Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-associated mortality in males and the sixth leading
cause in females worldwide (1). In
addition, HCC is ranked as the second leading cause of
cancer-related mortality in China (2). Over 50% of the annual HCC cases
reported worldwide are diagnosed in the Chinese population
(3), while hepatitis B virus (HBV)
infection is responsible for 80% of all HCC causes in China
(4). While a number of studies have
revealed that HBV infection is the key pathogenic factor for the
development of HCC, male gender and cirrhosis have also been found
to be independent risk factors for the occurrence of HCC (5–7).
However, few studies exist on patients presenting all these risk
factors.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
initially considered to be an essential glycolytic enzyme that is
expressed in all prokaryotic and eukaryotic organisms. GADPH plays
a major role in cellular metabolism, converting
glyceraldehyde-3-phosphate to 1,3-diphosphoglycerate (8). In addition, studies have demonstrated
that GAPDH is involved in certain important physiological
functions, including the transport of transfer RNA (9), translational control (10) and binding with viral RNAs (11,12).
Furthermore, increased GAPDH expression has been previously
reported in renal cell carcinoma (13), lung cancer (14), breast cancer (15), prostate carcinoma (16) and HCC (17). A number of studies have demonstrated
that the elevated expression of GAPDH is correlated with
chemotherapy-induced DNA damage response (18,19). The
induction of cell cycle arrest in p53-proficient carcinoma cells
through GAPDH abrogation indicates that GAPDH-depleting agents may
have a cytostatic effect in cancer cells (20). In addition, higher intranuclear GAPDH
expression has been found to be correlated with higher cell
sensitivity to mercaptopurine treatment in human leukemia cell
lines (21). Therefore,
investigating the underlying mechanism resulting in aberrant
expression of GAPDH in the development of HCC in patients
presenting the aforementioned risk factors is essential.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) is a powerful tool used to detect the level of
gene expression in certain tissues under normal and disease
conditions (22,23). The authors of the present study have
previously used RT-qPCR to analyze the stability of six candidate
genes in paired tumor and non-tumor tissues from 33 male with
untreated HBV-associated HCC and cirrhosis, using the geNorm and
NormFinder software (24).
C-terminal banding protein 1 (CTBP1) and hypoxanthine
phosphori-bosyltransferase 1 (HPRT1) were demonstrated to be
evidently more stable compared with GAPDH, while CTBP1 was found to
have the most stable gene expression (24). The present study aimed to investigate
whether GAPDH expression was aberrantly altered in the tumors of
male HCC patients with chronic HBV infection. The clinical
significance was also addressed.
Materials and methods
Patient information and sample
collection
HCC tumor and paired non-tumor tissue samples were
obtained from 72 untreated male patients, suffering from
HBV-associated HCC with cirrhosis. The patients underwent
hepatectomy at the Henan Cancer Hospital (Zhengzhou, China) between
July 2009 and October 2010. The selected HCC patients met all the
following criteria: seropositive for HBV surface antigen (HBsAg) or
HBV DNA-positive in tumor tissues; received no chemotherapy prior
to surgery; male gender; and suffered from liver cirrhosis. Liver
cirrhosis and HCC were evaluated by two pathologists independently.
The Ishak scoring system was used to assess fibrosis stage
(25). Samples were collected from
the tumor and paired non-tumor tissues following hepatectomy and
the specimens were snap-frozen in liquid nitrogen. The age range of
the 72 patients was 34–76 years (mean age, 51.25±9.91 years). The
Institutional Review Board of Peking University (Beijing, China)
approved all the procedures in the present study. Written informed
consent was obtained from the patients/patients' families prior to
their participation.
Analysis of GAPDH mRNA expression
levels in tumor and non-tumor tissues from HCC patients using
RT-qPCR
The tissue specimens were ground in liquid nitrogen
and homogenized in TRIzol (Invitrogen Life Technologies, Carlsbad,
CA, USA) using a mortar. Total RNA was extracted with TRIzol
(Invitrogen Life Technologies) according to the manufacturer's
instructions. Genomic DNA contamination was removed by on-column
digestion using the RNase-free DNase kit (Takara Bio Inc., Otsu,
Japan). The concentration of the isolated total RNA was calculated
by measuring the absorbance (A) at 260 and 280 nm using a NanoDrop
ND-2000 spectrophoto-meter (Thermo Fisher Scientific Inc.,
Wilmington, DE, USA). A260/A280 ratio of >1.90 and 28S/18S ratio
of ≥1.7 were the threshold values for inclusion of the RNA samples
in this study. The integrity of the RNA samples was confirmed by
electrophoresis on a 1% agarose gel. First-strand cDNA was
synthesized using a random primer and the RevertAid First Strand
cDNA Synthesis kit (Fermentas, Vilnius, Lithuania), according to
the manufacturer's instructions. The primers used in the RT-qPCR
assays of GAPDH were designed using the Primer Premier 5.0 software
(Premier Biosoft, Palo Alto, CA, USA). The Roche LightCycler 480
detection system (Roche Diagnostics GmbH, Mannheim, Germany) was
used for RT-qPCR analysis. The reactions were performed in a final
volume of 20 µl, containing 10 µl of SYBR® Green master mix (Roche
Diagnostics GmbH), 0.5 µl of each 10 µM primer (500 nM), 1 µl cDNA
and 8 µl nuclease-free sterile water. All the standard solutions
and samples were analyzed in triplicate on 96-well reaction plates.
The cycling conditions were set as follows: 10 min template
denaturation at 95°C, 40 cycles of denaturation at 95°C for 30 sec
and elongation at 72°C for 30 sec. Melting-curve analysis was
performed following RT-qPCR and the baseline and cycle threshold
values (Ct values) were automatically determined for all the plates
using the Roche LightCycler 480 software. A Ct value difference
between triplicates of ≤1 was considered as acceptable and was used
to calculate the average Ct values. Genes exhibiting an >2-fold
increase (2−ΔCt>2) or an <0.5-fold decrease
(2−ΔCt<0.5) in their expression levels were
considered to be differentially expressed, whereas genes with an
≤2-fold increase or ≥0.5-fold decrease (0.5≤2−ΔCt≤2)
were defined as equally expressed, as previously reported (26).
