Introduction
Non-obese diabetes (NOD) mice are one of the most
commonly used animal models for the study of autoimmune diseases,
and exhibit a susceptibility to the spontaneous development of
autoimmune insulin-dependent diabetes mellitus (1). A number of factors are associated with
the development of diabetes in NOD mice, including the release of
self-reactive cytotoxic T lymphocytes (CTL) from negative selection
in the thymus (central tolerance) and the loss of regulatory T
(Treg) cell function (peripheral tolerance) (2). In addition, natural killer (NK) and NKT
cells play a critical role in the progression of type I diabetes
(TID) (3–5).
In 1990, adjuvant immunotherapy was first reported
to be effective in preventing the development of TID in
pro-diabetic NOD mice (6), and
subsequent studies (7,8) suggested that complete Freunds adjuvant
(CFA) therapy was effective in treating new-onset NOD mice
(9), although the underlying
mechanisms remain unclear. Numerous studies have attempted to
reveal the underlying immunotherapy mechanism and have found that
adjuvant treatment in mice induces the differentiation of
regulatory cell populations in vivo, inhibiting the onset of
TID (10–12). Mice treated with immunological
adjuvants exhibit an altered ratio of T helper (Th)1 and Th2 cells,
which promotes an antibody response, but prevents Th1-mediated
auto-reactive T cell responses to pancreatic β-cell surface
antigens (13). Furthermore,
upregulation of pro-inflammatory cytokines, including tumor
necrosis factor (TNF)-α, has been reported following adjuvant
treatment (14). A previous study
found that NOD mice treated with the bacillus Calmette-Guerin (BCG)
vaccine were protected against TID (15). CFA and the BCG vaccine contain
inactivated M. tuberculosis; however, incomplete Freund's
adjuvant (IFA) does not contain M. tuberculosis. Since IFA
is unable to prevent TID development in NOD mice as effectively as
CFA (16,17), M. tuberculosis has been
hypothesized to play an important role in the modulation of the
immune response in cases of TID.
A previous study demonstrated that the
pro-inflammatory cytokine, interleukin (IL)-17, plays a critical
role in the pathogenesis of TID in NOD mice (18). In addition, treatment with CFA or
M. tuberculosis has been reported to induce IL-17
expression. However, this increase in IL-17 expression was produced
primarily by CD8+ (19)
or γδ T cells (20), rather than
CD4+ Th17 cells. Further studies have indicated that NKT
cells are involved in CFA-mediated protection against TID in NOD
mice via the activation of NK cells (21), which are the primary source of
interferon (IFN)-γ in the pro-diabetic NOD mice (12,22).
Mechanism studies show that these NKT cells are activated directly
by M. tuberculosis, possibly via CD1d recognition of
specific long fatty acyl chains (23) on the surface of M.
tuberculosis. Activated NKT cells, including Vα19 NKT cells,
produce IL-17 and other immunoregulatory cytokines, such as IL-4,
−10 and IFN-γ (24).
In the present study, NOD mice were treated with a
combined therapy of IFA and inactivated L. monocytogenes, a
microbe that often infects the liver in humans and mice and whose
elimination is in part dependent on activated invariant NKT cells
(25). Heat-killed L.
monocytogenes has been previously used as an adjuvant to induce
strong Th1 responses in mice (26).
L. monocytogenes shares numerous characteristics with M.
tuberculosis; however, L. monocytogenes cannot induce
IL-17 secretion in NKT cells as effectively as M.
tuberculosis. The effects of a combined IFA + L.
monocytogenes treatment on the development of TID was
investigated in a NOD mouse model.
Materials and methods
Mice and immunizations
A total of 108 female NOD mice (aged five weeks;
17–20 g) were purchased from Shanghai Animal Laboratory Center
(Shanghai, China) and housed in the East Hospital of Tongji
University (Shanghai, China). Mice were immunized by a hypodermic
injection into their back with one of the three treatments. The IFA
+ L. monocytogenes group mice received heat-killed L.
monocytogenes (108 bacteria/mouse) in 100 µl IFA. A
second group was injected with CFA, while a third IFA-only group
received a control injection containing no bacteria. Another 10
mice were administered twice with IFA + L. monocytogenes
immunization with the same dose at 5 weeks and 8 weeks of age.
Blood sugar levels were measured every three days following
immunization and the mice whose blood sugar levels were >11.8
mmol/L were defined as positive for TID. All animal experiments
were performed in accordance with protocols approved by the Animal
Care and Use Committee of East Hospital of Tongji University.
