Introduction
Cerebral infarction is a severe disease of the
central nervous system that has a high incidence rate. Thrombolytic
therapy is considered to be the only safe and effective method to
recover the blood supply; however, an effective method of providing
neuronal protection against ischemia and ischemia-reperfusion
injury has not yet been identified. In addition, a method for
inducing angiogenesis, which may aid the establishment of
collateral circulation and secondary prevention, has not yet been
developed (1).
Gene therapy has been considered to be prospective
in the treatment of infarction and neuron protection (2,3). A
number of studies assessing monogenic function have been conducted,
where the protective roles of neurotrophic factors, antiapoptosis
genes and angiogenic growth factors have been investigated
(4). However, the mechanisms
underlying cerebral ischemic diseases are complicated and involve a
variety of courses; thus, it was proposed that regulating two genes
that exhibit positive feedback on each other may aid further
understanding (5). Human thioredoxin
(hTRX) is a type of stress-induced protein that protects neurons
against oxidative stress (6,7). hTRX has been demonstrated to possess
the ability to scavenge free radicals, and also ease the
inflammatory response by regulating nuclear factors or
mitogen-activated protein kinases. In addition, since hTRX is a
type of natural human protein with low immunogenicity, hTRX can be
used as a frame protein to construct a gene fusion system, in which
the solubility and activity of the expression product can be
increased significantly (8,9).
Antibacterial peptide (PR39), a proline- and
arginine-rich peptide consisting of 39 amino acids, is considered
to be the switch for angiogenesis (10). PR39 is able to inhibit the
degradation of hypoxia inducible factor (HIF)-1α, which
subsequently elevates the expression of vascular endothelial growth
factor (VEGF), kinase insert domain containing-receptor, fms-like
tyrosine kinase and fibroblast growth factor receptor (FGFR)-1 to
promote vascularization (11,12).
This mechanism of action is similar to the mechanism of
vascularization that is observed under conditions of hypoxia. Sun
et al indicated that adeno-associated virus (AAV)-PR39 may
serve as a novel therapeutic agent for the treatment of myocardial
infarction (13). As a short
peptide, PR39 is unstable; thus, hTRX may be used to provide a
frame structure for PR39. Following the insertion of the hTRX frame
structure, the aptamer (PR39) is more stable compared with the free
peptide. Furthermore, the cell-penetrating ability of hTRX
(14,15) may enable PR39 to pass through the
blood-brain barrier, which is conducive to enabling the full
function of PR39.
In the present study, it was hypothesized that the
recombinant gene, hTRX-PR39, may exhibit multiple functions in the
protection of neurons and the vasculature. Thus, the aim of the
present study was to investigate the therapeutic roles of hTRX-PR39
in hypoxia.
Materials and methods
Recombinant virus construction
The pGEM-T-hTRX-PR39 cloning vector containing
hTRX-PR39 full-length gene sequence was constructed as previously
described (16). First, the forward
and reverse primers of PR39 were designed and synthesized. Using
PCR, the fragment encoding PR39 was produced, including
EcoR721 and BamHI restriction enzyme sites, and the
new hTRX cDNA including EcoR721 and EcoRI restriction
enzyme sites was generated. Next, the synthesized fragments were
cloned into a pGEM-T vector. The positive clone was identified
using restriction enzymes, and the cloned amplified fragments were
sequenced by the dideoxy-mediated chain-termination method. The
cloned hTRX and PR39 cDNA were compared with the GenBank sequence
(http://www.ncbi.nlm.nih.gov/genbank/)
using DNASIS software (MiraiBio Group, San Francisco, CA, USA).
Subsequently, pGEM-T-hTRX and pGEM-T-PR39 were digested by
BamHI and EcoRI, the PR39 BamHI, EcoRI
was cloned into the recombinant vector pGEM-T-hTRX BamHI,
EcoRI. Thus, the recombinant vector pGEM-T-hTRX-PR39 was
produced. The pSSCMV viral vector, adenovirus plasmid pAAV/Ad,
Escherichia coli TOP10, ECV304 and HEK293 cell lines were
provided by Xian Huaguang Biological Engineering Co., Ltd. (Xian,
China) (17).