Construction of GAPDH expression
vectors
The 1026 bp GAPDH cDNA containing the entire open
reading frame was amplified using RT-qPCR and cloned into the
expression vector, pIRES2-EGFP (Clontech Laboratories, Inc., Palo
Alto, CA, USA). The GAPDH primers used were as follows: sense,
5′-CCGGAATTCATGGGGAAGGTGAAGG-3′; and antisense,
5′-GACGTCGACTTACTCCTTGGAGGCCATG-3′. The GAPDH-expressing vector,
pGAPDH-IRES2-EGFP, was constructed and confirmed by automated
sequencing, in order to establish the direction of cloning and
determine whether the sequence was correct.
HepG2 and PLC/PRF/5 cell cultures,
drug treatment and viability assay
HepG2 cells (American Type Culture Collection,
Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (Gibco Life
Technologies, Carlsbad, CA, USA), while PLC/PRF/5 cells (American
Type Culture Collection) were maintained in RPMI 1640 medium
supplemented with 10% fetal bovine serum. In this study, the stock
solutions of 104 µM epirubicin (Pfizer, New York, NY,
USA) or 106 µM araC (Sigma-Aldrich, St. Louis, MO, USA)
were used, which were dissolved in phosphate-buffered saline and
stored at −20°C.
Cell viability was determined using MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium; Promega
Corporation, Madison, WI, USA]. HepG2 cells (7,000 cells/well) and
PLC/PRF/5 cells (8,000 cells/well) were plated into 96-well plates
and cultured for two days with various drug concentrations
(10−3-10 µM epirubicin or 5–5×104 µM araC).
The IC50 values, representing the half maximal
inhibitory concentration, were calculated using the GraphPad Prism
5.0a software (GraphPad Software Inc., San Diego, CA, USA)
software. To evaluate the effect of GAPDH expression on drug
resistance, the cells were treated with araC or epirubicin at 48 h
after transfection.
The cells were seeded in six-well plates or in 60-mm
dishes and grown for 24 h before transfection. Plasmids and small
interfering RNA (siRNA) molecules were transfected into the cells
using Lipofectamine™ 2000 transfection reagent (Invitrogen Life
Technologies) according to the manufacturer's instructions. A GAPDH
siRNA kit (#NM-002046) and scrambled control siRNA were purchased
from Sigma-Aldrich. The primer sequences used were as follows:
GAPDH siRNA sense, 5′-GGUUUACAUGUUCCAAUAUdTdT-3′, and anti-sense,
5′-AUAUUGGAACAUGUAAACCdTdT-3′; siRNA negative control sense,
5′-UUCUCCGAACGUGUCACGUTT-3′, and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′. Initially, western blot analysis was
performed to validate the expression of GAPDH in the transfected
plasmid (27).
The primary antibodies used in the present study
included mouse p21 [MBL(K0081-3); 1:500; Santa Cruz Biotechnology,
Inc., Dalla, TX, USA], mouse GAPDH [MBL(M171-3); 1:3,000 Santa Cruz
Biotechnology, Inc.] and rabbit β-actin (sc-1616; 1:1,000; Santa
Cruz Biotechnology, Inc.). The cultured cells were collected and
the proteins were lysed in RIPA buffer (Applygen Technologies Inc.,
Beijing, China). Protein samples (30 µg each) were loaded onto 12%
sodium dodecyl sulfate-polyacrylamide gels, electrophoresed and
transferred onto nitrocellulose membranes (Amersham Biosciences,
Uppsala, Sweden). Briefly, membranes were blocked with 5% dried
milk in phosphate-buffered saline (PBS) for 2 h followed by
incubation with the primary antibodies for 2 h. After 3 washes with
PBS containing 0.1% Tween-20, the membranes were incubated with the
IRDye® 680 goat anti-rabbit (#926-32221; 1:8,000; LI-COR
Biosciences, Cambridge, UK) and IRDye® 680 goat anti-mouse
(#926-32220; 1:8,000; LI-COR Biosciences) secondary antibodies for
1 h at room temperature. Protein-antibody complexes were visualized
using the secondary antibodies conjugated with Cy5.5
(Amersham-Pharmacia Biotech, Piscataway, NJ, USA) and the LI-COR
Odyssey® IR Imaging system (LI-COR Biosciences). Subsequently,
siRNA-transfected cells were treated with araC or epirubicin at 48
h after transfection and MTT assay was performed to measure the
cell viability.
Statistical analyses
Student's t-test or Wilcoxon signed-rank test were
used for statistical analyses with the SAS software (version 9.1;
SAS Institute Inc., Cary, NC, USA). A Kaplan-Meier survival curve
was generated to analyze the patients' survival rates following
surgery. p≤0.05 was considered to indicate a statistically
significant difference. The results are expressed the mean ±
standard error of mean of three independent experiments.
