Introduction
Osteoporosis is one of the main geriatric problems
worldwide, occurring frequently in postmenopausal females (1), while postmenopausal osteoporosis is a
common systemic skeletal system disease occurring in middle-aged
females. The functional decline of the ovaries leads to decreased
estrogen levels, which triggers osteoporotic changes (2). Osteoporosis becomes a clinical issue
when fragility fractures occur in weakened bones. Osteoporotic
fractures of the hip and spine can lead to serious complications,
including loss of mobility and independence, and even mortality.
With the aging of a large portion of the worldwide population,
considerable sums of money are spent managing osteoporosis and
associated fractures (3,4).
A large portion of osteoporosis research is aimed at
prevention, medical treatment of low bone mass and surgical
treatment of osteoporotic fractures. Thus, large animal models that
accurately portray human osteoporotic changes are required for
these studies. In previous studies, rats (5), large osteopenic animal models, such as
nonhuman primates and sheep, have been used as models (6,7).
However, the time required to establish an accurate osteoporosis
model is unknown (8–12). Therefore, the aim of the present
study was to use locally available Chinese goats to establish a
large osteopenic animal model through application of an ovariectomy
(OVX), with a follow-up period of 24 months.
Materials and methods
Ovariectomized goat animal model
In total, 14 skeletally mature female Chinese
mountain goats, with a body weight between 27 and 32 kg, were used
for the study. The goats were randomly divided into an
ovariectomized group (OVX, n=7) or a sham group (SHAM, n=7). The
animals were aged 2.5 years, and skeletal maturity was determined
by radiographical confirmation of the closure of the distal femoral
and proximal tibia growth plates (12). The goats were housed on a farm and
cared for by a qualified veterinarian during the entire study.
Animal Research Ethics approval was obtained from the Research
Ethics Committee of Shanghai Ninth People's Hospital (Shanghai,
China). A bilateral ovariectomy was performed under general
anesthesia, using a standard aseptic surgical technique, on the
seven goats in the OVX group. The same surgical procedure was
performed on the seven goats in the SHAM group, without the
ligation of the oviduct and the excision of the ovary. All the
goats were housed for 24 months, and no goats were excluded from
the study due to disease or any other reasons. The development of
osteopenia was confirmed by measuring the changes in bone mineral
density (BMD), bone microstructure and alterations in biomechanical
properties at several skeletal sites.
Measurement of the serum estrogen
concentration
A 20-ml blood sample was collected from the jugular
vein of the goats at the beginning of study and prior to being
euthanized with pentobarbital sodium (100 mg/kg, intravenously).
After leaving the blood samples to stand for 30 min at room
temperature, the blood was centrifuged for 10 min at 1,000 × g. The
serum estrogen (pg/ml) concentration was measured using a
radioimmunoassay (RIA) following manufacturers instructions. A
gamma counter (University of Science and Technology of China,
Hefei, China) was employed and RIA kits were obtained from the
Institute of Radioactive Medicine at Fudan University (#1031990;
Shanghai, China).
BMD measurement by dual-energy X-ray
absorptiometry (DXA)
DXA (Discovery DXA System; Hologic, Bedford, MA,
USA) was used to measure the BMD (g/cm2) of the first to
fourth lumber vertebrae, femoral neck, femoral diaphysis and tibial
diaphysis. The BMD of the vertebral body was measured at the
baseline and at 24 months after surgery. All measurements were
obtained by the same individual.
Microstructure analysis by
micro-computed tomography (micro-CT)
Vertebral bodies from the goats were isolated by
carefully removing the surrounding muscles, ligaments and
intervertebral discs. The femoral head and femoral neck bone
specimens were subjected to a similar treatment. All the samples
were scanned using micro-CT (µCT 80; Scanco Medical AG,
Brüttisellen, Switzerland) at 70 kVp, 117 mA and 20-µm slice
thickness. After scanning, a constant volume of interest (VOI)
centered over the specimen was selected for analysis of all the
study samples. Three-dimensional (3-D) images were reconstructed
based on the VOI. The bone volume fraction (BV/TV; %), trabecular
thickness (Tb.Th; µm), specific bone surface (BS/BV; %), trabecular
number (Tb.N; 1/mm), trabecular spacing (Tb.Sp; mm), connectivity
density (Conn.D; 1/mm3) and structure model index (SMI;
%) were calculated using the Image Processing Language software,
version 4.29d (Scanco Medical AG) provided with the instrument. The
SMI is a topological index used to estimate the characteristic form
of bone in terms of the plates and rods that compose the 3-D
structure. This index assumes integer values of 0 and 3 for ideal
plates and rods, respectively; for a mixed structure containing
plates and rods, the value lies between 0 and 3 (13). Cortical bone porosity (%) in the
vertebral body and femoral neck was also measured.
