose the
renal tumors for excision and suturing, ensure the closure of the
section plane, therefore reducing warm ischemia time, protect
residual renal function and prevent intraoperative complications,
such as hemorrhage.
In the present study, which began in 2012, the
renal-rotation technique was applied in a series of surgeries,
including RPN of ventral side or inferior pole renal tumors. It has
been demonstrated that this method can significantly increase the
operating space, facilitate surgical procedures and shorten kidney
warm ischemia time. Postoperative complications were found to be
rare. The renal-rotation technique is, therefore, safe, feasible
and worthy of wider clinical application.
Acknowledgements
The authors would like to thank numerous individuals
who participated in this study and Dr Jiahui Wang for manuscript
preparation.
References
|
1
|
Rini BI, Rathmell WK and Godley P: Renal
cell carcinoma. Curr Opin Oncol. 20:300–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campbell SC, Novick AC, Belldegrun A, et
al; Practice Guidelines Committee of the American Urological
Association. Guideline for the management of the clinical T1 renal
mass. J Urol. 182:1271–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Porpiglia F, Volpe A, Billia M and Scarpa
RM: Laparoscopic versus open partial nephrectomy: analysis of the
current literature. Eur Urol. 53:732–743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gill IS, Matin SF, Desai MM, et al:
Comparative analysis of laparoscopic versus open partial
nephrectomy for renal tumors in 200 patients. J Urol. 170:64–68.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gill IS, Desai MM, Kaouk JH, et al:
Laparoscopic partial nephrectomy for renal tumor: duplicating open
surgical techniques. J Urol. 167:469–477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Zhou LQ, Li NC, Zhang XC and Na YQ:
Analysis of complications of retroperitoneal laparoscopic surgery
(with experience of 1560 cases). Zhonghua Mi Niao Wai Ke Za Zhi.
28:810–812. 2007.(In Chinese).
|
|
8
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: Kidney. AJCC Cancer Staging Manual. 7th ed.
Springer Verlag; New York: pp. 479–489. 2010
|
|
9
|
Wang K, Zhang YL, Lin CH, et al:
Application of self-retaining bidirectional barbed absorbable
suture in retroperito-neoscopic partial nephrectomy. Int Braz J
Urol. 40:220–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marszalek M, Chromecki T, Al-Ali BM, et
al: Laparoscopic partial nephrectomy: a matched-pair comparison of
the transperitoneal versus the retroperitoneal approach. Urology.
77:109–113. 2011. View Article : Google Scholar
|
|
11
|
Allaf ME, Bhayani SB, Rogers C, et al:
Laparoscopic partial nephrectomy: evaluation of long-term
oncological outcome. J Urol. 172:871–873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kijvikai K, Viprakasit DP, Milhoua P,
Clark PE and Herrell SD: A simple, effective method to create
laparoscopic renal protective hypothermia with cold saline surface
irrigation: clinical application and assessment. J Urol.
184:1861–1866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson RH, Lane BR, Lohse CM, et al:
Every minute counts when the renal hilium is clamped during partial
nephrectomy. Eur Urol. 58:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|