Results
Significantly increased GAPDH
expression levels in tumor tissues when compared with non-tumor
tissues
Using the HPRT1 housekeeping gene as a reference
gene, the mRNA expression levels of GAPDH were detected in 33 out
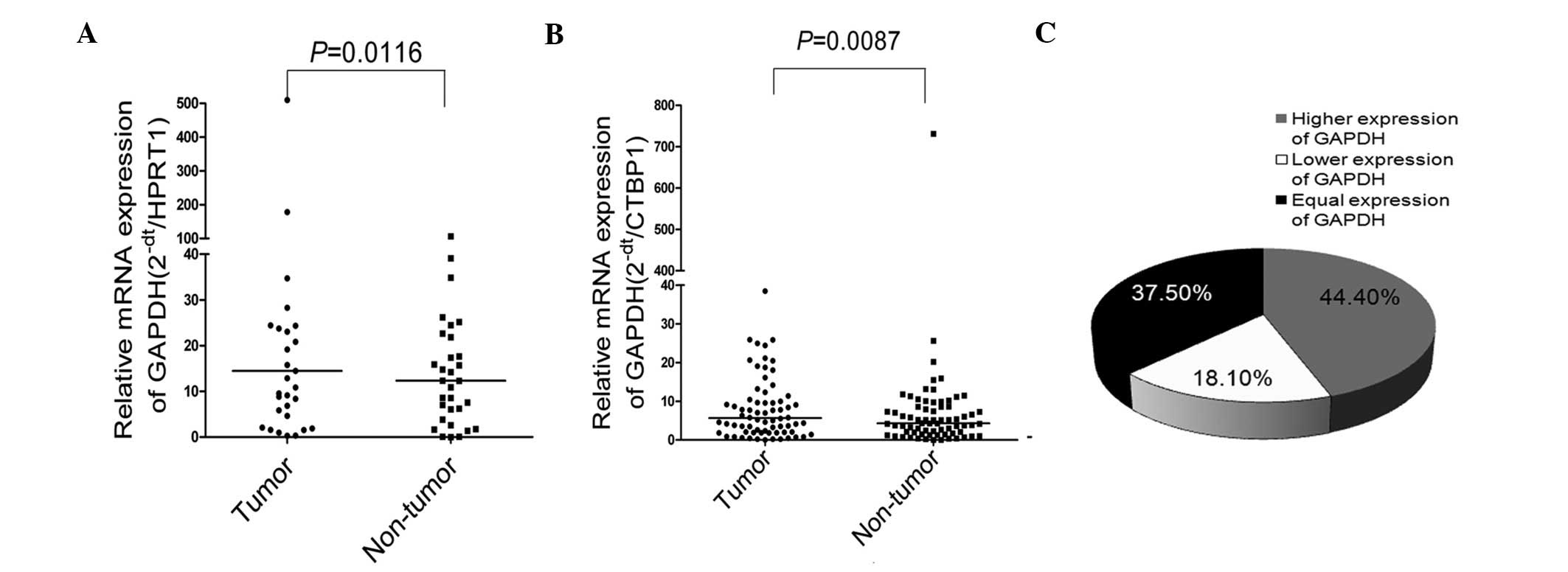
of the 72 cases. Compared with non-tumor tissues, significantly
higher GAPDH expression was observed in the tumor tissues
(P=0.0116; Fig. 1A). In order to
validate this observation in the 72 patients with HCC, CTBP1 was
used as the reference gene, since it has been previously
demonstrated to be the most stable gene among six candidate
housekeeping genes (24). The
relative expression of GAPDH was measured in the 72 HCC cases using
RT-qPCR. The results of the Wilcoxon signed-rank test demonstrated
that GAPDH expression was significantly increased in the HCC tumor
tissues when compared with the non-tumor tissues (P=0.0087;
Fig. 1B). In total, 37.5% of cases
(27/72 patients) presented increased expression of GAPDH
(2−ΔCt>2), 18.1% of cases (13/72 patients) presented
reduced expression of GAPDH (2−ΔCt<0.5) and 44.4% of
cases (32/72 patients) presented unchanged expression of GAPDH
(0.5≤2−ΔCt≤2). Fig. 1C
shows the distribution of the different expression levels of GAPDH
detected in the HCC patients.
In order to investigate whether there was an
association between the mRNA levels of GAPDH and the clinical
characteristics, the patients were further divided into the
increased (n=27) and non-increased expression group (n=45), which
included the cases presenting reduced and unchanged expression
levels of GAPDH. Statistical analysis revealed that the increased
expression group was associated with a lower score of liver
fibrosis (Ishak score I-III vs. IV-VI; 46.7%, vs. 22.2%; P=0.0394;
Table I) (25). This indicated that increased GAPDH
expression was significantly associated with a lower liver fibrosis
score. However, other clinicopathological characteristics,
including age, abdominal dropsy, preoperative α-fetoprotein value
and portal vein cancerous thrombus, did not induce a statistically