(Shanghai, China).
Fluorescence-activated cell sorting
and intracellular staining
Cytokine secretion in the lymphocytes was analyzed
using Cytofix/Cytoperm™ Plus (BD Biosciences, Franklin Lakes, NJ,
USA), according to the manufacturer's instructions. Spleen cells
were collected and incubated with 50 ng/ml phorbol 12-myristate
13-acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA), 5 µM calcium
ionophore A23187 (Sigma-Aldrich) and GolgiStop™(BD Biosciences) at
37°C for 4 h. Surface staining was performed using
anti-CD3e-PerCP/Cy5.5 antibodies (BioLegend, Inc., San Diego, CA,
USA) for 20 min at 4°C. Cells were subsequently permeabilized with
Cytofix/Cytoperm™ solution for 20 min at 4°C, and intracellular
cytokine staining was performed with anti-IL-17A-Alexa Fluor 647
(cat. no. 560224; BD Biosciences) and phycoerythrin (PE)-IFN-γ
antibodies (cat. no. 557735; BD Biosciences). For Treg staining,
spleen cells were fixed and stained using anti-T cell receptor
(TCR)b-fluorescein isothiocyanate (cat. no. 553171; BD
Biosciences), anti-CD25-PE (cat. no. 553075; BD Biosciences) and
intercellular anti-Foxp3-Alexa Fluor 647 (cat. no. 560402; BD
Biosciences) antibodies. Antibodies were used in a 1:100 dillution
(BioLegend) or 1:50 dillution (BD Biosciences), according to the
manufacturer's instructions.
Antibody levels in the blood
serum
Total levels of IgG, IgG1 and IgG2a were examined by
ELISA. In brief, 96-well plates (Nunc; Thermo Fisher Scientific,
Waltham, MA, USA) were coated with 300 ng/well goat anti-mouse IgG
antibodies (Life Technologies, Grand Island, NY, USA) in
phosphate-buffered saline (PBS) and incubated overnight at 4°C.
After blocking with 5% skim milk in PBS-Tween-20, the plates were
incubated for 1 h at 37°C with serially-diluted serum samples.
Following three washes with PBS-Tween-20, the samples were reacted
with sheep anti-mouse IgG, IgG1 or IgG2a antibodies conjugated to
horseradish peroxidase (BD Biosciences). Plates were developed by
adding tetramethylbenzidine (Endogen®; Pierce Biotechnology, Inc.,
Rockford, IL, USA) and incubating in the dark. The reaction was
stopped using 1 mol/L H2SO4, and the optical
densities (OD) were read at 450 nm using an ELISA reader (Thermo
Fisher Scientific). ELISA end-point titers were expressed as the
reciprocal of the highest sample dilution that yielded an OD two
times the mean value of the blank control.
ELISA analysis of the expression of
IL-17
A total of 1 × 106 lymphocytes were
collected from pancreas-draining lymph nodes and cultured with
RPMI-1640 medium with 10% FCS and 200 U/ml mouse IL-2 (cat. no
CYT-370; ProSpec-Tany Technogene Ltd. East Brunswick, NJ, USA) in
96-well plates with plate-coated 0.5 µg/ml functional anti-CD3e
(cat. no. 16-0031-81; eBioscience, Inc., San Diego, CA, USA) and
0.5 µg/ml soluble CD28 (cat. no. 16-0821-81; eBioscience, Inc.) for
two days in the presence of recombinant mouse IL-2 (40 U/ml,
R&D Systems, Minneapolis, MN, USA). The expression of IL-17 in
the medium was detected using a Mouse IL-17A Platinum ELISA kit
(cat. no. M17AF0; eBioscience Inc.), according to the
manufacturer's instructions.
