Role of the Th1/Th2 response in RA
Rheumatoid arthritis (RA) is an autoimmune disease
that is characterized by persistent intense immunological activity,
local destruction of bone and cartilage, and a variety of systemic
manifestations (1). Although the
pathogenesis is yet to be fully resolved, the T helper (Th)l/Th2
response is known to play an important role in the development of
RA (2).
Adult patients with active RA have been shown to
have a dominant Th1 cell-mediated immune response (Th1 advantage),
as reported in experimental, clinical and epidemiological studies
in vivo and in vitro (1,2). In
addition, inducing a Th2-type response was found to be beneficial
for the treatment of RA in an in vivo rat model induced by
type Ⅱ collagen, while a drug-induced Th2 response was able to
inhibit the inflammation observed in RA induced by excessive Th1
responses in vitro (2). To
date, studies investigating the balance of the Th1/Th2 response in
peripheral blood mononuclear cells (PBMCs) for the clinical
treatment of RA are limited, and reliable conclusions are yet to be
established.
Children aged <16 years suffering from RA are
classified as having juvenile RA (JRA), which is now known as
juvenile idiopathic arthritis (JIA). This condition is clinically
and genetically distinct from that observed in adult patients
(3). In previous studies on children
with JIA, the majority of cases have presented with a dominant Th2
cell-mediated immune response (Th2 advantage) (4,5). In
addition, the longer the course of the disease, the more
significant the Th2 response (5).
Furthermore, treatment with Th1-type cytokines, such as interferon
(IFN)-γ, may be useful for the control of JIA (6). However, a type 1 phenotype of synovial
fluid T cells has also been identified in patients with JIA,
suggesting a high IFN-γ to interleukin (IL)-4 ratio in the synovial
fluid, which indicates that specific activation events have
occurred in the synovial T cells that may differ from PMBC T cells
(7).
In addition, longitudinal studies in human patients
with RA have revealed that the production of Th1/Th2-type cytokines
in different stages, particularly in the early and late stages, are
not the same, which suggests that there may be a shift in the
Thl/Th2 balance at different development stages of RA. A Th2
response dominates in the PBMCs at early stages of RA, while
long-term chronic RA exhibits a Th1 dominant response (8). Patients with early inflammatory
arthritis, who subsequently developed RA, had a distinct but
transient synovial fluid cytokine profile. The levels of type 2
cytokines, such as IL-4 and IL-13, were significantly elevated in
these patients within 3 months after symptom onset, as compared
with the early arthritis patients who did not develop RA. In
addition, this cytokine profile was not present in patients with
established RA. By contrast, patients with non-rheumatoid
persistent synovitis exhibited elevated levels of IFN-γ at the
initiation of the disease, which suggested that early synovitis
destined to develop into RA may be characterized by a distinct and
transient synovial fluid cytokine profile (9). However, in an adult patient with active
RA, a dominant Th1 response was initially observed in the synovium,
while a dominant Th2 response was observed in the PBMCs.
Subsequently, a Th0 and Th1 response became dominant in the
synovium, which was associated with disease inflammation (10).
Therefore, whether the Th1 or Th2 response is
dominant in RA may depend on a variety of factors, including the
age of the patients (JIA or RA), the stage of RA (early or late)
and where the condition is located (PBMCs or synovial fluid).
However, the mechanisms underlying the mediation of the imbalance
in the Th1/Th2 response, particularly during the early stages of
RA, remain unclear. In our clinical experience (data not
published), the majority of newly diagnosed JIA cases were in the
early stage, while the diagnosis of adult RA cases occurred
predominantly during the interim or late stage of the disease.
Based on these observations, a Th2 imbalanced response may be more
important in the early stage of RA.
Role of basophils in the Th1/Th2
response
In recent years, research into the effector
functions and immunoregulatory effects of basophils has made
considerable progress with marked achievements (11). Falcone et al described the
current insights into the roles of basophils in allergic responses
and innate immunity (12).