Mechanical testing
Prior to mechanical testing, samples were defrosted
overnight in a 0.15-M NaCl solution at 5°C. At 3 h prior to
mechanical testing, the samples were removed from the refrigerator
and allowed to reach room temperature. A previously described
mechanical testing method was applied (8), which utilized a testing machine (model
8874; Instron Corporation, Norwood, MA, USA). The samples were kept
moist with a saline-soaked gauze throughout the experiment. Prior
to the cyclic loading the vertebral body, femoral head and femoral
neck were preloaded to 50 N and cycled for 200 cycles between 50
and 450 N at 1 Hz for preconditioning. Immediately after cyclic
loading, the sample was compressed under displacement control at a
rate of 2 mm/min. During compression, load and displacement data
were recorded. Each specimen was compressed in a longitudinal
direction between two plates at a rate of 2 mm/min. The test
concluded upon the failure of the specimen. The ultimate stress and
elastic modulus were obtained from the stress-strain curve.
The frozen tibial samples were thawed prior to the
three-point bending assessment. The tibia was placed on a lateral
surface on two rounded support bars spaced 2.4 cm apart. A preload
was applied at the medial surface of the diaphysis by lowering a
third rounded bar. A constant displacement rate of 2 mm/min was
applied until failure occurred.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The Student's t-test was used to compare the mean values
between the OVX and SHAM groups, while a paired t-test was used to
compare the baseline data and the data collected at 24 months after
surgery. All statistical analyses were performed using a commercial
software package (SPSS 16.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum estrogen levels in the
blood
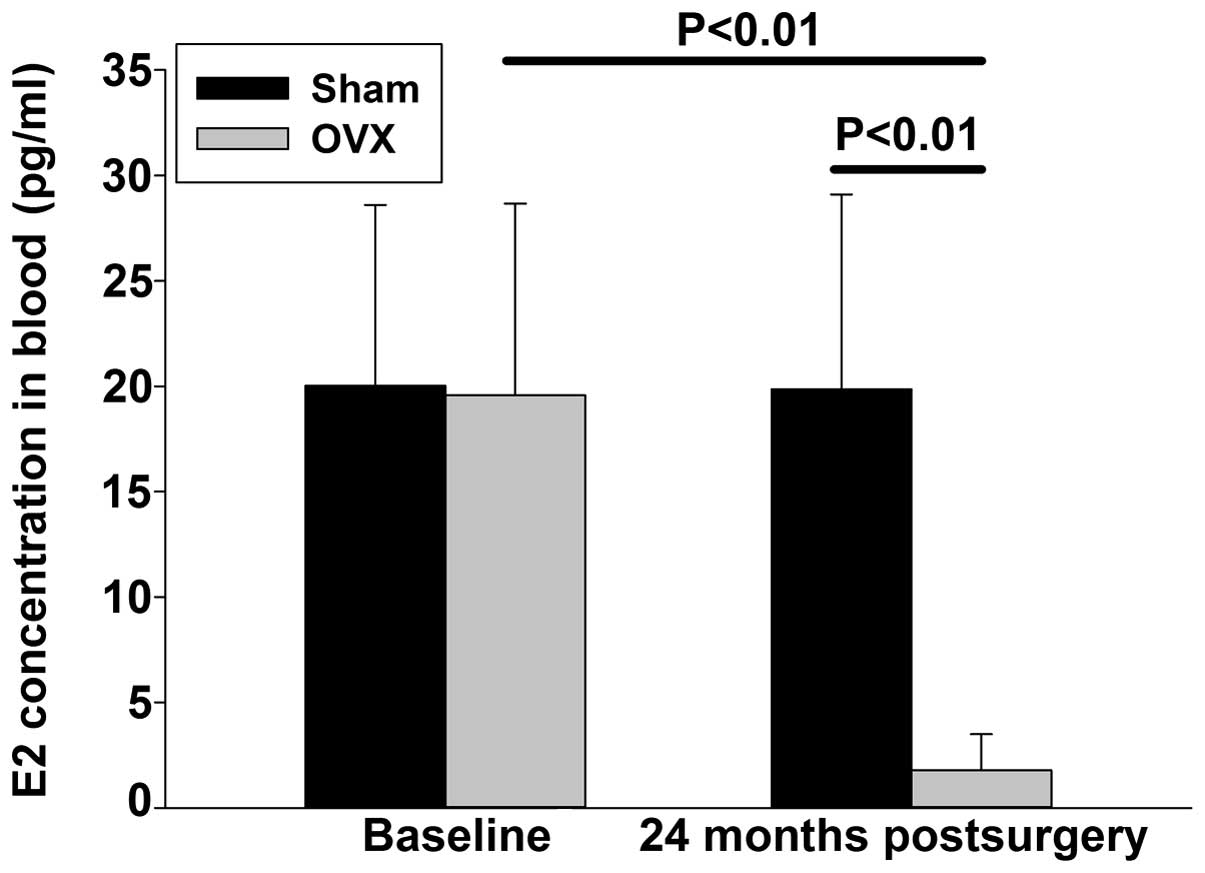
At 24 months after the ovariectomy, the serum
estrogen levels in the OVX group (1.78±1.71 pg/ml) were
significantly lower compared with those in the SHAM group
(19.86±9.24 pg/ml). When compared with the baseline levels, the
24-month estrogen level of the SHAM group did not change
significantly, whereas a statistically significant decrease in the
estrogen levels of the OVX group was observed (Fig. 1).