Statistical analysis
Data were analyzed using GraphPad Prisms® 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA) and are
expressed as the mean ± standard deviation. Differences between the
groups were analyzed by one-way analysis of variance with a
post-test comparison using the t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
IFA and L. monocytogenes treatment in
pro-diabetic NOD mice delays TID development
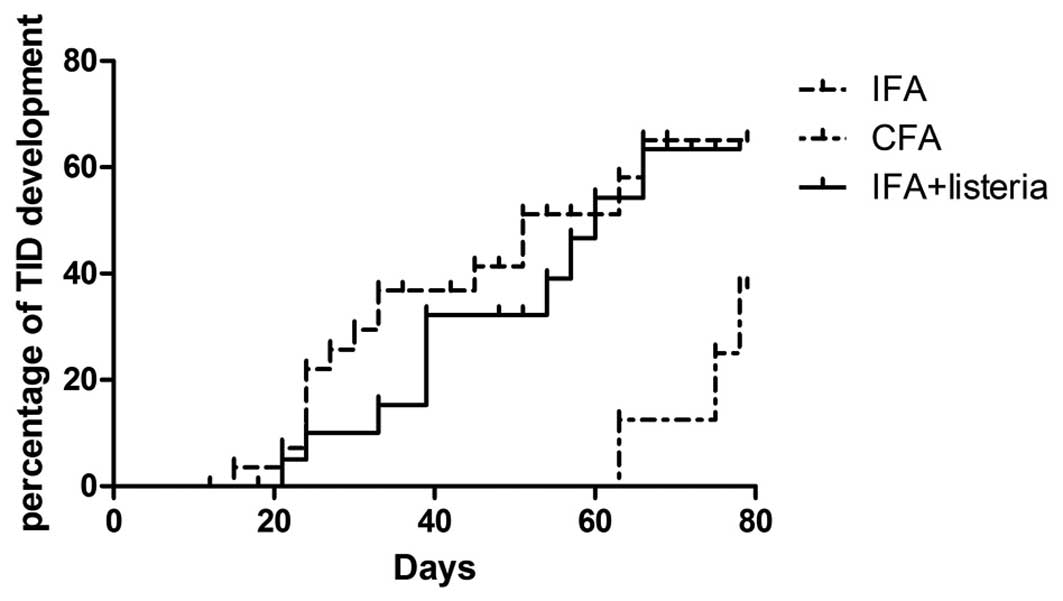
TID development in NOD mice is associated with
numerous factors, including age, diet and living environment. Blood
sugar levels of >11.8 mmol/L were observed in the mice aged 8–10
weeks (data not shown). As previously reported (6), treatment with CFA in pro-diabetic
(five-week old) NOD mice was able to effectively block TID
development, which was confirmed in the present study. In order to
determine the effects of alternative immune therapies, heat-killed
L. monocytogenes (108 bacteria/mouse) was used
instead of M. tuberculosis in CFA. The results indicated
that IFA + L. monocytogenes was unable to totally prevent
TID development, as >50% of the mice became diabetic within 12
weeks of treatment, while in the CFA treatment group, this figure
was <30% (Fig. 1). However,
increased blood sugar levels were not detected in the IFA + L.
monocytogenes group mice until they were 12 weeks-old (seven
weeks after treatment), suggesting that IFA + L.
monocytogenes treatment resulted in a delayed disease
progression when compared with the IFA-only group. Another 10
pro-diabetic NOD mice received a second administration of IFA +
L. monocytogenes at three weeks following the initial
immunization (age, eight weeks), in order to study whether an
additional immunization was able to further delay TID development.
However, no significant change in disease progression was observed
in the twice-immunized mice when compared with the once-immunized
mice (data not shown).
IFA + L. monocytogenes treatment has
no effect on IL-17-producing T cell populations
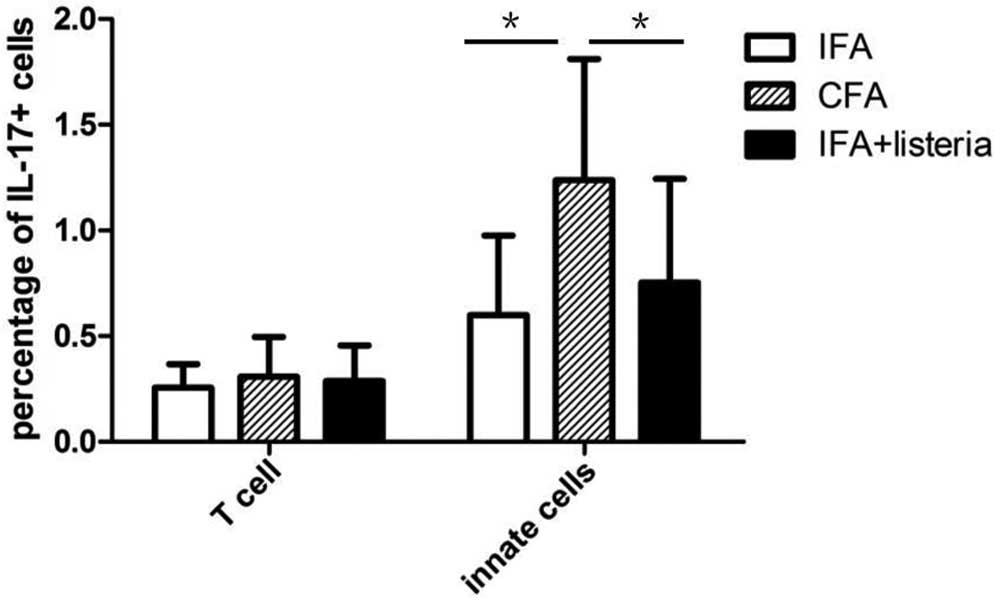
Spleen cells were collected three weeks after
adjuvant treatment and were incubated with PMA/calcium ionophore.