Karasuyama et al referred to basophils as a neglected
minority that have gained a new respect following recent
immunological studies (13).
The major immunoregulatory role of basophils in the
regulation of the Th1/Th2 balance is the induction of Th2 immunity
(14). Basophils are able to induce
Th2 immunity by primarily secreting key Th2-inducing cytokines,
namely IL-4 and thymic stromal lymphopoietin (TSLP), and by
functioning as professional antigen presenting cells. Basophils are
an efficient producer of Th2-type cytokines, such as IL-4 and
IL-13, which are able to enhance the differentiation of Th0 into
Th2 and inhibit the differentiation of Th0 to Th1 (15,16). As
such, following stimulation with allergens and innate IgE-dependent
triggers or other activation methods, a novel immunoregulatory role
of basophils has been identified in the regulation of the Th1/Th2
balance in vitro and in vivo (17). In addition, TSLP produced by
basophils in the lymph nodes is important for the initiation of Th2
differentiation in vivo and in vitro (18). In a T cell-independent pathway,
basophils induce an isotype switch toward IgE in human tonsillar B
cells (19), which subsequently
enhances the Th2-type humoral immune response (18). Therefore, basophils are able to
regulate the Th1/Th2 balance by enhancing Th2 immunity, and may
participate in the pathogenesis of various autoimmune diseases.
Basophils may play a key role in the
development of RA
A study that included 800 adult patients with RA
found that the number of peripheral basophils was significantly
decreased, although the cells were activated (20). In addition, the results of our study
exhibited a similar trend in adult RA patients (21). However, the reason for the decreased
number of peripheral basophils remains unclear, and the role of the
decreased level of activated basophils in the development of RA,
which is Th1 response dominant, requires further investigation.
A number of inflammatory effector cells, including
macrophages and lymphocytes, have been observed to infiltrate into
inflammatory sites in RA, such as synovial joint tissues.
Furthermore, in animal models, such as guinea pigs, basophils have
been shown to infiltrate into tissues during cutaneous
hypersensitivity responses (cutaneous basophil hypersensitivity)
(22,23). Basophil infiltration has also been
observed in allergen-induced late-phase cutaneous responses in
human atopic subjects (24), and has
been implicated in allergic human diseases (25). Previous observations in mice have
clearly demonstrated that basophils are essential initiator cells
of IgE-mediated chronic allergic inflammation (26), and are capable of functioning as a
source of IL-4 and contributing to Th2-type immunity (27). An explanation for the concomitant
recruitment of basophils may be due to the common expression of C-C
chemokine receptor (CCR)3. Eotaxin 1 and 3, which are ligands of
CCR3, are produced by several types of cell in response to Th2-type
cytokines, such as IL-4 and IL-13.
In addition, basophils have been observed in the
lymph node tissues of patients with systemic lupus erythematosus.
Basophils may migrate to the lymph nodes due to the higher
expression of the adhesion molecule, CD62L (28). Therefore, it was hypothesized that
basophils become activated following migration to the lymph nodes
or local inflammatory tissue, where they are involved in the
inflammatory response, subsequently leading to a reduction in the
number of peripheral blood basophils, and thus participating in the
pathogenesis of adult RA (Fig.
1).