BMD
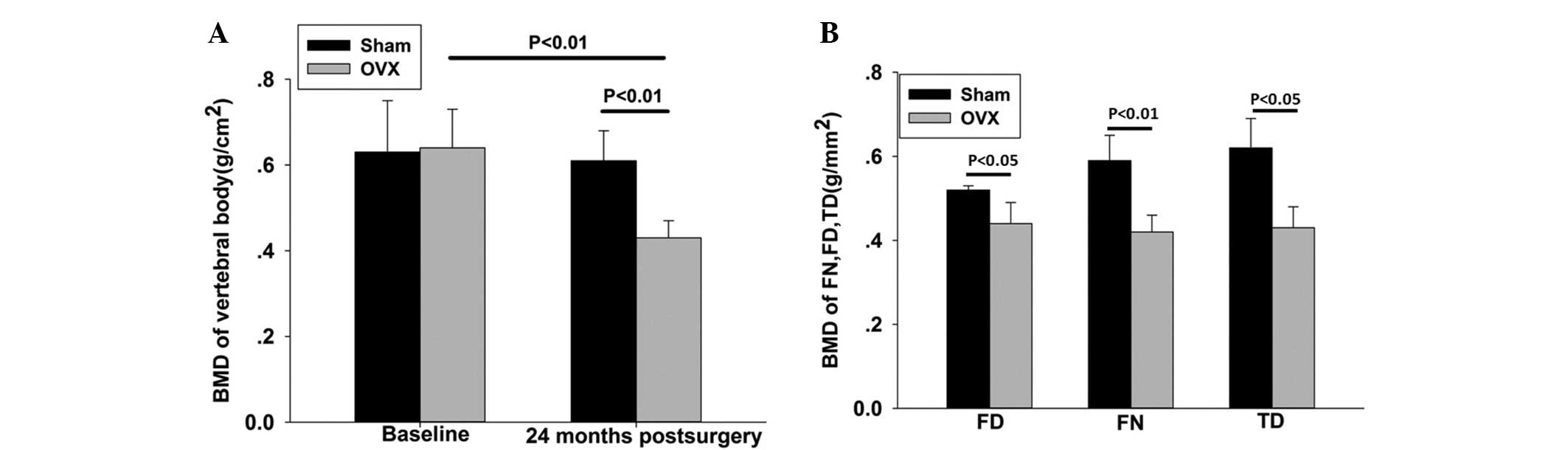
At 24 months after the ovariectomy, when compared
with the SHAM group, the OVX group exhibited a significantly
reduced BMD in the lumbar spine, femoral neck, femoral diaphysis
and tibial diaphysis [29.5, 28.5 (P<0.01), 23 and 28.8%
(P<0.05), respectively] (Fig.
2).
Microstructure analysis by
micro-CT
The trabecular microstructure of the vertebral body
revealed the following results. Compared with the SHAM group, the
OVX group exhibited a significantly decreased BV/TV, Tb.N, Conn.D
and DA (37.1, 36.7, 17.3 and 16.5%, respectively; P<0.01), Tb.Th
(0.5%; P<0.05) and significantly increased BS/BV and Tb.Sp (37.7
and 62%, respectively; P<0.01). In addition, the SMI was lower
in the SHAM group compared with the OVX group; however, the
difference was not statistically significant (P>0.05). The BV/TV
and DA of the femoral head were significantly lower in the OVX
group (15.5 and 12.7%, respectively; P<0.05) when compared with
the SHAM group. BS/BV, SMI and Tb.Sp exhibited upward trends, while
the remaining parameters exhibited a significant downward trend;
however, no statistically significant differences were observed.
The Tb.Th and Conn.D of the femoral neck were lower in the OVX
group compared with the SHAM group, while the other parameters