Transfection
The recombinant virus was seeded into the culture
medium of ECV304 cells (Xi'an Huaguang Biological Engineering Co.,
Ltd.) and incubated for 24 h. For the control, adenoviruses were
seeded into the culture medium of ECV304 cells instead of the
recombinant virus. The control and transfection groups were
subsequently divided into three subgroups that were separately
incubated in a hypoxic (1 and 5% O2) or normoxic
environment (20% O2) for 72 h.
Reverse transcription-quantitative
polymerase chain reaction (PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reverse
transcribed to cDNA using a Moloney Murine Leukemia Virus (M-MLV)
reverse transcriptase PCR kit (Promega Corporation, Madison, WI,
USA). Briefly, 3 µl RNA was reverse transcribed to cDNA at 37°C for
1 h in a 20-µl reaction system that contained 1 µl M-MLV reverse
transcriptase, 4 µl 5X M-MLV buffer, 0.5 µl RNase inhibitor, 1 µl
oligo-dT and 1 µl dNTP (Promega Corporation, Madison, WI, USA). For
quantitative PCR, the PCR amplification mixture (20 µl) consisted
of 2 µl cDNA mixture, 10 µl SYBR Green (Takara Biotechnology Co.,
Ltd., Dalian, China), 2 µl primers and 6 µl deionized water.
β-actin was used as a control. The amplification conditions were as
follows: Initial denaturation at 95°C for 2 min, followed by 40
cycles of 95°C for 10 sec, 58°C for 30 sec and 72°C for 30 sec.
Nested PCR was performed using a 2-µl sample of the PCR product as
a template under the aforementioned PCR conditions. Bio-Rad IQ5.0
Optical System software (Bio-Rad Laboratories, Hercules, CA, USA)
was used for the detection of the quantitative PCR products that
were specific for VEGF, vascular endothelial growth factor receptor
(VEGFR)-1, VEGFR-2, FGFR-1, syndecan-4, PR39 and β-actin. The
primer sequences used for PCR are shown in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Genes | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product (bp) |
|---|
| VEGF |
TCTACCTCCACCATGCCAAGT |
GCTGCGCTGATAGACATCCA | 104 |
| VEGFR-1 |
TCCCTTATGATGCCAGCAAGT |
CCAAAAGCCCCTCTTCCAA | 79 |
| VEGFR-2 |
CTTCGAAGCATCAGCATAAGAAACT |
TGGTCATCAGCCCACTGGAT | 156 |
| FGFR-1 |
ACTCTGTGGTGCCTTCTGAC |
CATTTCCTTGTCGGTGGTAT | 317 |
| Syndecan-4 |
CTGCTGCTGTTCTTCGTAGG |
CTTTGAGCTGTCTGGCTCTG | 153 |
| PR39 |
CTCTACCGCCTCCTGGAGCT |
GGCCCTTCATAATATCCCCCA | 117 |
Effects of AAV-hTRX-PR39 transfection
on the hypoxic chick embryo
Effects of AAV-hTRX-PR39 on the hypoxic chick embryo
were analyzed using a chick embryo (Xi'an Huaguang Biological
Engineering Co., Ltd.) chorioallantoic membrane (CAM). In total,
120 fertilized chicken eggs (age, seven days; weight, 50–55 g) were
incubated under 65–70% relative air humidity at 37°C. On day four
of incubation, 100 µl AAV-hTRX-PR39 or adenovirus (control) was
gently pipetted onto the CAM surface using a transfer pipette. The
eggs were subsequently placed in an incubator for three days. Next,
the CAMs were incubated for 8 h in a hypoxic (1 and 5%
O2) or normoxic (20% O2) environment, after
which they were incubated for 11 days in a normoxic environment.