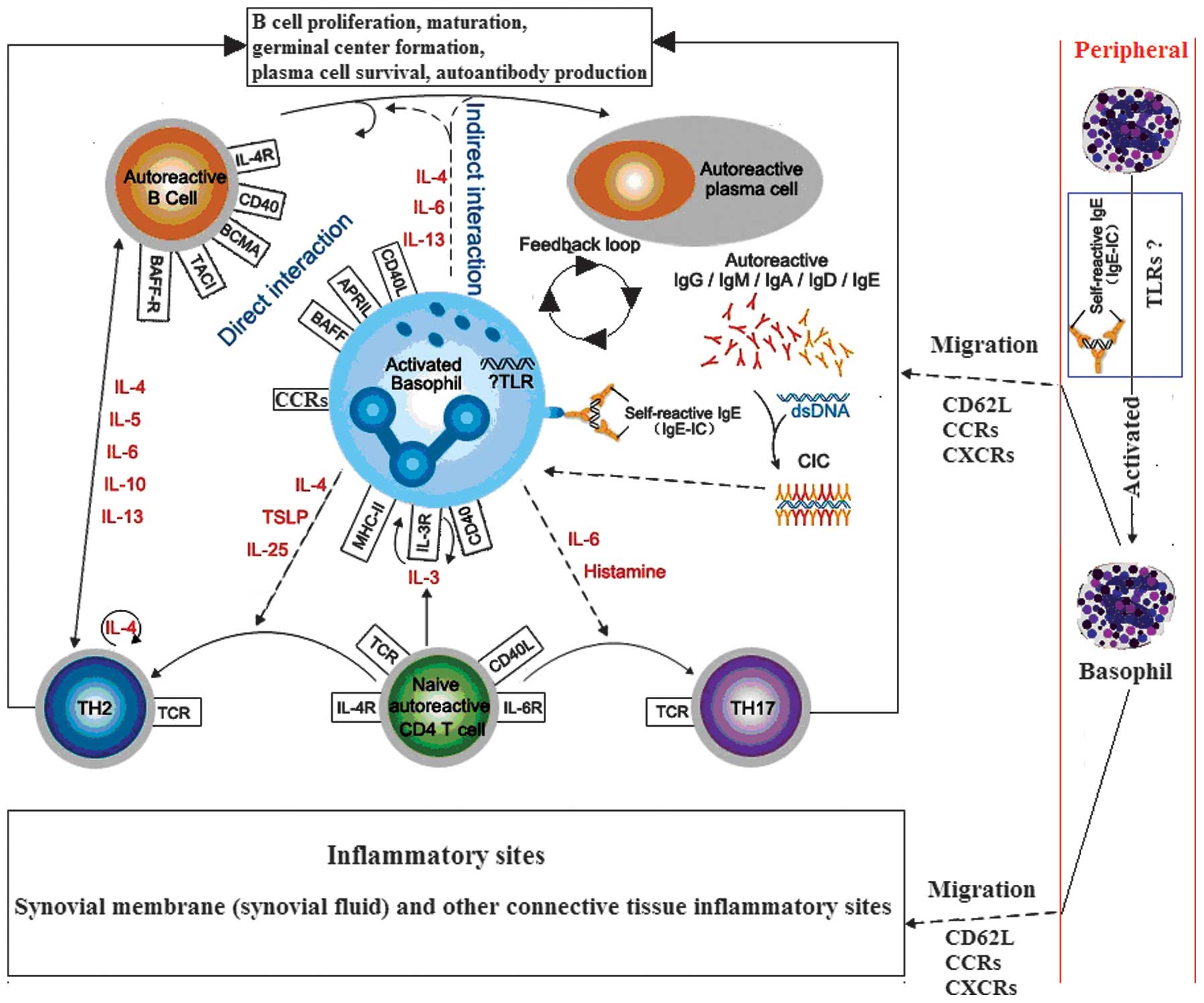 | Figure 1.Hypothesis of the interactions
between basophils, B and T cells during rheumatoid arthritis (RA;
dotted line indicates the hypothesis sections). Autoreactive T and
B cells trigger autoantibody production and CIC formation during
RA, which may be mediated via activated basophils. These basophils
may migrate from the blood into tissue sites, such as the lymph
nodes, via the expression of CD62L, where they can interact with B
and T cells, or inflammatory sites, via the expression of
transcripts of CCR1, CCR2, CCR3, and CCR5 and CXCR1, CXCR2 and
CXCR4, where they exert effector functions through the release of
diverse, proinflammatory mediators (29–32). IL,
interleukin; TSLP, thymic stromal lymphopoietin; BCMA, B cell
maturation antigen; TACI, transmembrane activator and calcium
modulator and cyclophilin ligand interactor; BAFF-R, B-cell
activating factor receptor; TH, T helper; TCR, T-cell receptor;
MHC, major histocompatibility complex; CCR, C-C chemokine receptor;
CXCR, C-X-C chemokine receptor; TLR, Toll-like receptor; CIC,
circulating antigen-antibody immune complexes. |
Number and activation degree of peripheral
blood basophils in JIA
Athreya et al (33,34)
reported that peripheral basophils were increased in absolute
number and in percentage in 11 out of 16 patients with JIA. The
increase was particularly significant in children with active
polyarticular arthritis.