showed no significant difference (P<0.05; Fig. 3).
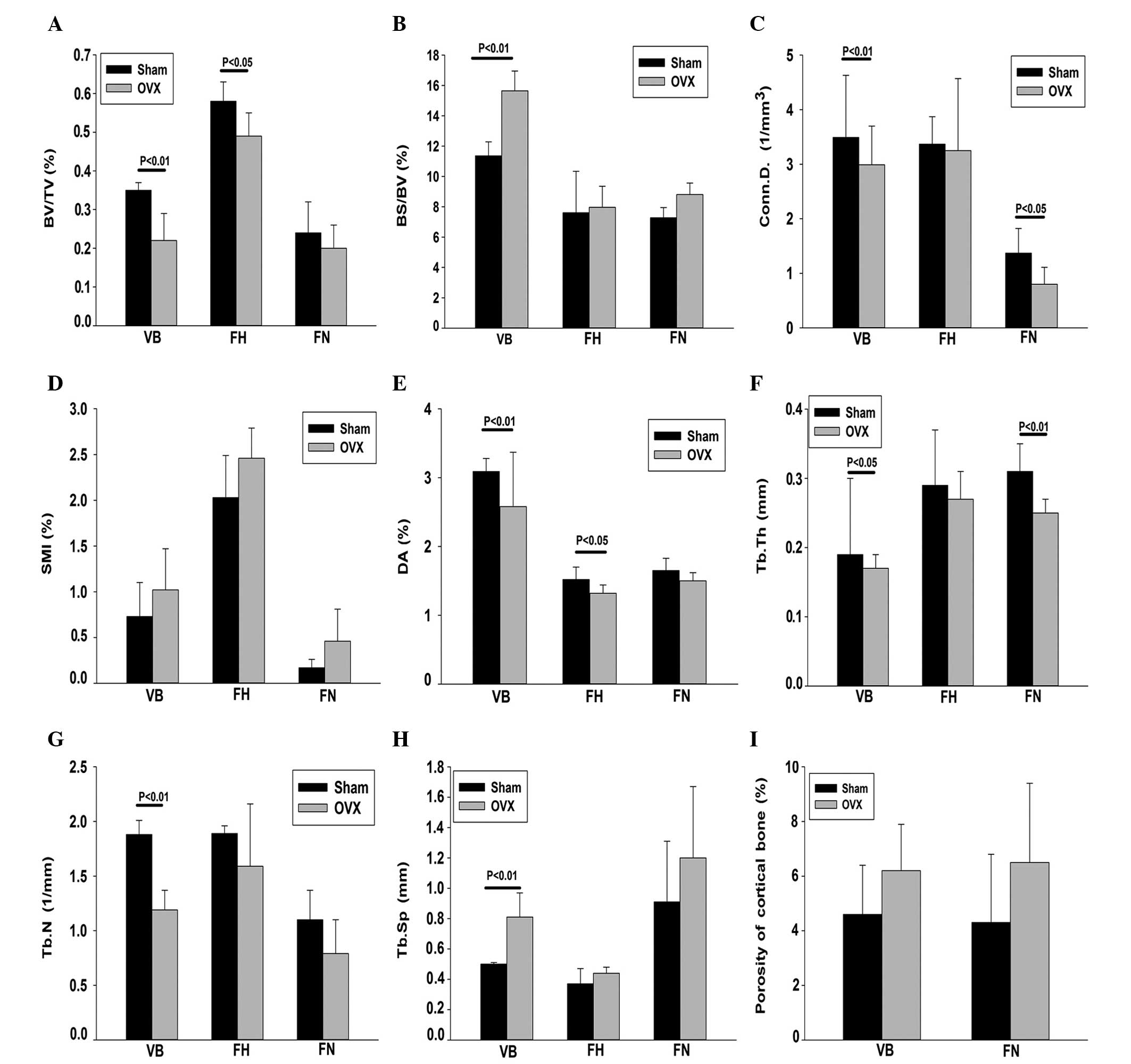 | Figure 3.Trabecular microstructure parameters
were measured using micro-computed tomography at 24 months after
surgery. (A) BV/TV; (B) BS/BV; (C) Conn.D; (D) SMI; (E) DA; (F)
Tb.Th; (G) Tb.N; (H) Tb.Sp; and (I) porosity of cortical bone. OVX,
ovariectomized; VB, vertebral body; FH, femoral head; FN, femoral
neck; BV/TV, bone volume fraction; BS/BV, specific bone surface;
Conn.D, connectivity density; SMI, structure model index; DA,
degree of anisotropy; Tb.Th, trabecular thickness; Tb.N, trabecular
number; Tb.Sp, trabecular spacing. |
The cortical bone porosity of the vertebral body and
femoral neck were higher in the OVX group when compared with the
SHAM group (P<0.05; Fig. 3).
Mechanical testing
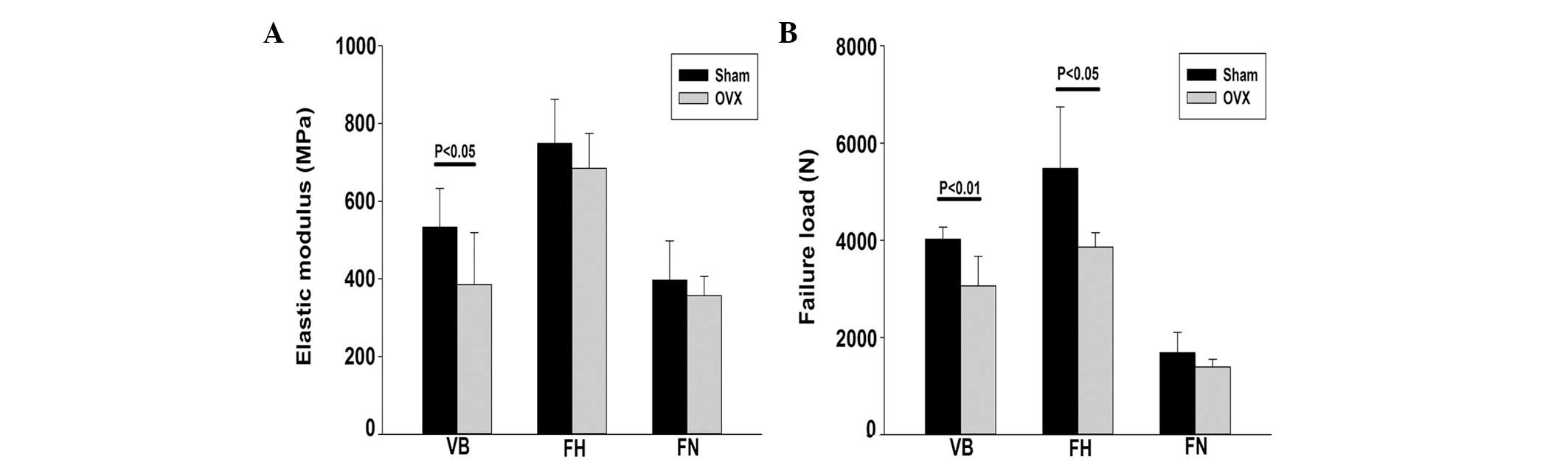
At 24 months after the surgery, the failure load and
elastic modulus of the vertebral body were significantly lower in
the OVX group compared with the SHAM group (P<0.05), with an
overall decrease of ~24%. The failure load of the femoral head and
femoral neck were also significantly lower in the OVX group when
compared with the SHAM group, decreasing by ~30% (P<0.05) and
17% (P>0.05), respectively (Fig.