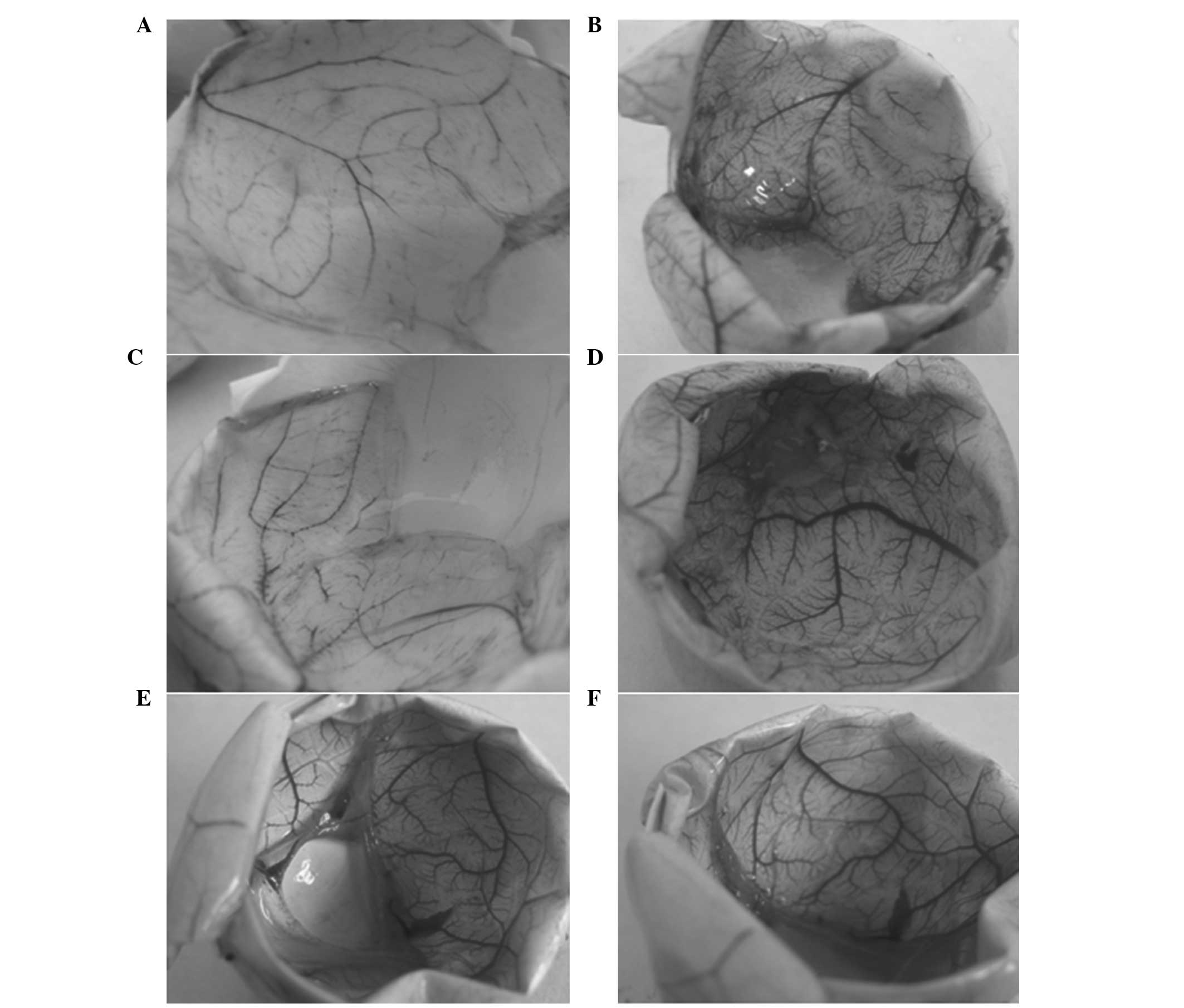
The CAMs were subsequently photographed using an Olympus DP73
digital camera (Olympus Corporation, Tokyo, Japan), as presented in
Fig. 1. The vessel density of the
CAMs was analyzed using Image-Pro Plus software (Media Cybernetics,
Inc., Rockville, MD, USA). For each study group, 10–15 domains were
selected for vessel quantification, and the mean values of the
vessel density were calculated. All animal studies were approved by
the Shandong University Institutional Animal Care and Use
Committee.
Statistical analysis
Data are expressed as the mean ± standard deviation,
and were analyzed using analysis of variance and the homogeneity of
variance test, according to a completely randomized design.