The percentage of IL-17-producing spleen cells was between 0.2 and
0.5%, which was relatively low and no significant difference was
observed between the CFA, IFA + L. monocytogenes and
IFA-only control groups (data not shown). Spleen cells were
separated using the T cell-specific surface marker, TCRb, into
TCRb+ T cells and TCRb−B220−
innate cell populations, which consisted primarily of macrophages,
dendritic and NK cells. It is generally accepted that γδ T cells
are innate immune cells, despite expressing the TCR on their
surface. CFA treatment induced significant IL-17 secretion in the
innate immune cells when compared with the IFA + L.
monocytogenes and IFA-only control groups (Fig. 2). Furthermore, adjuvant treatment
appeared to have no influence on the TCRb+ T cell
population, since no statistically significant difference was
observed between the three groups. In addition, IFN-γ expression
was compared among the groups, and adjuvant treatment was shown to
induce a notable increase in IFN-γ expression in T cells of the
IFA-only control group. However, no significant difference in IFN-γ
expression was observed in the T cells of the CFA and IFA + L.
monocytogenes groups (data not shown).
CFA treatment induces strong IL-17
expression in the pancreatic draining lymph nodes
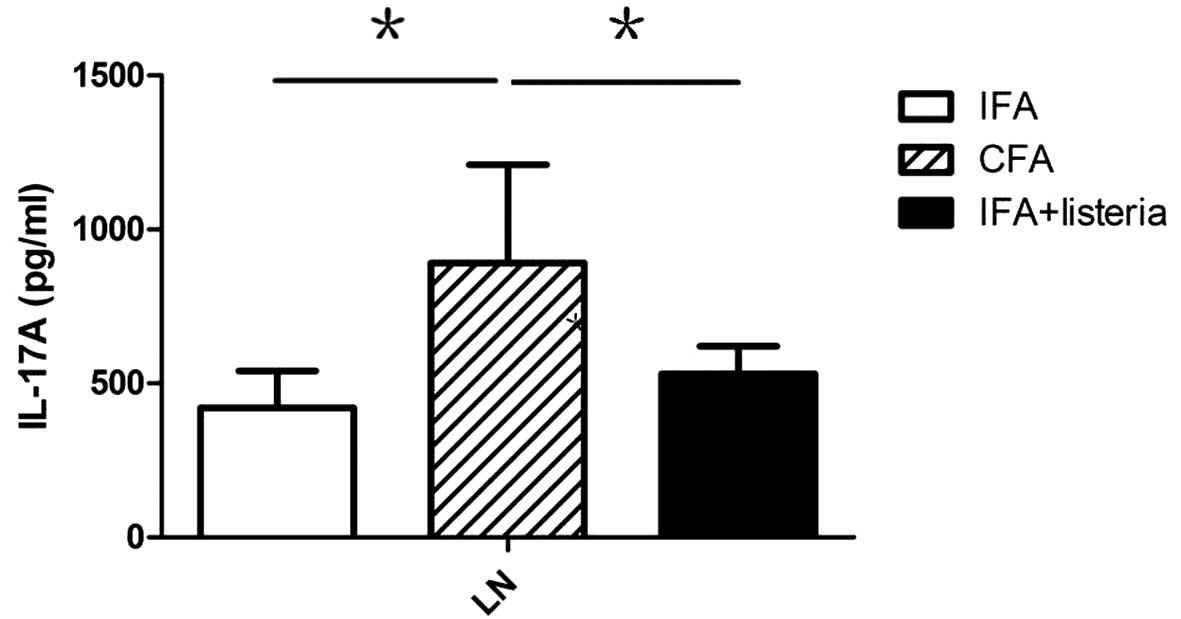
Considering that IL-17 is a pro-inflammatory
cytokine that is primarily involved in local inflammation, the
expression of IL-17 in the pancreatic draining lymph nodes was
analyzed. Lymphocytes from pancreatic draining lymph nodes were
collected from eight week-old NOD mice (three weeks after
treatment) and cultured in vitro for two days, after which
secreted IL-17 was captured by plate-coated antibodies. ELISA
results revealed that CFA treatment induced strong IL-17 expression
in the pancreatic draining lymph nodes when compared with the IFA +
L. monocytogenes and IFA-only groups (Fig. 3). The source of this extra IL-17
expression in the CFA group remains unclear, but it may have been
secreted by Th17 and/or innate immune cells, such as NKT cells.