4).
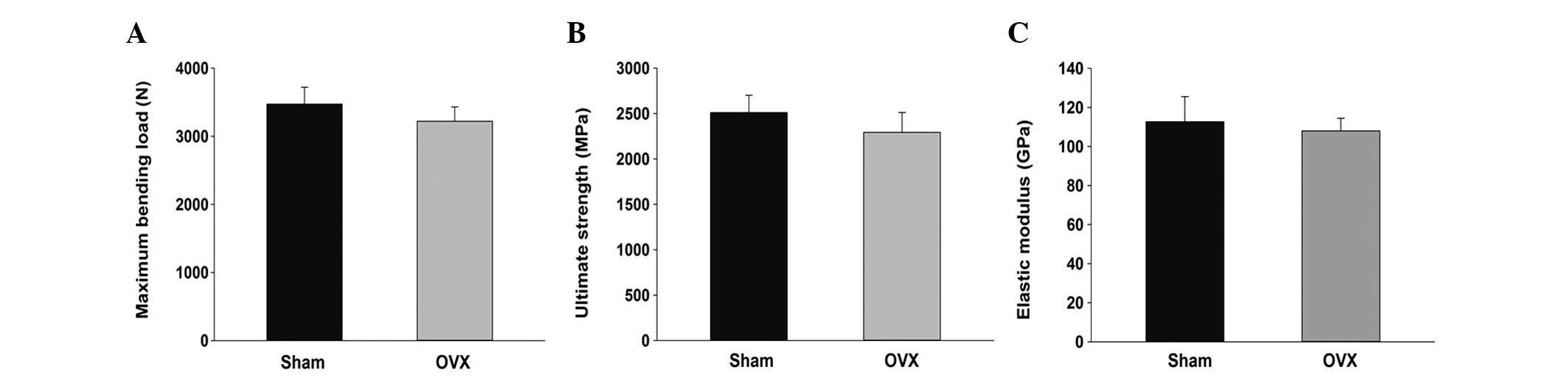
Results of the three-point bending test revealed
that the maximum bending load of the tibia in the OVX group was
less than that in the SHAM group, with a decrease of ~7%; however,
this difference was not statistically significant (P>0.05). The
ultimate strength and elastic modulus in the OVX group decreased by
4 and 7%; however, the differences were not statistically
significant (P>0.05; Fig. 5).
Discussion
Loss of bone mass and damaged bone microstructure
significantly weakens the mechanical strength of the bone.
Osteoporotic bones are prone to brittle fractures, which seriously
threaten the quality of life in elderly female patients (1). The pathogenesis of osteoporosis is very
complex, and research into the condition is expensive and
time-consuming. Estrogen plays a fundamental role in skeletal
growth and bone homeostasis in males and females. In postmenopausal
females, longitudinal loss of bone mass increases in association
with reduced levels of endogenous estrogen (8,14).
Although marked progress has been made in understanding how
estrogen deficiency causes bone loss, the mechanisms involved are
complex and multifaceted (15).
Therefore, selecting and establishing an ideal experimental animal
model is essential for further osteoporosis research.
Sheep have been widely used as an animal model in
orthopedic research (16), and as
osteoporosis models in numerous studies (17–19).
However, the time required to establish an accurate osteoporosis
model remains inconsistent between studies. A number of previous
studies (20–23) have found that bone formation in sheep
continues to decline between 10 weeks and 6 months after an
ovariectomy, with certain studies reporting that the BMD
significantly decreases 6 months after an ovariectomy (24); however, other studies have not
observed these results (25).
A number of scholars have proposed that an
osteoporosis model can be established within 6 months through the
use of various testing methods, such as biomechanical testing, bone
histophotometry analysis or DXA (18,26–28);
however, there are limitations, including a small number of samples
and single specimen testing methods. Recent research has
demonstrated that the BMD of the vertebral body in ovariectomized
sheep exhibits a significant downward trend after 1 year; however,
no statistically significant difference was identified when
compared with a sham-control group (18). It has been suggested that a
short-term ovariectomized sheep model should be defined as an
osteopenia model, rather than an osteoporosis model (29). Lill et al (12) hypothesized that >1 year was
required to establish an osteoporosis model using an ovariectomy
alone. Short-term ovariectomies are unable to guarantee the
establishment of an effective osteoporosis model. Therefore, the
time required to establish an osteoporosis model remains
controversial. In the present study, the changes in BMD, bone
microstructure and biomechanical properties were analyzed in
different skeletal locations in ovariectomized goats for 24 months
to further clarify the time period required to establish an
effective osteoporosis model.