Comparisons between groups were performed using the two-sample
t-test. SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of VEGF, VEGFR-1,
VEGFR-2, FGFR-1, syndecan-4 and PR39
Under normoxic conditions of 20% O2, the
mRNA expression levels of PR39, VEGF, VEGFR-1, VEGFR-2, FGFR-1 and
syndecan-4 exhibited no statistically significant difference when
comparing the hTRX-PR39-transfected ECV304 cells and the control
group (P>0.05). However, under hypoxic conditions of 1%
O2, the quantitative PCR results demonstrated increased
mRNA expression levels of VEGF, VEGFR-1, VEGFR-2, FGFR-1 and
syndecan-4, as well as sharply increased expression levels of PR39,
in the hTRX-PR39-transfected group (P<0.05), as compared with
the control group. The results are shown in Table II. The 5% O2 condition
was not examined as the 20% and 1% O2 conditions
successfully demonstrated that transfection with rAAV/hTRX-PR39
increased the expression levels of VEGF, VEGFR-1, VEGFR-2, FGFR-1
and syndecan-4 under hypoxic condition (1% O2).
 | Table II.Expression levels of the angiogenic
growth factors in the control and PR39-transfected groups. |
Table II.
Expression levels of the angiogenic
growth factors in the control and PR39-transfected groups.
| 20%
O2 | 1%
O2 |
|---|
|
|
|---|
| Gene
expression | Control 1
group | PR39 | Control 2
group | PR39 |
|---|
| VEGF | 0.3±0.06 | 0.33±0.08 | 0.47±0.22 |
0.75±0.25a |
| VEGFR-1 | 0.14±0.07 | 0.15±0.10 | 0.31±0.09 |
0.49±0.21a |
| VEGFR-2 | 0.16±0.05 | 0.15±0.11 | 0.35±0.10 |
0.54±0.12a |
| FGFR-1 | 0.23±0.06 | 0.28±0.16 | 0.42±0.14 |
0.67±0.20a |
| Syndecan-4 | 0.16±0.05 | 0.15±0.04 | 0.29±0.06 |
0.39±0.11a |
| PR39 | 0.00±0.00 | 0.10±0.12 | 0.00±0.00 |
1.43±0.25a |
Effects of AAV-hTRX-PR39 transfection
on the hypoxic chick embryo
Under 20% O2 conditions, the survival
rate of the chick embryos was 100% in the transfected and
non-transfected groups (P>0.05). By contrast, under hypoxic
conditions (5 or 1% O2), the survival rate of the chick
embryos was shown to increase in the transfected group when
compared with the non-transfected group (5% O2, 17/20
vs. 3/20; 1% O2, 16/20 vs. 0/20; P<0.05).
As shown in Table
III, the wet weight of the chick embryos under normoxic
conditions did not significantly differ between the transfected or
non-transfected groups (P>0.05). However, the wet weight of the
chick embryos under hypoxic conditions (5 or 1% O2)
increased significantly in the transfected group when compared with
the non-transfected group (P<0.05).
 | Table III.Effects of AAV-hTRX-PR39 transfection
on the hypoxic chick embryo. |
Table III.
Effects of AAV-hTRX-PR39 transfection
on the hypoxic chick embryo.
| Group | Survival rate
(n) | Wet weight (g) | Density of vessels
(%) |
|---|
| 1%
O2 |
| Control
1 |
0/20c |
8.5±3.56c |
2.21±0.4c |
|
PR39 |
16/20a |
32.5±4.5a |
10.6±0.6a |
| 5%
O2 |
| Control
2 |
3/20c |
20.4±8.56c |
5.65±0.6c |
|
PR39 |
17/20b |
34.31±6.51b |
11.9±0.5b |
| 20%
O2 |
| Control
3 |
20/20 |
38.8±4.27 |
12.5±0.5 |
|
PR39 |
20/20 |
34.2±5.44 |
10.1±0.5 |
Under a normoxic environment, no statistically
significant difference was observed with regard to the density of
the vessels between the transfected and non-transfected groups
(P>0.05). However, the density of the vessels in the chick
embryos subjected to mild hypoxia (5% O2) and severe
hypoxia (1% O2) decreased significantly in the
non-transfected group when compared with the 20% O2
control group. In addition, the density of the vasculature
increased significantly in the transfected groups when compared
with the respective non-transfected groups (P<0.05). The results
are presented in Table III.