IFA + L. monocytogenes treatment
promotes Treg proliferation and increases IgG2a levels in the blood
serum
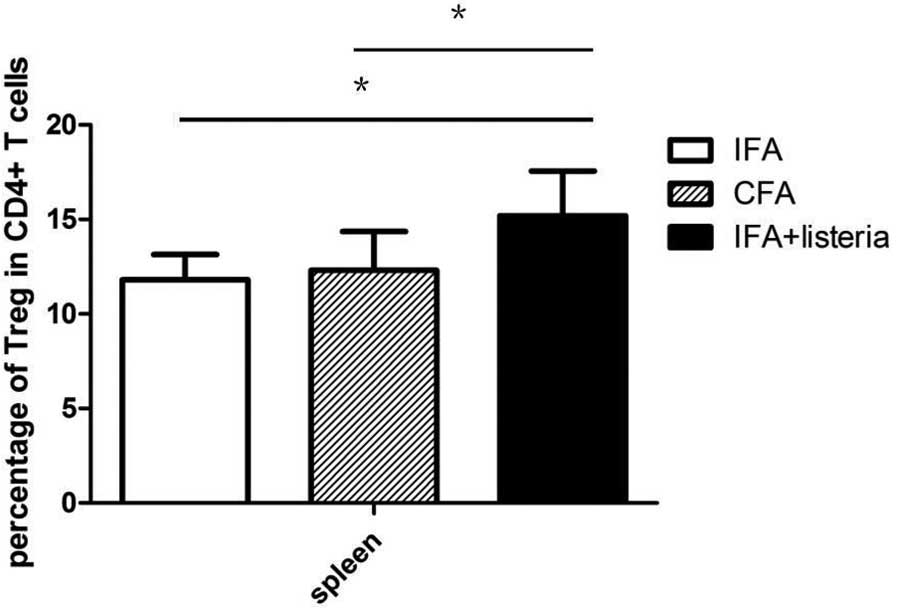
IFA + L. monocytogenes treatment delayed
disease progression, but did not alter the secretion of cytokines,
including IL-17, in the innate immune response, which indicates
that alternative mechanisms were involved. The Treg cell
populations in the spleen of the mice were analyzed and the IFA +
L. monocytogenes group mice were found to exhibit a
significantly higher percentage of Treg cells in the
CD4+ T cell population when compared with the CFA and
IFA-only groups (Fig. 4). Treg cells
are the major immunoregulatory cells in the development of TID in
NOD mice; thus, the increased Treg cell population was hypothesized
to be associated with the protective effects of the IFA + L.
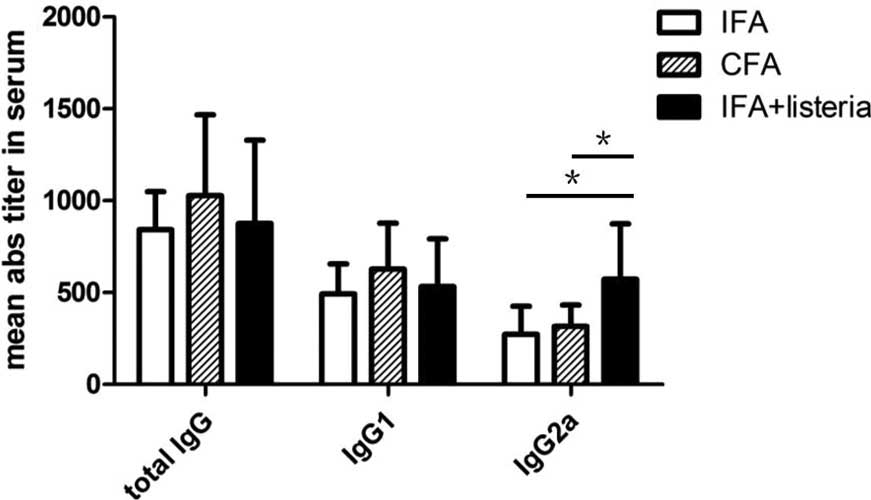
monocytogenes treatment against TID. In addition, Th1 and Th2
responses to the treatment were assessed via serum antibody-subtype
analysis, which revealed no change in the total serum levels of
IgG. However, the IFA + L. monocytogenes group mice
exhibited increased levels of the IgG2a subtype when compared with
the CFA and IFA-only groups (Fig.