significantly difference on the expression of GAPDH (P>0.05;
Table I).
 | Table I.Patient characteristics according to
the expression levels of GAPDH in tumor tissues. |
Table I.
Patient characteristics according to
the expression levels of GAPDH in tumor tissues.
|
Characteristics | Increased group
(n=27), n | Non-increased group
(n=45), n | P-value |
|---|
| Age |
|
| 0.9036 |
| <50
years | 13 | 21 |
|
| ≥50
years | 14 | 24 |
|
| Abdominal
dropsy |
|
| 0.9374 |
|
Yes | 5 | 8 |
|
| No | 22 | 37 |
|
| Portal vein
cancerous thrombus |
|
| 0.5118 |
|
Complete | 7 | 15 |
|
|
Incomplete | 20 | 30 |
|
| Preoperative AFP
value |
|
| 0.7873 |
| ≤20
μg/l | 7 | 13 |
|
| >20
μg/l | 20 | 32 |
|
| Liver
cirrhosis |
|
| 0.0394 |
|
I-III | 21 (46.7%) | 24 |
|
|
IV-VI | 6 (22.2%) | 21 |
|
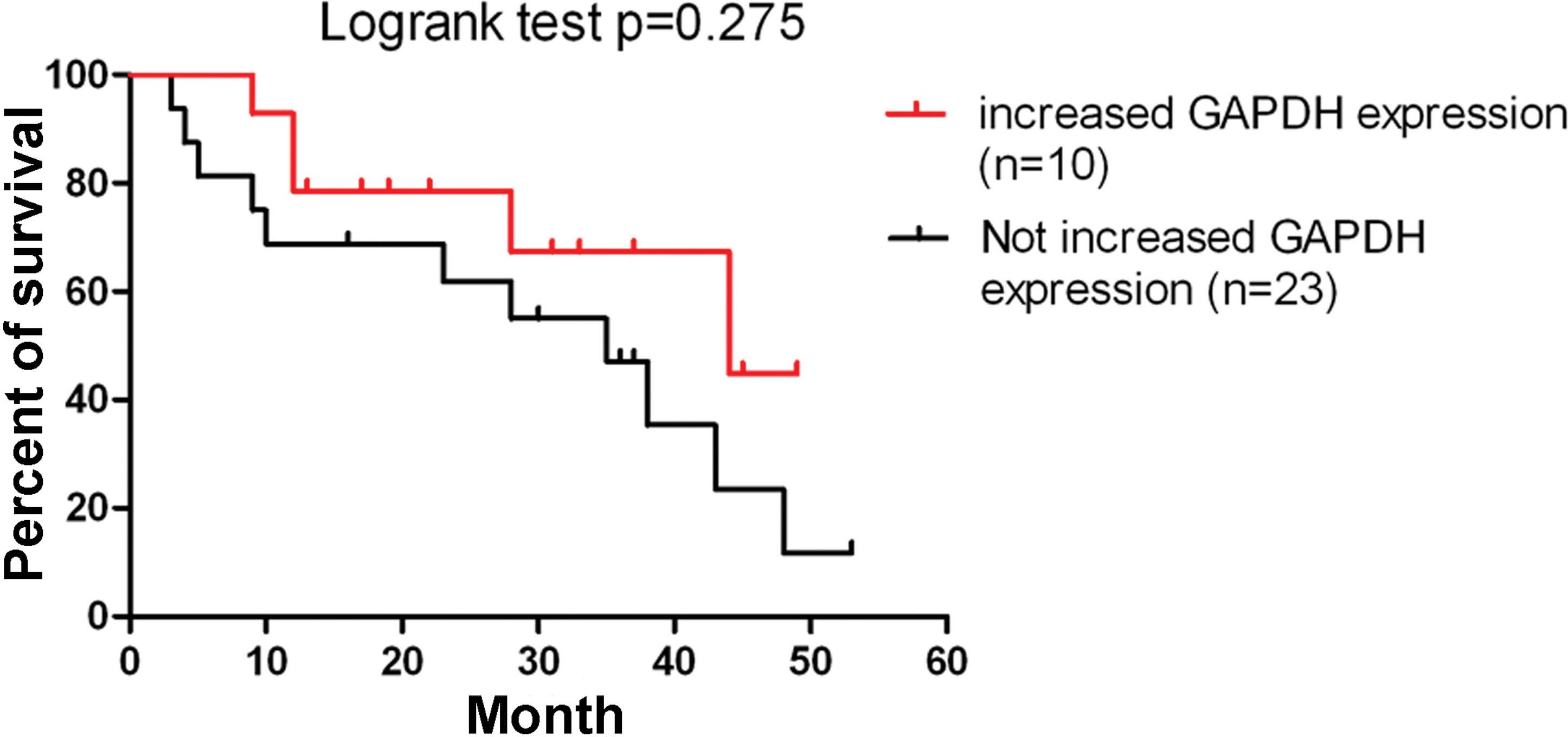
To investigate whether an association exists between
the elevated GAPDH expression and patient survival rates,
Kaplan-Meier survival analysis was conducted and patients with
increased GAPDH expression presented higher overall survival rates;
however, no statistically significant difference was observed
(P=0.275; Fig. 2).
Knockdown of GAPDH expression results
in reduced cell sensitivity to chemotherapy with araC in HepG2
cells, but not in PLC/PRF/5 cells
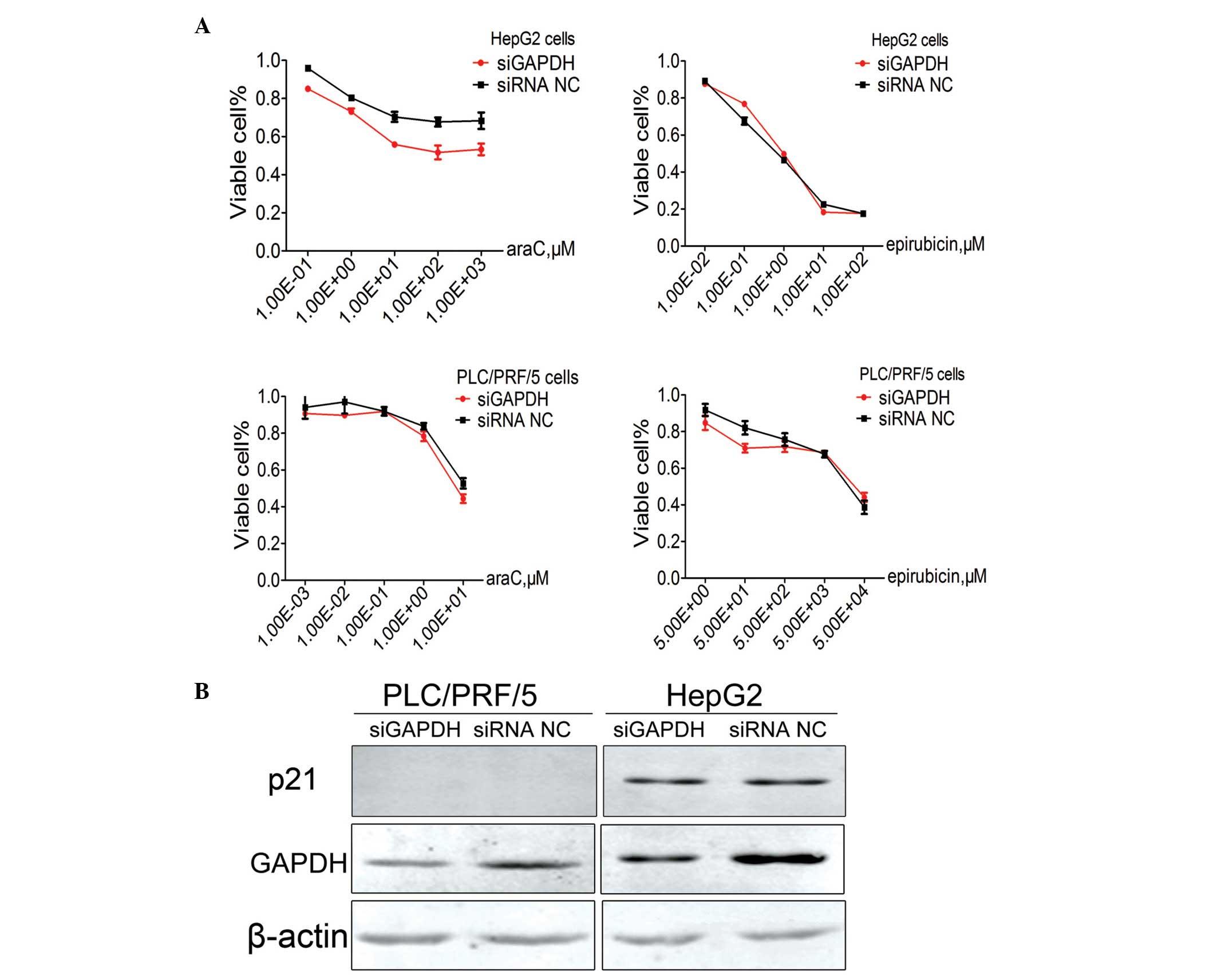
HepG2 and PLC/PRF/5 cells were initially transiently
transfected with GAPDH siRNA (siGAPDH) or siRNA NC. Subsequently,
the sensitivity of each cell line to araC or epirubicin was assayed
individually. Notably, GAPDH expression knockdown was found to
significantly alter the HepG2 cell chemotherapy sensitivity to araC
(308.28 µM in the siGAPDH group, vs. 67.68 µM µM in the siRNA NC
group; P=0.01), but not to epirubicin (10.37 µM in the siGAPDH
group, vs. 8.96 µM in the siRNA NC group). However, GAPDH knockdown
did not significantly alter the PLC/PRF/5 cell chemotherapy
sensitivity to araC (4719.05 µM in the siGAPDH group vs. 6834.30 µM
in the siRNA NC group) or epirubicin (6.15 µM in the siGAPDH group
vs. 13.99 µM in the siRNA NC group; Fig.