Discussion
hTRX is a micromolecular protein that functions as
an oxidant and a reductant. The hTRX gene is 13 kb in length and
encodes 104 amino acids (18). hTRX
is known to play a number of important roles, including regulating
the redox reaction, scavenging free radicals and exerting
antiapoptosis effects (19–21). Previously, hTRX protein homology was
demonstrated to consist of a protein cross-frame feature that
provides available sites for the binding of an active aptamer
(9,22). Following the insertion of the aptamer
into the cross-frame structure, the aptamer becomes more stable
compared with the free peptide and is more prone to transfer into
cells. PR39, a short peptide that is extremely unstable, is prone
to inactivating conformational changes. Thus, therapeutic use of
PR39 requires expression as a recombinant protein. The hTRX protein
can provide a framework for the expression of PR39 as a therapeutic
aptamer. As hTRX is a natural human protein with low
immunogenicity, hTRX can serve as a cross-frame protein for the
construction of a gene fusion expression system, which may
significantly increase the activity of the expression products and
activated soluble proteins. Previous studies have demonstrated that
hTRX-PR39 can reduce the number of apoptotic ECV304 cells under
hypoxic conditions (23,24). Thus, the hTRX-PR39 chimeric protein
provides structural compatibility to ensure the directed
bioavailability of PR39 at the target site, in addition to the
added stability of the protein. Furthermore, in the present study,
the mRNA expression levels of PR39 and the various growth factors
were only activated under conditions of hypoxia, but not under
conditions of 20% O2, indicating that the application of
hTRX-PR39 is controllable.
In the present study, the mRNA expression levels of
VEGF, VEGFR-2, FGFR-1 and syndecan-4 were shown to increase in the
PR39-transfected groups when compared with the respective control
groups, indicating that PR39 may activate these growth factors and
receptors. To assess the effect on vascularization, the density of
the allantoic sac vasculature were calculated. The results
demonstrated that the density of the vasculature was markedly
increased in the transfected groups. Accordingly, the survival
rates of the chick embryos were also improved in the transfected
groups when compared with the respective control groups. Therefore,
the results indicated that hTRX-PR39 was able to induce tolerance
to hypoxia.
PR39 has been previously demonstrated to prevent the
degradation of HIF-1α, which results in the upregulation of
HIF-1α-dependent genes, including VEGF and VEGFR-1 (24,25). In
addition, PR39 is known to upregulate the expression of the FGFRs,
FGFR-1 and syndecan-4, which subsequently activate FGF signaling
(24,26). The improvement in blood supply may
further increased the level of PR39 (27,28). In
the present study, increases in the expression levels of angiogenic
growth factors and vascularization were confirmed in the
PR39-transfected groups; thus, it was hypothesized that these
factors may be the main mechanisms underlying the protective role
of PR39 in hypoxia. In addition, PR39 has been hypothesized to play
a role in the protection of IAP-2 (an inhibitor of apoptosis) and
the decreased activity of caspase-3, leading to the suppression of
apoptosis (29). According to these
results, PR39 may stimulate an angiogenic response, which may be
used as a therapeutic intervention in ischemic areas. In future
studies, hTRX-PR39 should be transfected into ischemic brain
tissues to observe the protective role in ischemic stroke.
According to previous studies (30–33) by
other groups, and our previous study (34), a recombinant AVV (rAAV) vector,
containing the hTRX-PR39 chimeric gene, was constructed to achieve
sustained, stable and efficient protein production. Vectors
containing hTRX-PR39 were transfected into chick embryonic tissues
that had been subjected to hypoxia, and the expression of PR39 was
shown to be activated, as well as that of downstream factors.
Expression activation was only observed under hypoxic conditions,
indicating that the expression is well-controlled. Thus,
rAAV/hTRX-PR39 was demonstrated to promote vascularization and cell
survival in hypoxia, and may have potential in the therapy of
cerebral ischemia.