Since sheep are most fertile in the autumn and
winter, an increase in hormone levels occurs during these seasons,
which can affect the result of an ovariectomy. Furthermore, the BMD
of ewes is influenced by seasons and is generally reduced in the
winter (30–32). To account for seasonal influences,
the present study was initiated in July and finished in the same
season 24 months later. Evaluation of the serum estrogen levels is
essential for the assessment of the model. Johnson et al
(25) found that an ovariectomy was
unable to completely eliminate 17β-estradiol synthesis (4–6 pg/ml)
in sheep. Furthermore, Karch et al (26) found that the normal estrogen level in
sheep was ~1 pg/ml, and estrogen levels were significantly lower
following an ovariectomy. The results of the present study
demonstrated that 24 months after an ovariectomy, the serum
estrogen levels were significantly decreased in the OVX group
(1.78±1.71 pg/ml) when compared with the SHAM group (19.86±9.24
pg/ml; P<0.001).
Geusens et al (29) observed that at 6 months after an
ovariectomy, bone mass in the femoral neck of sheep decreased by
between 3 and 9%; however, no statistically significant difference
was observed when compared with a control group (33). Lill et al (9,12) found
that following application of an ovariectomy and restricted calcium
intake, the BMD of the radius in sheep decreased by 5.5%. In the
study by Turner et al (32),
the BMD was shown to decrease by 3–8%. These observations indicate
that short-term estrogen deficiency can increase bone turnover by
up to 10% in an ovariectomized sheep model. However, whether
short-term ovariectomized sheep can effectively simulate the
long-term human postmenopausal process that leads to osteoporosis
is yet to be determined. A previous study demonstrated that
limiting calcium and vitamin D intake can enhance the effect of
bone mass loss caused by estrogen deficiency (34). Food-induced metabolic acidosis may
also enhance the effects of an ovariectomy (35). In the present study, long-term
estrogen deficiency (2 years) was investigated. The BMDs of the
lumbar spine, femoral neck and femoral head were significantly
lower in the OVX group when compared with the SHAM group,
decreasing by 28.5, 28.8 and 23% (P<0.05), respectively. The
BMDs of the femoral and tibial shafts were also reduced by 15.3 and
30.6%, respectively; however, the decreases were not statistically
significant when compared with those in the SHAM group (P>0.05).
Changes in the microstructure of the cortical bone play an
important role in bone quality. Increased cortical bone porosity
can lead to decreased structural integrity (35) and is closely associated with the
occurrence of fractures (36). The
results of the present study revealed that at 24 months after the
ovariectomy, the cortical bone porosity in the vertebral body and
femoral neck significantly increased by 6.2±1.7 and 6.5±2.9%,
respectively. These changes may have been caused by long-term
estrogen deficiency.
In addition to a reduction in bone mass, the
trabecular spatial microstructure is altered during estrogen
deficiency (37). Bone
microstructure is strongly associated with the mechanical
properties of bone (35), and the
measurement of bone microstructure is an important predictor of
fracture risk (13). Estrogen
deficiency for two years has been shown to influence the bone
microstructure primarily by affecting the SMI (36,38).
However, previously used osteoporosis models have been studied for
no longer than 18 months following the ovariectomy (35). In the present study, the observation
time was increased to 24 months to further investigate the effect
of estrogen deficiency on bone microstructure. After 24 months, the
BV/TV decreased by 37.1% (P<0.05), while the DA decreased by
16.5% (P<0.001), as compared with the SHAM group. The mechanical
property of bone is mostly determined by the BV/TV and DA. When the
BV/TV and DA decrease, the mechanical properties also decrease.
Furthermore, the axial compressive load and elastic modulus of the
vertebral body were found to be significantly lower in the OVX
group when compared with the SHAM group (P<0.05), with the
maximum compressive load reduced by ~24%. The important
characteristics of trabecular bone degeneration include changes to
the rod-like structure and the appearance of perforations (40). In the present study, the trabecular
bone structure became significantly rod-like at 24 months after the
ovariectomy. In addition, for the vertebral body, the SMI in the
OVX group increased by 40%, and the BS/BV increased by 37.7%
(P<0.001). Furthermore, the Tb.N decreased by 36.7% (P<0.01),
the Conn.D increased by 17.3% (P<0.01). In the femoral head,
only the BV/TV (P<0.05) and DA (P<0.05) decreased
significantly in the OVX group when compared with the SHAM group;
this change was proportional to a change in the maximum axial
compression load. When comparing the structure of the femoral neck
between the OVX and SHAM groups, only the BS/BV, Tb.Th and Conn.D
were significantly different, whereas the mechanical test results
revealed no statistically significant differences. The parameters
of the trabecular microstructure changed unevenly, which may have
been the result of adaptive changes triggered by the decrease in
BMD and alterations in mechanical properties (41). By reorganizing the trabecular
direction, the trabecular bone is able to maintain mechanical
properties. Results of the three-point bending test revealed that
the maximum tibial bending loads were lower in the OVX group when
compared with the SHAM group, showing a decrease of 7%; however,
the difference was not statistically significant (P>0.05). The
ultimate strength in the OVX group decreased by 4% (P>0.05) when
compared with the SHAM group, while the elastic modulus decreased
by ~7% (P>0.05). These decreases may have been caused by the
high rate of bone turnover triggered by the estrogen deficiency,
which subsequently increased the porosity of the trabecular
bone.