3A).
To investigated the underlying mechanism, the
protein level of p21, which is a cyclin-dependent kinase (CDK)
inhibitor, was further evaluated by Western blot analysis using the
p21-specific antibody. However, no statistically significant
difference was observed in the protein levels of p21 between cells
transfected with siGAPDH or siRNA NC. In addition, p21 expression
in PLC/PRF/5 cells was below the detectable levels (Fig. 3B).
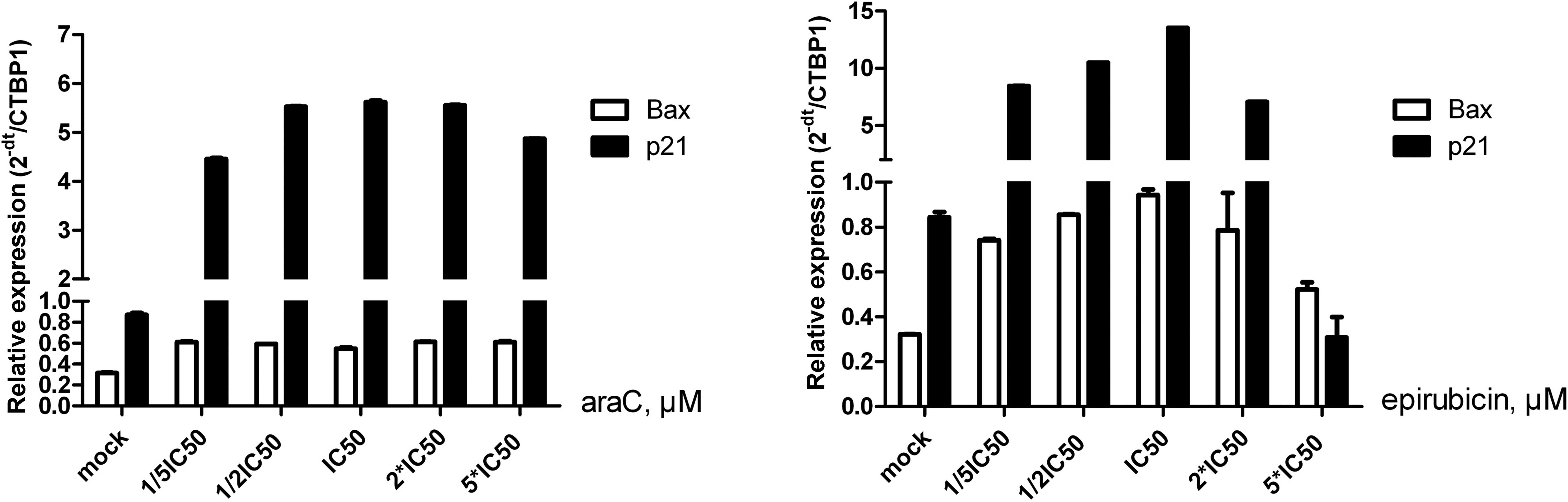
In order to investigate the effect of araC on HepG2
cellular sensitivity at the mRNA level of p21 or B-cell lymphoma
2-associated X protein (Bax), the expression levels of these genes
were detected following treatment with five different
concentrations of araC or epirubicin for 48 h. Compared with the
control group (mock), the mRNA expression levels of p21 increased
with increasing concentrations of araC or epirubicin; however, a
decrease was observed between 2×IC50 and
5×IC50 doses. By contrast, the mRNA expression of Bax
following araC treatment was slightly altered. Following epirubicin
treatment, the mRNA expression of Bax increased until the
IC50 dose and then decreased (Fig. 4).