JRA is an autoimmune disease which mainly causes the
inflammation of joints. For the purpose of improving the
description and classification of several forms of JRA, ILAR
(International League of Associations for Rheumatology) recently
redefined the disease. The name of JRA was changed to JIA.
Furthermore, the classification of JIA has been expanded to include
two further conditions, spondyloarthropathy and psoriatic
arthritis, which were not previously classified under JRA (35,36).
Mechanisms underlying periphery basophil
activation in RA
Basophils are an important source of Th2-type
cytokines. Basophils collected from humans and mice have been shown
to rapidly secrete large quantities of IL-4, as compared with Th2
cells, in response to various stimulations, including signaling
through the FcεRI (37–40). Moreover, upon IgE-receptor cross
linking in basophils, a number of cell surface membrane antigens
appear to be translocated from cytoplasmic membranes onto the cell
surface (41–48). These upregulated cell surface
membrane molecules include CD11b, CD13, CD63, CD107a, CD107b and
CD203c (49).
In addition, in RA patients, higher levels of IgE
type antibodies, which primarily form immune complexes (47) and circulating immune complexes
(IgE-CIC) containing IgE, can be detected in the peripheral blood
and synovial fluid (51–53). Furthermore, IgE-CIC-positive RA
patients exhibit higher disease activity compared with negative
patients (51). IgE-CIC can activate
inflammatory effector cells, such as mast cells, neutrophils and
mononuclear cells (51,55), which subsequently promotes RA disease
progression. These observations indicate that IgE immune complexes
may mediate basophil activation in RA.
As reported, several animal models of arthritis are
associated with a Th1 skew, and arthritis in such models can be
ameliorated by therapeutic intervention aimed at restoring the
Th1/Th2 balance (57–59). However, further investigation of the
potential function of basophils in restoring the Th1/Th2 balance in
RA, particularly in the early stage of RA, may be a novel
therapeutic strategy in RA and requires further investigation.
Basophils may be involved in the development of RA
by affecting the Th1/Th2 balance, particularly in the early stages
of RA. Therefore, targeting basophils may be a novel therapeutic
strategy for the treatment of RA; however, further studies are
required to confirm this.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81202346 and 81471530), the
Natural Science Foundation of Guangdong Province, China (no.
S2013010011568) and the Zhanjiang Planning Project of Science and
Technology (no. 2013B01086).
References
|
1
|
Harris ED Jr: Rheumatoid arthritis.
Pathophysiology and implications for therapy. N Engl J Med.
322:1277–1289. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schulze-Koops H and Kalden JR: The balance
of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin
Rheumatol. 15:677–691. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woo P and Wedderburn LR: Juvenile chronic
arthritis. Lancet. 351:969–973. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raziuddin S, Bahabri S, Al-Dalaan A, et
al: A mixed Th1/Th2 cell cytokine response predominates in systemic
onset juvenile rheumatoid arthritis: immunoregulatory IL-10
function. Clin Immunol Immunopathol. 86:192–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Q, Zhang H and Dong Z: A study on
immune response of Th1/Th2 in children with juvenile rheumatoid
arthritis. Acta Acad Med Qingdao Univ. 40:134–136. 2004.
|
|
6
|
Tang AT, Lau YL, Jones B, et al:
Cefadroxil reduces the production of IgE in a 3 year old asthmatic
with juvenile rheumatoid arthritis. Allergol Immunopathol (Madr).