In conclusion, the present study investigated the
osteoporosis outcome in goats after extending the estrogen
deficiency time to 24 months. After 24 months, the OVX goats
exhibited features of osteoporosis (osteopenia). Therefore, based
on the results, the following hypotheses can be concluded. Firstly,
24 months after an ovariectomy in goats, a pathological state
similar to osteoporosis is produced. Secondly, goats may be a
suitable animal model for the study of osteoporosis. Finally, a
reduction in the BMD, alterations in biomechanical properties and a
change in the microstructure are associated with estrogen
deficiency. These results may aid the study into the long-term
effects of different therapeutic protocols for osteoporosis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation for Youth (no. 11002090), the Shanghai
Natural Science Foundation (no. 10ZR1417900) and the Program for
Key Disciplines of the Shanghai Municipal Education Commission (no.
J50206).
References
|
1
|
Amarante F, Vilodre LC, Maturana MA and
Spritzer PM: Women with primary ovarian insufficiency have lower
bone mineral density. Braz J Med Biol Res. 44:78–83. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Syed FA, Oursler MJ, Hefferanm TE,
Peterson JM, Riggs BL and Khosla S: Effects of estrogen therapy on
bone marrow adipocytes in postmenopausal osteoporotic women.
Osteoporos Int. 19:1323–1330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA FDA Guidelines and animal models for osteoporosis. Bone.
17:(4 Suppl). 125S–133S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau EM: Epidemiology of osteoporosis in
urbanized Asian populations. Osteoporos Int. 7:(Suppl 3). S91–S95.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lirani-Galvão AP, Bergamaschi CT, Silva OL
and Lazaretti-Castro M: Electrical field stimulation improves bone
mineral density in ovariectomized rats. Braz J Med Biol Res.
39:1501–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagi CM, Hanson N, Andresen C, Pero R,
Lariviere R, Turner CH and Laib A: The use of micro-CT to evaluate
cortical bone geometry and strength in nude rats: Correlation with
mechanical testing, pQCT and DXA. Bone. 38:136–144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longcope C, Hoberg L, Steuterman S and
Baran D: The effect of ovariectomy on spine bone mineral density in
rhesus monkeysBone. 10:341–344. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egermann M, Goldhahn J and Schneider E:
Animal models for fracture treatment in osteoporosis. Osteoporos
Int. 16:(Suppl 2). S129–S138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lill CA, Gerlach UV, Eckhardt C, Goldhahn
J and Schneider E: Bone changes due to glucocorticoid application
in an ovariectomized animal model for fracture treatment in
osteoporosis. Osteoporos Int. 13:407–414. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Les CM, Spence CA, Vance JL,
Christopherson GT, Patel B, Turner AS, Divine GW and Fyhrie DP:
Determinants of ovine compact bone viscoelastic properties: Effects
of architecture, mineralization, and remodeling. Bone. 35:729–738.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Macleay JM, Olson JD and Turner AS: Effect
of dietary-induced metabolic acidosis and ovariectomy on bone
mineral density and markers of bone turnover. J Bone Miner Metab.
22:561–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lill CA, Fluegel AK and Schneider E:
Effect of ovariectomy, malnutrition and glucocorticoid application
on bone properties in sheep: a pilot study. Osteoporos Int.
13:480–486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hildebrand T and Rüegsegger P:
Quantification of bone microarchitecture with the structure model
index. Comput Methods Biomech Biomed Engin. 1:15–23. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ng AC, Melton LJ III, Atkinson EJ,
Achenbach SJ, Holets MF, Peterson JM, Khosla S and Drake MT:
Relationship of adiposity to bone volumetric density and
microstructure in men and women across the adult lifespan. Bone.
55:119–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fazzalari NL, Forwood MR, Smith K, Manthey
BA and Herreen P: Assessment of cancellous bone quality in severe
osteoarthrosis: Bone mineral density, mechanics, and microdamage.
Bone. 22:381–388. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giavaresi G, Fini M, Torricelli P, Martini
L and Giardino R: The ovariectomized ewe model in the evaluation of
biomaterials for prosthetic devices in spinal fixation. Int J Artif
Organs. 24:814–820. 2001.PubMed/NCBI
|
|
17
|
Goldhahn J, Neuhoff D, Schaeren S, Steiner
B, Linke B, Aebi M and Schneider E: Osseointegration of hollow
cylinder based spinal implants in normal and osteoporotic
vertebrae: A sheep study. Arch Orthop Trauma Surg. 126:554–561.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Les CM, Vance JL, Christopherson GT,
Turner AS, Divine GW and Fyhrie DP: Long-term ovariectomy decreases
ovine compact bone viscoelasticity. J Orthop Res. 23:869–876. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Zhao J, Geusens P, Liao EY,
Adriaensens P, Gelan J, Azria M, Boonen S, Caulin F, Lynch JA, et
al: Femoral neck trabecular microstructure in ovariectomized ewes
treated with calcitonin: MRI microscopic evaluation. J Bone Miner
Res. 20:125–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosekilde L, Weisbrode SE, Safron JA,
Stills HF, Jankowsky ML, Ebert DC, Danielsen CC, Søgaard CH, Franks
AF, Stevens ML, et al: Evaluation of the skeletal effects of
combined mild dietary calcium restriction and ovariectomy in
Sinclair S-1 minipigs: A pilot study. J Bone Miner Res.