GAPDH overexpression did not alter
HepG2 cellular sensitivity to chemotherapy with araC or
epirubicin
Knockdown of GAPDH expression did not alter p21
protein level expression. Therefore, the effect of the
overexpression of GAPDH on HepG2 cell sensitivity to araC or
epirubicin was investigated. The HepG2 cells were initially
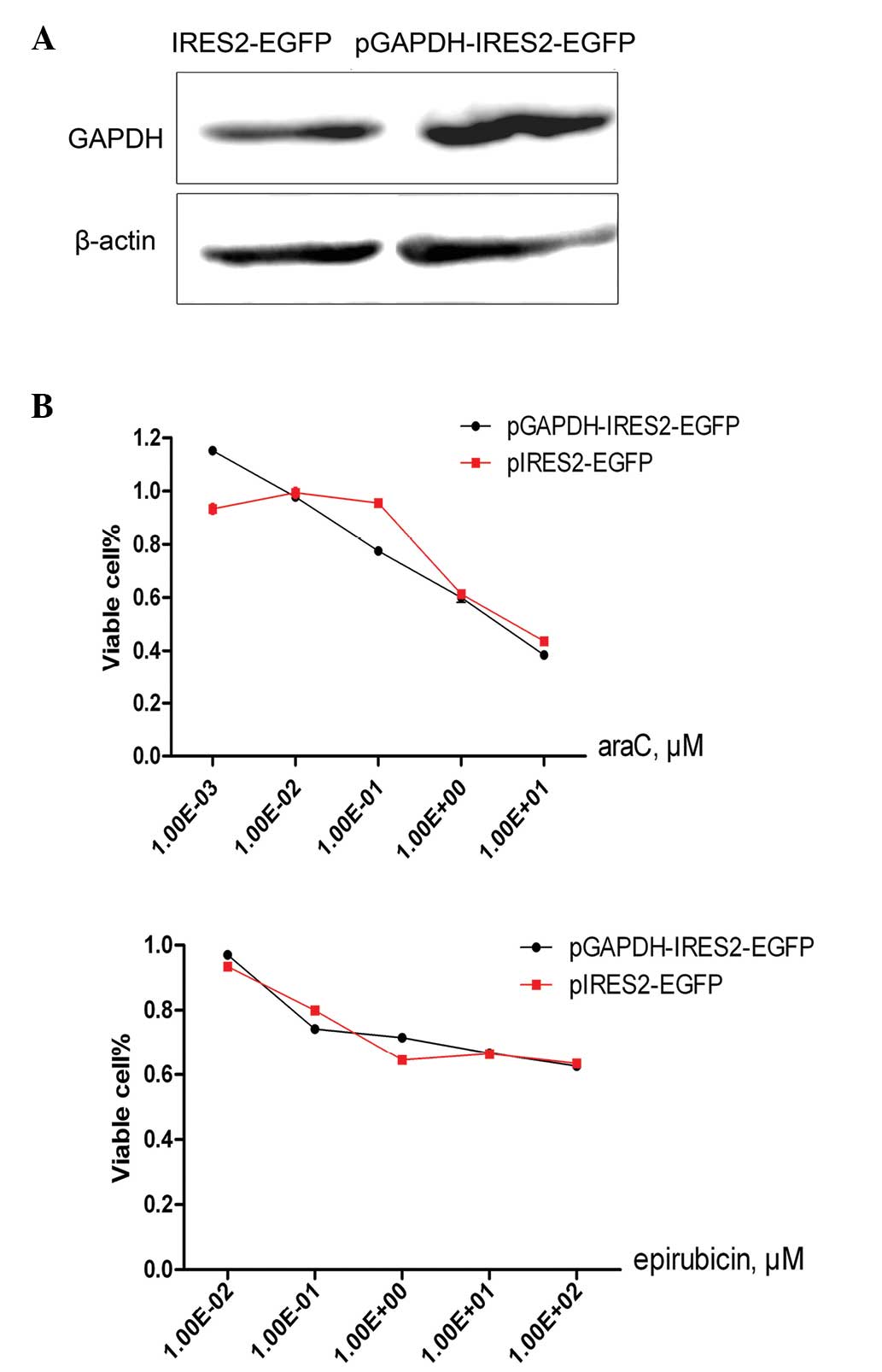
transiently transfected with 2 µg pGAPDH-IRES2-EGFP or pIRES2-EGFP
and the transfection was validated by western blot analysis
(Fig. 5A). Subsequently, the
transfected HepG2 cells were treated with araC or epirubicin for 24
h. As opposed to the expected result, the GAPDH overexpression was
not found to alter the cellular sensitivity to araC (2.11 µM vs.
2.11 µM) or epirubicin treatment (23.48 µM vs. 21.01 µM; Fig. 5B).
Discussion
Since the implementation of the national HBV vaccine
immunization program in China, the overall rate of the population
carrying the HBsAg declined from 9.75% in 1992 to 7.18% in 2006
(28). However, a previous study has
estimated that 93 million individuals of the Chinese population are
infected with chronic HBV, which may eventually result in
significant public health problems in the future (29). Patients with HBV infection that is
accompanied by cirrhosis are in high risk of hepatocarcinogenesis.
In addition, evidence revealed that males are more prone to HCC
development, compared with females (3).
GAPDH has been widely used as a reference gene in
RT-qPCR analysis performed in HCC cells; however, elevated mRNA and
protein expression levels of GAPDH in HCC patients have been
reported in a number of studies (30,31).
These studies investigated patients with HBV-associated HCC,
hepatitis C virus-associated HCC and HCC without viral hepatitis
infection history. Therefore, it is essential to investigate the
expression of GAPDH in male patients with HBV-associated HCC and
cirrhosis.
The group of the present study has previously
demonstrated the presence of aberrant GAPDH expression in male
patients with HBV-associate HCC and cirrhosis (24). In addition, GAPDH expression was
found to be the one of the most unstable genes among the six
housekeeping gene investigated, which was consistent with a
previous study on HBV-associated HCC (32).
In the present study, ∼56% of tumor tissue samples
exhibited differential expression of GAPDH in tumor tissues
(including 37.5% of samples with increased expression and 18.1%
with decreased expression) compared with non-tumor tissues, with an
>2-fold difference. A large number of studies have demonstrated
that GAPDH is involved in multiple basic cellular metabolism
functions, as observed by the altered GAPDH expression levels in
tumor tissues (9–12). Therefore, in the present study, HCC
patients were divided into the increased and non-increased GAPDH
expression groups. Notably, increased expression of GAPDH was found
to be significantly associated with a lower liver fibrosis score
(P=0.0394); to the best of our knowledge, the present study is the
first to report this in male patients with HBV-associated HCC and
cirrhosis. These results are in agreement with the findings of a
previous study comparing healthy controls and patients with
hepatitis B or C virus, which identified that GAPDH expression
levels were significantly increased in patients with cirrhosis and
the presence of HCC was closely associated with high GAPDH levels
(33).