21:131–135. 1993.PubMed/NCBI
|
|
7
|
Wedderburn LR, Robinson N, Patel A, et al:
Selective recruitment of polarized T cells expressing CCR5 and
CXCR3 to the inflamed joints of children with juvenile idiopathic
arthritis. Arthritis Rheum. 43:765–774. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerli R, Bistoni O, Russano A, et al: In
vivo activated T cells in rheumatoid synovitis. Analysis of Th1-
and Th2-type cytokine production at clonal level in different
stages of disease. Clin Exp Immunol. 129:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raza K, Falciani F, Curnow SJ, et al:
Early rheumatoid arthritis is characterized by a distinct and
transient synovial fluid cytokine profile of T cell and stromal
cell origin. Arthritis Res Ther. 7:R784–R795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aarvak T, Chabaud M, Thoen J, et al:
Changes in the Th1 or Th2 cytokine dominance in the synovium of
rheumatoid arthritis (RA): a kinetic study of the Th subsets in one
unusual RA patient. Rheumatology (Oxford). 39:513–522. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chirumbolo S: State-of-the-art review
about basophil research in immunology and allergy: is the time
right to treat these cells with the respect they deserve? Blood
Transfus. 10:148–164. 2012.PubMed/NCBI
|
|
12
|
Falcone FH, Zillikens D and Gibbs BF: The
21st century renaissance of the basophil? Current insights into its
role in allergic responses and innate immunity. Exp Dermatol.
15:855–864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karasuyama H, Mukai K, Tsujimura Y and
Obata K: Newly discovered roles for basophils: a neglected minority
gains new respect. Nat Rev Immunol. 9:9–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Min B: Basophils induce Th2 immunity: Is
this final answer? Virulence. 1:399–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sokol CL, Barton GM, Farr AG and Medzhitov
R: A mechanism for the initiation of allergen-induced T helper type
2 responses. Nat Immunol. 9:310–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Charles N, Watford WT, Ramos HL, et al:
Lyn kinase controls basophil GATA-3 transcription factor expression
and induction of Th2 cell differentiation. Immunity. 30:533–543.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh K, Shen T, Le Gros G and Min B:
Induction of Th2 type immunity in a mouse system reveals a novel
immunoregulatory role of basophils. Blood. 109:2921–2927.
2007.PubMed/NCBI
|
|
18
|
Denzel A, Maus UA, Gomez Rodriguez M, et
al: Basophils enhance immunological memory responses. Nat Immunol.
9:733–742. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gauchat JF, Henchoz S, Mazzei G, et al:
Induction of human IgE synthesis in B cells by mast cells and
basophils. Nature. 365:340–343. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao E and Xiang J: Basophils for the
diagnosis of rheumatoid arthritis activity. Foreign Medical
Sciences (Clinical Biochemistry and Laboratory Medicine Volume).
3:401986.[(In Chinese)].
|
|
21
|
Chen Q, Tang P, Lan Q, Xie T, Wu P and Pan
Q: The number of activation of periphery basophils from patients
with rheumatoid arthritis. The 19th National Annual Conference of
the Chinese Rheumatology Association. 1622014.[(In Chinese)].
|
|
22
|
Askenase PW: Cutaneous basophil
hypersensitivity in contact-sensitized guinea pigs. I. Transfer
with immune serum. J Exp Med. 138:1144–1155. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Askenase PW, Graziano F and Worms M:
Basophils and mast cells. Immunobiology of cutaneous basophil
reactions. Monogr Allergy. 14:222–235. 1979.PubMed/NCBI
|
|
24
|
Ying S, Robinson DS, Meng Q, Barata LT,
McEuen AR, Buckley MG, et al: C-C chemokines in allergen-induced
late-phase cutaneous responses in atopic subjects: association of
eotaxin with early 6-hour eosinophils, and of eotaxin-2 and
monocyte chemoattractant protein-4 with the later 24-hour tissue
eosinophilia and relationship to basophils and other C-C chemokines
(monocyte chemoattractant protein-3 and RANTES). J Immunol.