5). In summary, the results indicated that treatment with IFA +
L. monocytogenes had a notable effect on T cell
differentiation and antibody responses during the development of
TID.
Discussion
In the present study, pro-diabetic NOD mice were
treated with IFA + L. monocytogenes, which was found to
delay the development of TID. However, the treatment was unable to
inhibit disease progression indefinitely. The levels of the
cytokines, IL-17 and IFN-γ, were examined in T cells and innate
immune cells in all three groups. CFA was found to induce the
expression of IL-17 in innate immune cells; however, IFA + L.
monocytogenes treatment induced only a small increase in IL-17
expression levels when compared with the IFA-only control group. No
statistically significant differences were observed in the levels
of IL-17-producing T cells and IFN-γ-producing Th1 cells between
the CFA and IFA + L. monocytogenes groups. CFA treatment
induced the production of IL-17 in innate immune cells, including
NKT and γδ T cells, which is consistent with previous studies
investigating the effects of CFA on TID development in NOD mice
(27). In the present study, IFA +
L. monocytogenes treatment delayed disease progression, but
did not induce IL-17 secretion in T cells and innate immune cells,
which suggests that alternative mechanisms may be involved in L.
monocytogenes-mediated protection against TID.
No significant difference was observed between the
groups in the levels of IFN-γ-producing Th1 and Th17 cells;
therefore, the levels of Treg cells were analyzed. Treg cells are
widely considered to play a critical role in the regulation of
autoimmune pathologies, such as TID (28). The results showed that the percentage
of Treg cells in the IFA + L. monocytogenes-treated mice was
higher compared with the CFA and IFA-only groups. Although
treatment with CFA and IFA-only had no effect on thymic Treg
levels, the IFA + L. monocytogenes treatment contained
components, such as the cell wall and microbial DNA, which
effectively activated innate immune cells. This activation was
hypothesized to induce local pro-inflammatory cytokine secretion
through Toll-like receptor signaling pathways, altering T cell
differentiation and promoting the proliferation of Treg cells.
However, the data from the current study are not sufficient to
confirm whether the IFA + L. monocytogenes treatment
increased the Treg cell population via this mechanism.
The levels of IgG antibody isotypes in the blood
serum were analyzed, and the IFA + L. monocytogenes group
mice were found to exhibit increased levels of IgG2a when compared
with the CFA and IFA-only groups. However, no statistically
significant difference was observed in the other antibody subtypes.
These results indicate that IFA + L. monocytogenes treatment
altered the Th1/Th2 balance in NOD mice, inducing the production of
IgG2a antibodies, which is closely associated with the Th1
response.
In conclusion, treatment with IFA + L.
monocytogenes was observed to delay disease progression in
pro-diabetic NOD mice. The mechanisms underlying this L.
monocytogenes-specific protection differed from those involved
in CFA treatment, since L. monocytogenes did not induce
IL-17 secretion in innate immune cells. However, IFA + L.
monocytogenes treatment was shown to affect the Th cell
subsets. Mice treated with IFA + L. monocytogenes exhibited
increased levels of Treg cells and IgG2a antibodies in the blood
serum, indicating a marked antibody response when compared with the
CFA or IFA-only treated mice. However, the mechanisms by which
these T cell subsets affect disease progression remain unclear, and
further experimental data are required.
Acknowledgements
Experimental support was provided by Shanghai Xuhai
Biological Technology Co., Ltd (Shanghai, China).