To demonstrate whether the aberrant expression of
GAPDH affects the survival rates of HCC patients within a period of
60 months, survival curve analysis was performed. Notably, patients
with increased GAPDH expression exhibited a tendency of improved
survival rates; however, no statistically significant differences
were observed (P=0.275) and, to the best of our knowledge, no
similar studies has been conducted.
Numerous studies have indicated that GAPDH is
involved in apoptosis and elevated GAPDH expression has been
observed in HCC (17) and other
cancer cells (13–16). Tumorigenesis has been hypothesized to
be due to the occurrence of increased cell necrosis compared with
apoptosis, which supports the observation of the present study that
patients with increased GAPDH expression present higher survival
rates.
Enrolling a group of patients with HBV-associated
HCC but without history of cirrhosis as the control group may have
provided valuable information in the present study. However, only a
few cases without cirrhosis were detected in >500 HBV-associated
HCC cases, and therefore these were not included in the present
study. A similar observation was identified by Obata et al,
reporting that HCC was developed only in 23% of patients who were
HBsAg-positive and suffered from cirrhosis and in only 5.9% of
patients who were HBsAg-negative and presented liver cirrhosis
(34).
Although several studies have indicated that GAPDH
is involved in chemotherapy-induced DNA damage response, the
specific mechanism of GAPDH in HCC chemotherapy treatment remains
unclear (18,19). araC is considered to inhibit the
proliferation of cells through incorporation into the DNA during
replication (35), by inhabiting the
DNA synthesis and arresting cell division; however, it does not
disturb the RNA synthesis (36).
Previous results have demonstrated that araC-induced apoptosis of
cerebellar granule cells involves the expression of GAPDH and p53,
while, similar to Bax, GAPDH is upregulated by p53 following
exposure to the apoptotic insult (37).
In the experiments of the current study, knockdown
of GAPDH expression significantly altered the sensitivity of
p53-proficient HepG2 cells to araC chemotherapy, but not to
epirubicin chemotherapy. However, the knockdown of GAPDH expression
in the p53 mutation of PLC/PRF/5 cells did not alter the cellular
chemotherapy sensitivity to araC or epirubicin. This is supported
by the results of previous studies, which identified that
araC-induced apoptosis was p53-dependent (38). In the present study, upon
transfection of HepG2 cells with siGAPDH, the cell proliferation
arrest in GAPDH-depleted cells occurred through the p53-induced
expression of p21. In order to investigate the specific mechanism
through which the GAPDH expression knockdown in HepG2 cells induced
resistance to araC treatment, the protein levels of CDK inhibitor,
p21waf1 cip1, were further investigated in the two cell lines. The
expression of p21waf1/cip1 in PLC/PRF/5 cells was found to be
undetectable at the protein level; therefore, its effect on
chemotherapy in PLC/PRF/5 cells was not evaluated.
The DNA synthesis inhibitor epirubicin eliminates
cancer cells mainly via inducing G2/M arrest and apoptosis
(39,40). The results of the present study
demonstrated that, in epirubicin treated HepG2 cells, significant
upregulation of the mRNA expression levels of cell cycle
progression inhibitor, p21waf1/cip1, and pro-apoptosis Bax was
detected (Fig. 4). However, in
araC-treated HepG2 cell, the expression of Bax was slightly
altered, while the expression of p21waf1/cip1 was significantly
upregulated. These differences were also observed in Fig. 3A, where epirubicin treatment
significantly higher HepG2 cell apoptosis with increasing
concentration.
In conclusion, to the best of our knowledge, this
study is the first to report that elevated GAPDH expression in
tumor tissues may be involved in the development of fibrosi. In
addition, a tendency towards higher survival rates was observed for
male patients with HBV-associated HCC patients and cirrhosis;
however, the underlying mechanism remains unclear. Furthermore,
increased GAPDH expression may enhance the sensitivity of HCC cells
to antimetabolite chemotherapy.
Acknowledgements
This study was supported by a grant from the Project
for the Major Infectious Diseases from the Ministry of Science and
Technology of the People's Republic of China (no.
2012ZX10002005).
Glossary
Abbreviations
Abbreviations:
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
CTBP1
|
C-terminal banding protein 1
|
|
HPRT1
|
hypoxanthine phosphoribosyltransferase
1
|
|
araC
|
cytosine arabinoside
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
|
References
|
1
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harada K, Hirohara J, Ueno Y, et al:
Incidence of and risk factors for hepatocellular carcinoma in
primary biliary cirrhosis: national data from japan. Hepatology.
57:1942–1949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MH, Yang HI, Liu J, et al: Prediction
models of long-term cirrhosis and hepatocellular carcinoma risk
risk in chronic hepatitis B patients: risk scores integrating host
and virus profiles. Hepatology. 58:546–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang HI, Sherman M, Su J, et al: Nomograms
for risk of hepatocellular carcinoma in patients with chronic
hepatitis B virus infection. J Clin Oncol. 28:2437–2444. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumura M, Ijichi M, Shiratori Y, et al:
Simple quantitative assay of α-fetoprotein mRNA in liver tissue
using the real-time detection polymerase chain reaction assay – its
application for clinical use. Hepatol Res. 20:84–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sirover MA: New insights into an old
protein: the functional diversity of mammalian
glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta.
1432:159–184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi M, Schultz DE and Lemon SM: Functional
significance of the interaction of hepatitis a virus rna with
glyceraldehyde 3-phosphate dehydrogenase (gapdh): opposing effects
of gapdh and polypyrimidine tract binding protein on internal
ribosome entry site function. J Virol. 74:6459–6468. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin SS, Chang SC, Wang YH, Sun CY and
Chang MF: Specific interaction between the hepatitis delta virus
rna and glyceraldehyde 3-phosphate dehydrogenase: an enhancement on
ribozyme catalysis. Virology. 271:46–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berry MD and Boulton AA:
Glyceraldehyde-3-phosphate dehydrogenase and apoptosis. J Neurosci
Res. 60:150–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vila MR, Nicolas A, Morote J, de I and
Meseguer A: Increased glyceraldehyde-3-phosphate dehydrogenase
expression in renal cell carcinoma identified by rna-based,
arbitrarily primed polymerase chain reaction. Cancer. 89:152–164.