8:1311–1321. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newton BI, Cooper RC, Gilbert JA, Johnson
RB and Zardiackas LD: The ovariectomized sheep as a model for human
bone loss. J Comp Pathol. 130:323–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leung KS, Siu WS, Cheung NM, Lui PY, Chow
DH, James A and Qin L: Goats as an osteopenic animal model. J Bone
Miner Res. 16:2348–2355. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chavassieux P, Garnero P, Duboeuf F, et
al: Effects of a new selective estrogen receptor modulator (MDL
103,323) on cancellous and cortical bone in ovariectomized ewes: A
biochemical, histomorphometric, and densitometric study. J Bone
Miner Res. 16:89–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosen CJ, Morrison A, Zhou H, Storm D,
Hunter SJ, Musgrave K, Chen T, Wei W and Holick MF: Elderly women
in northern New England exhibit seasonal changes in bone mineral
density and calciotropic hormones. Bone Miner. 25:83–92. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnson RB, Gilbert JA, Cooper RC, Dai X,
Newton BI, Tracy RR, West WF, DeMoss TL, Myers PJ and Streckfus CF:
Alveolar bone loss one year following ovariectomy in sheep. J
Periodontol. 68:864–871. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karsch FJ, Dahl GE, Evans NP, Manning JM,
Mayfield KP, Moenter SM and Foster DL: Seasonal changes in
gonadotropin-releasing hormone secretion in the ewe: alteration in
response to the negative feedback action of estradiol. Biol Reprod.
49:1377–1383. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baird DT, Campbell B, de Souza C and
Telfer E: Long-term ovarian function in sheep after ovariectomy and
autotransplantation of cryopreserved cortical strips. Eur J Obstet
Gynecol Reprod Biol. 113:(Suppl 1). S55–S59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gaynor JS, Monnet E, Selzman C, Parker D,
Kaufman L, Bryant HU, Mallinckrodt C, Wrigley R, Whitehill T and
Turner AS: The effect of raloxifene on coronary arteries in aged
ovariectomized ewes. J Vet Pharmacol Ther. 23:175–179. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geusens P, Boonen S, Nijs J, Jiang Y,
Lowet G, Van Auderkercke R, Huyghe C, Caulin F, Very JM, Dequeker
XJ and Van der Perre G: Effect of salmon calcitonin on femoral bone
quality in adult ovariectomized ewes. Calcif Tissue Int.
59:315–320. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Newman E, Turner AS and Wark JD: The
potential of sheep for the study of osteopenia: Current status and
comparison with other animal models. Bone. 16:(Suppl). 277S–284S.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turner AS: Animal models of
osteoporosis-necessity and limitations. Eur Cell Mater. 1:66–81.
2001.PubMed/NCBI
|
|
32
|
Turner AS, Alvis M, Myers W, Stevens ML
and Lundy MW: Changes in bone mineral density and bone-specific
alkaline phosphatase in ovariectomized ewes. Bone. 17:(Suppl).
395S–402S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
MacLeay JM, Olson JD, Enns RM, Les CM,
Toth CA, Wheeler DL and Turner AS: Dietary-induced metabolic
acidosis decreases bone mineral density in mature ovariectomized
ewes. Calcif Tissue Int. 75:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aldini NN, Fini M, Giavaresi G, Giardino
R, Greggi T and Parisini P: Pedicular fixation in the osteoporotic
spine: A pilot in vivo study on long-term ovariectomized sheep. J
Orthop Res. 20:1217–1224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ammann P and Rizzoli R: Bone strength and
its determinants. Osteoporos Int. 14:(Suppl 3). S13–S18.
2003.PubMed/NCBI
|
|
36
|
Bolotin HH: Inaccuracies inherent in
dual-energy X-ray absorptiometry in vivo bone mineral densitometry
may flaw osteopenic/osteoporotic interpretations and mislead
assessment of antiresorptive therapy effectiveness. Bone.
28:548–555. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bord S, Horner A, Beavan S and Compston J:
Estrogen receptors alpha and beta are differentially expressed in
developing human bone. J Clin Endocrinol Metab. 86:2309–2314. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Linde F and Hvid I: The effect of
constraint on the mechanical behaviour of trabecular bone
specimens. J Biomech. 22:485–490. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sigrist IM, Gerhardt C, Alini M, Schneider
E and Egermann M: The long-term effects of ovariectomy on bone
metabolism in sheep. J Bone Miner Metab. 25:28–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu XS, Walker MD, McMahon DJ, Udesky J,
Liu G, Bilezikian JP and Guo XE: Better skeletal microstructure
confers greater mechanical advantages in Chinese-American women
versus white women. J Bone Miner Res. 26:1783–1792. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding M, Danielsen CC, Hvid I and Overgaard
S: Three-dimensional microarchitecture of adolescent cancellous
bone. Bone. 51:953–960. 2012. View Article : Google Scholar : PubMed/NCBI
|