In conclusion, the hTRX-PR39 chimeric protein
provides structural compatibility to ensure the directed
bioavailability of PR39 at the target site, in addition to added
stability of the protein. Furthermore, transfection with
rAAV/hTRX-PR39 was shown to increase the expression levels of VEGF,
VEGFR-1, VEGFR-2, FGFR-1 and syndecan-4, and promote angiogenesis
and cell survival under hypoxic conditions. Thus, rAAV/hTRX-PR39
may provide a novel therapeutic method for the treatment of
ischemia.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 30970992). The authors
thank Professor Qingyong Liu (Jinan Central Hospital Affiliated
with Shandong University, Jinan, China) for providing support and
assistance with regard to the study design and the provision of
reagents; Shibao Zhang, Bo Liu and Shuangqing Liu (Jinan Central
Hospital Affiliated with Shandong University) for their assistance
in data collection, statistical analyses and image acquisition;
Professor Jianzhong Bi (Second Affiliated Hospital of Shandong
University, Jinan, China) for providing research guidance; and
Professor Aiqin Song (Department of Statistics, Jining Medical
College, Jining, China) for providing statistical support.
References
|
1
|
Alderazi YJ and Grotta JC: Acute
antithrombotic treatment of ischemic stroke. Curr Vasc Pharmacol.
12:353–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madonna R and Rokosh G: Insights into gene
therapy for critical limb ischemia: the devil is in the details.
Vascul Pharmacol. 57:10–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Navarro-Yepes J, Zavala-Flores L, Anandhan
A, et al: Antioxidant gene therapy against neuronal cell death.
Pharmacol Ther. 142:206–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoki M and Morishita R: Therapeutic
angiogenesis for ischemic diseases. Nihon Rinsho. 64:762–768.
2006.[(In Japanese)]. PubMed/NCBI
|
|
5
|
Su H, Joho S, Huang Y, et al:
Adeno-associated viral vector delivers cardiac-specific and
hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc
Natl Acad Sci USA. 101:16280–16285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simonato M, Bennett J, Boulis NM, et al:
Progress in gene therapy for neurological disorders. Nat Rev
Neurol. 9:277–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spector A, Yan GZ, Huang RR, McDermott MJ,
Gascoyne PR and Pigiet V: The effect of H2O2
upon thioredoxin-enriched lens epithelial cells. J Biol Chem.
263:4984–4990. 1988.PubMed/NCBI
|
|
8
|
Anbanandam A, Albarado DC, Tirziu DC,
Simons M and Veeraraghavan S: Molecular basis for proline- and
arginine-rich peptide inhibition of proteasome. J Mol Biol.
384:219–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borghouts C, Kunz C, Delis N and Groner B:
Monomeric recombinant peptide aptamers are required for efficient
intracellular uptake and target inhibition. Mol Cancer Res.
6:267–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muinck ED, Nagy N, Tirziu D, et al:
Protection against myocardial ischemia-reperfusion injury by the
angiogenic Masterswitch protein PR 39 gene therapy: the roles of
HIF1alpha stabilization and FGFR1 signaling. Antioxid Redox Signal.
9:437–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Lecker S, Post MJ, et al:
Inhibition of ubiquitin-proteasome pathway-mediated I kappa B alpha
degradation by a naturally occurring antibacterial peptide. J Clin
Invest. 106:439–448. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerber HP, Condorelli F, Park J and
Ferrara N: Differential transcriptional regulation of the two
vascular endothelial growth factor receptor genes. Flt-1, but not
Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem.
272:23659–23667. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Hao Y, Nie X, Zhang X, Yang G and
Wang Q: Construction of PR39 recombinant AAV under control of the
HRE promoter and the effect of recombinant AAV on gene therapy of
ischemic heart disease. Exp Ther Med. 4:811–814. 2012.PubMed/NCBI
|
|
14
|
Zorko M and Langel U: Cell-penetrating
peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv
Rev. 57:529–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deshayes S, Morris MC, Divita G and Heitz
F: Cell-penetrating peptides: tools for intracellular delivery of
therapeutics. Cell Mol Life Sci. 62:1839–1849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruan XY, Bi JZ, Liu QY, et al:
Construction and identification of recombinant plasmids expressing
hTRX-PR39. Shandong Daxue Xuebao: Yixue Ban. 47:30–34. 2009.[(In
Chinese)].
|
|
17
|
Liu QY, Ruan XY, Liu XG, et al: Cloning
and expression of a cDNA sequence for human thioredoxin. Xian
Jiaotong Daxue Xuebao. 15:183–188. 2003.[(In Chinese)].
|
|
18
|
Powis G and Montfort WR: Proprties and
biological activities of thioredoxins. Annu Rev Pharmacol Toxicol.