2000. View Article : Google Scholar
|
|
14
|
Tokunaga K, Nakamura Y, Sakata K, et al:
Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase
gene in human lung cancers. Cancer Res. 47:5616–5619.
1987.PubMed/NCBI
|
|
15
|
Revillion F, Pawlowski V, Hornez L and
Peyrat JP: Glyceraldehyde-3-phosphate dehydrogenase gene expression
in human breast cancer. Eur J Cancer. 36:1038–1042. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harada N, Yasunaga R, Higashimura Y, et
al: Glyceraldehyde-3-phosphate dehydrogenase enhances
transcriptional activity of androgen receptor in prostate cancer
cells. J Biol Chem. 282:22651–22661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeguchi M, Hirooka Y and Kaibara N:
Quantitative analysis of apoptosis-related gene expression in
hepatocellular carcinoma. Cancer. 95:1938–1945. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meyer-Siegler K, Mauro DJ, Seal G, et al:
A human nuclear uracil dna glycosylase is the 37-kda subunit of
glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA.
88:8460–8464. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azam S, Jouvet N, Jilani A, et al: Human
glyceraldehyde-3-phosphate dehydrogenase plays a direct role in
reactivating oxidized forms of the dna repair enzyme ape1. J Biol
Chem. 283:30632–30641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phadke MS, Krynetskaia NF, Mishra AK and
Krynetskiy E: Glyceraldehyde 3-phosphate dehydrogenase depletion
induces cell cycle arrest and resistance to antimetabolites in
human carcinoma cell lines. J Pharmacol Exp Ther. 331:77–86. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krynetski EY, Krynetskaia NF, Gallo AE,
Murti KG and Evans WE: A novel protein complex distinct from
mismatch repair binds thioguanylated dna. Mol Pharmacol.
59:367–374. 2001.PubMed/NCBI
|
|
22
|
Liu S, Zhu P, Zhang L, et al: Selection of
reference genes for RT-qPCR analysis in tumor tissues from male
hepatocellular carcinoma patients with hepatitis B infection and
cirrhosis. Cancer Biomark. 13:345–349. 2013.PubMed/NCBI
|
|
23
|
Heid CA, Stevens J, Livak KJ and Williams
PM: Real time quantitative pcr. Genome Res. 6:986–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gibson UE, Heid CA and Williams PM: A
novel method for real time quantitative rt-pcr. Genome Res.
6:995–1001. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knodell RG, Ishak KG, Black WC, et al:
Formulation and application of a numerical scoring system for
assessing histological activity in asymptomatic chronic active
hepatitis. Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Claverie JM: Computational methods for the
identification of differential and coordinated gene expression. Hum
Mol Genet. 8:1821–1832. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Q, Chen X, Lu F, et al: Aberrant
expression of microrna 155 may accelerate cell proliferation by
targeting sex-determining region y box 6 in hepatocellular
carcinoma. Cancer. 118:2431–2442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang X, Bi S, Yang W, et al:
Epidemiological serosurvey of hepatitis B in China – declining hbv
prevalence due to hepatitis B vaccination. Vaccine. 27:6550–6557.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu FM, Li T, Liu S and Zhuang H:
Epidemiology and prevention of hepatitis B virus infection in
China. j viral hepat. 17 (Suppl 1):4–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong Y, Cui L and Minuk GY: Comparison of
glyceraldehyde-3-phosphate dehydrogenase and 28s-ribosomal rna gene
expression in human hepatocellular carcinoma. Hepatology.
23:734–737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun W, Xing B, Sun Y, et al: Proteome
analysis of hepatocellular carcinoma by two-dimensional difference
gel electrophoresis: novel protein markers in hepatocellular
carcinoma tissues. Mol Cell Proteomics. 6:1798–1808. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu LY, Jia HL, Dong QZ, et al: Suitable
reference genes for real-time pcr in human hbv-related
hepatocellular carcinoma with different clinical prognoses. BMC
Cancer. 9:492009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shibuya A and Ikewaki N: High serum
glyceraldehyde-3-phosphate dehydrogenase levels in patients with
liver cirrhosis. Hepatol Res. 22:174–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Obata H, Hayashi N, Motoike Y, et al: A
prospective study on the development of hepatocellular carcinoma
from liver cirrhosis with persistent hepatitis B virus infection.
Int J Cancer. 25:741–747. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hedley DW and McCulloch EA: Generation of
reactive oxygen intermediates after treatment of blasts of acute
myeloblastic leukemia with cytosine arabinoside: role of bcl-2.
Leukemia. 10:1143–1149. 1996.PubMed/NCBI
|
|
36
|
Karon M, Henry P, Weissman S and Meyer C:
The effect of 1-beta-d-arabinofuranosylcytosine on macromolecular
synthesis in kb spinner cultures. Cancer Res. 26:166–171.
1966.PubMed/NCBI
|
|
37
|
Chen RW, Saunders PA, Wei H, et al:
Involvement of glyceraldehyde-3-phosphate dehydrogenase (gapdh) and
p53 in neuronal apoptosis: evidence that gapdh is upregulated by
p53. J Neurosci. 19:9654–9662. 1999.PubMed/NCBI
|
|
38
|
Anderson CN and Tolkovsky AM: A role for
mapk/erk in sympathetic neuron survival: protection against a
p53-dependent, jnk-independent induction of apoptosis by cytosine
arabinoside. J Neurosci. 19:664–673. 1999.PubMed/NCBI
|
|
39
|
Essmann F, Wieder T, Otto A, et al: Gdp
dissociation inhibitor d4-gdi (rho-gdi 2), but not the homologous
rho-gdi 1, is cleaved by caspase-3 during drug-induced apoptosis.
Biochem J. 346:777–783. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun WL, Chen J, Wang YP and Zheng H:
Autophagy protects breast cancer cells from epirubicin-induced
apoptosis and facilitates epirubicin-resistance development.
Autophagy. 7:1035–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|