163:3976–3984. 1999.PubMed/NCBI
|
|
25
|
Mitchell EB and Askenase PW: Basophils in
human disease. Clin Rev Allergy. 1:427–448. 1983.PubMed/NCBI
|
|
26
|
Obata K, Mukai K, Tsujimura Y, Ishiwata K,
Kawano Y, Minegishi Y, et al: Basophils are essential initiators of
a novel type of chronic allergic inflammation. Blood. 110:913–920.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sokol CL, Barton GM, Farr AG and Medzhitov
R: A mechanism for the initiation of allergen-induced T helper type
2 responses. Nat Immunol. 9:310–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Charles N, Hardwick D, Daugas E, Illei GG
and Rivera J: Basophils and the T helper 2 environment can promote
the development of lupus nephritis. Nat Med. 16:701–707. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamada H, Hirai K, Miyamasu M, Iikura M,
Misaki Y, Shoji S, Takaishi T, Kasahara T, Morita Y and Ito K:
Eotaxin is a potent chemotaxin for human basophils. Biochem Biophys
Res Commun. 231:365–368. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uguccioni M, Mackay CR, Ochensberger B,
Loetscher P, Rhis S, LaRosa GJ, Rao P, Ponath PD, Baggiolini M and
Dahinden CA: High expression of the chemokine receptor CCR3 in
human blood basophils. Role in activation by eotaxin, MCP-4 and
other chemokines. J Clin Invest. 100:1137–1143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krieger M, Brunner T, Bischoff SC, von
Tscharner V, Walz A, Moser B, Baggiolini M and Dahinden CA:
Activation of human basophils through the IL-8 receptor. J Immunol.
149:2662–2667. 1992.PubMed/NCBI
|
|
32
|
Ochensberger B, Tassera L, Bifrare D, Rihs
S and Dahinden CA: Regulation of cytokine expression and
leukotriene formation in human basophils by growth factors,
chemokines and chemotactic agonists. Eur J Immunol. 29:11–22. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Athreya BH, Moser G and Raghavan TE:
Increased circulating basophils in juvenile rheumatoid arthritis. A
preliminary report. Am J Dis Child. 129:935–937. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Athreya BH and Nichols WW: Basophils in
juvenile rheumatoid arthritis. Pediatr Res. 8:397. 1974. View Article : Google Scholar
|
|
35
|
Petty RE, Southwood TR, Baum J, et al:
Revision of the proposed classification criteria for juvenile
idiopathic arthritis: Durban 1997. J Rheumatol. 25:1991–1994.
1998.PubMed/NCBI
|
|
36
|
Petty RE, Southwood TR, Manners P, et al:
International League of Associations for Rheumatology
Classification of juvenile idiopathic arthritis: second revision,
Edmonton2001. J Rheumatol. 31:390–392. 2004.PubMed/NCBI
|
|
37
|
Seder RA, Paul WE, Dvorak AM, Sharkis SJ,
Kagey-Sobotka A, Niv Y, et al: Mouse splenic and bone marrow cell
populations that express high-affinity Fc epsilon receptors and
produce interleukin 4 are highly enriched in basophils. Proc Natl
Acad Sci USA. 88:2835–2839. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schroeder JT, MacGlashan DW Jr and
Lichtenstein LM: Human basophils: mediator release and cytokine
production. Adv Immunol. 77:93–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Voehringer D, Shinkai K and Locksley RM:
Type 2 immunity reflects orchestrated recruitment of cells
committed to IL-4 production. Immunity. 20:267–277. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Min B, Prout M, Hu-Li J, et al: Basophils
produce IL-4 and accumulate in tissues after infection with a
Th2-inducing parasite. J Exp Med. 200:507–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bühring HJ, Seiffert M, Giesert C, et al:
The basophil activation marker defined by antibody 97A6 is
identical to the ectonucleotide pyrophosphatase/phosphodiesterase
3. Blood. 97:3303–3305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hauswirth AW, Natter S, Ghannadan M, et
al: Recombinant allergens promote expression of CD203c on basophils
in sensitized individuals. J Allergy Clin Immunol. 110:102–109.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Platz IJ, Binder M, Marxer A, Lischka G,
Valent P and Bühring HJ: Hymenoptera-venom-induced upregulation of
the basophil activation marker ecto-nucleotide
pyrophosphatase/phosphodiesterase 3 in sensitized individuals. Int
Arch Allergy Immunol. 126:335–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bühring HJ, Streble A and Valent P: The
basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell
activation and allergy diagnosis. Int Arch Allergy Immunol.