41:261–295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yegorova S, Yegorov O and Lou MF:
Thioredoxin induced antioxidant gene expressions in human lens
epithelial cells. Exp Eye Res. 83:783–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi-Miura M, Nakamura H, Yodoi J and
Shiota K: Thioredoxin, an anti-oxidant protein, protects mouse
embryos from oxidative stress-induced developmental anomalies. Free
Radic Res. 36:949–956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Das SK, Sharma NK, Hasstedt SJ, et al: An
integrative genomics approach identifies activation of
thioredoxin/thioredoxin reductase-1-mediated oxidative stress
defense pathway and inhibition of angiogenesis in obese non
diabetic human subjects. J Clin Endocrinol Metab. 96:E1308–E1313.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Umekawa T, Sugiyama T, Kihira T, et al:
Overexpression of thioredoxin-1 reduces oxidative stress in the
placenta of transgenic mice and promotes fetal growth via glucose
metabolism. Endocrinology. 149:3980–3988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Post MJ, Sato K, Murakami M, et al:
Adenoviral PR39 improves blood flow and myocardial function in a
pig model of chronic myocardial ischemia by enhancing collateral
formation. Am J Physiol Regul Integr Comp Physiol. 290:R494–R500.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Post M, Volk R, et al: PR39, a
peptide regulator of angiogenesis. Nat Med. 6:49–55. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Hao Y, Nie X, et al: Recombinant
AAV-PR39-mediated hypoxia-inducible factor 1 alpha gene expression
attenuates myocardial infarction. Int J Mol Med. 33:171–177.
2014.PubMed/NCBI
|
|
26
|
Simons M: Integrative signaling in
angiogenesis. Mol Cell Biochem. 264:99–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen YA, Yu XL, Xia QF, Cen D, Liu JB and
Tu ZG: Macrophage-specific overexpression of antimicrobial peptide
PR-39 inhibits intracellular growth of Salmonella enterica serovar
Typhimurium in macrophage cells. Int J Antimicrob Agents.
34:315–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rodríguez-Martínez S, Cancino-Diaz JC,
Vargas-Zuñiga LM and Cancino-Diaz ME: LL-37 regulates the
overexpression of vascular endothelial growth factor (VEGF) and
c-IAP-2 in human keratinocytes. Int J Dermatol. 47:457–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramanathan B, Wu H, Ross CR and Blecha F:
PR-39, a porcine antimicrobial peptide, inhibits apoptosis:
Involvement of caspase-3. Dev Comp Immunol. 28:163–169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen F, Kuo R, Milon-Camus M, et al:
Intravenous delivery of adeno-associated viral vector serotype 9
mediates effective gene expression in ischemic stroke lesion and
brain angiogenic foci. Stroke. 44:252–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan H, Ji D, Barnard AR, Lipinski DM, You
Q, Lee EJ, Kamalden TA, et al: AAV-mediated gene transfer of human
x-linked inhibitor of apoptosis protects against oxidative cell
death in human RPE cells. Invest Ophthalmol Vis Sci. 52:9591–9597.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Blouin V, Brument N, Bello-Roufai
M and Francois A: Production and purification of recombinant
adeno-associated vectors. Methods Mol Biol. 807:361–404. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bockstael O, Foust KD, Kaspar B and
Tenenbaum L: Recombinant AAV delivery to the central nervous
system. Methods Mol Biol. 807:159–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruan XY, Yuan ZG, Du YF, Yang GX and Wang
QY: Recombinant adeno-associated virus delivered human
thioredoxin-PR39 prevents hypoxia-induced apoptosis of ECV304
cells. Neural Regen Res. 7:708–713. 2012.PubMed/NCBI
|