133:317–329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hennersdorf F, Florian S, Jakob A, et al:
Identification of CD13, CD107a and CD164 as novel
basophil-activation markers and dissection of two response patterns
in time kinetics of IgE-dependent upregulation. Cell Res.
15:325–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sonneck K, Baumgartner C, Rebuzzi L, et
al: Recombinant allergens promote expression of aminopeptidase-n
(CD13) on basophils in allergic patients. Int J Immunopathol
Pharmacol. 21:11–21. 2008.PubMed/NCBI
|
|
47
|
Chirumbolo S, Vella A, Ortolani R, et al:
Differential response of human basophil activation markers: a
multi-parameter flow cytometry approach. Clin Mol Allergy.
6:122008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sainte-Laudy J and Ouk C: Use of lipid
rafting for the analysis of human basophil activation by flow
cytometry. Inflamm Res. 59 (Suppl 2):S193–S195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Valent P: Basophil activation antigens:
molecular mechanisms and clinical implications. Open Allergy J.
3:52–59. 2010. View Article : Google Scholar
|
|
50
|
Millauer N, Zuercher AW, Miescher SM, et
al: High IgE in rheumatoid arthritis (RA) patients is complexed
with anti-IgE autoantibodies. Clin Exp Immunol. 115:183–188. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Clerck LS, Struyf NJ, Bridts CH and
Stevens WJ: Activation of inflammatory cells by immune complexes
containing IgE in serum and synovial fluid of patients with
rheumatoid arthritis: a study using flow cytometric analysis. Ann
Rheum Dis. 50:379–382. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Van Offel JF, De Clerck LS and Kersschot
IE: Cholesterol crystals and IgE-containing immune complexes in
rheumatoid pericarditis. Clin Rheumatol. 10:78–80. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
De Clerck LS, Struyf NJ, Bridts CH, et al:
IgE-containing immune complexes in synovial fluid of patients with
rheumatoid arthritis. Clin Rheumatol. 9:176–181. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bartholomew JS, Evanson JM and Woolley DE:
Serum IgE anti-cartilage collagen antibodies in rheumatoid
patients. Rheumatol Int. 11:37–40. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Permin H, Wiik A and Djurup R:
Phagocytosis by normal polymorphonuclear leukocytes of immune
complexes from serum of patients with Felty's syndrome and
rheumatoid arthritis with special reference to IgE immune
complexes. Acta Pathol Microbiol Immunol Scand C. 92:37–42.
1984.PubMed/NCBI
|
|
56
|
Schuerwegh AJ, Ioan-Facsinay A, Dorjée AL,
et al: Evidence for a functional role of IgE anticitrullinated
protein antibodies in rheumatoid arthritis. Proc Natl Acad Sci USA.
107:2586–2591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Müller B, Gimsa U, Mitchison NA, Radbruch
A, Sieper J and Yin Z: Modulating the Th1/Th2 balance in
inflammatory arthritis. Springer Semin Immunopathol. 20:181–196.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nicholson LB and Kuchroo VK: Manipulation
of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol.
8:837–842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Joosten LA, Lubberts E, Durez P, Helsen
MM, Jacobs MJ, Goldman M and van den Berg WB: Role of interleukin-4
and interleukin-10 in murine collagen-induced arthritis. Protective
effect of interleukin-4 and interleukin-10 treatment on cartilage
destruction. Arthritis Rheum. 40:249–260. 1997. View Article : Google Scholar : PubMed/NCBI
|