References
|
1
|
Makino S, Kunimoto K, Muraoka Y, Mizushima
Y, Katagiri K and Tochino Y: Breeding of a non-obese, diabetic
strain of mice. Jikken Dobutsu. 29:1–13. 1980.PubMed/NCBI
|
|
2
|
Aoki CA, Borchers AT, Ridgway WM, Keen CL,
Ansari AA and Gershwin ME: NOD mice and autoimmunity. Autoimmun
Rev. 4:373–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehuen A, Diana J, Zaccone P and Cooke A:
Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol.
10:501–513. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novak J, Griseri T, Beaudoin L and Lehuen
A: Regulation of type 1 diabetes by NKT cells. Int Rev Immunol.
26:49–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Godfrey DI and Kronenberg M: Going both
ways: immune regulation via CD1d-dependent NKT cells. J Clin
Invest. 114:1379–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadelain MW, Qin HY, Lauzon J and Singh B:
Prevention of type I diabetes in NOD mice by adjuvant
immunotherapy. Diabetes. 39:583–589. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mori Y, Kodaka T, Kato T, Kanagawa EM and
Kannagawa O: Critical role of IFN-gamma in CFA-mediated protection
of NOD mice from diabetes development. Int Immunol. 21:1291–1299.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kukeja A, Costi G, Marker J, Zhang CH,
Sinha S and Maclaren N: NKT cell defects in NOD mice suggest
therapeutic opportunities. J Autoimmun. 19:117–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryu S, Kodama S, Ryu K, Schoenfeld DA and
Faustman DL: Reversal of established autoimmune diabetes by
restoration of endogenous beta cell function. J Clin Invest.
108:63–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McInerney MF, Pek SB and Thomas DW:
Prevention of insulitis and diabetes onset by treatment with
complete Freund's adjuvant in NOD mice. Diabetes. 40:715–725. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee IF, Qin H, Trudeau J, Dutz J and Tan
R: Regulation of autoimmune diabetes by complete Freund's adjuvant
is mediated by NK cells. J Immunol. 172:937–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee IF, van den Elzen P, Tan R and Priatel
JJ: NKT cells are required for complete Freund's adjuvant-mediated
protection from autoimmunediabetes. J Immunol. 187:2898–2904. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin HY, Elliott JF, Lakey JR, Rajotte RV
and Singh B: Endogenous immune response to glutamic acid
decarboxylase (gad67) in NOD mice is modulated by adjuvant
immunotherapy. J Autoimmun. 11:591–601. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin HY, Chaturvedi P and Singh B: In vivo
apoptosis of diabetogenic T cells in nod mice by
IFN-gamma/TNF-alpha. Int Immunol. 16:1723–1732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin HY and Singh B: Bcg vaccination
prevents insulin-dependent diabetes mellitus (iddm) in NOD mice
after disease acceleration with cyclophosphamide. J Autoimmun.
10:271–278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin HY, Sadelain MW, Hitchon C, Lauzon J
and Singh B: Complete Freund's adjuvant-induced T cells prevent the
development and adoptive transfer of diabetes in nonobese diabetic
mice. J Immunol. 150:2072–2080. 1993.PubMed/NCBI
|
|
17
|
Tisch R, Wang B and Serreze DV: Induction
of glutamic acid decarboxylase 65-specific Th2 cells and
suppression of autoimmune diabetes at late stages of disease is
epitope dependent. J Immunol. 163:1178–1187. 1999.PubMed/NCBI
|
|
18
|
Alam C, Valkonen S, Palagani V, Jalava J,
Eerola E and Hӓnninen A: Inflammatory tendencies and overproduction
of il-17 in the colon of young NOD mice are counteracted with diet
change. Diabetes. 59:2237–2246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tigno-Aranjuez JT, Lehmann PV and
Tary-Lehmann M: Dissociated induction of cytotoxicity and dth by
cfa and cpg. J Immunother. 32:389–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lockhart E, Green AM and Flynn JL: Il-17
production is dominated by gammadelta t cells rather than cd4 t
cells during mycobacterium tuberculosis infection. J Immunol.
177:4662–4669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duarte N, Stenström M, Campino S, et al:
Prevention of diabetes in nonobese diabetic mice mediated by
cd1d-restricted nonclassical nkt cells. J Immunol. 173:3112–3118.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee IF, Qin H, Priatel JJ and Tan R:
Critical role for ifn-gamma in natural killer cell-mediated
protection from diabetes. Eur J Immunol. 38:82–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramanian L, Blumenfeld H, Tohn R, et
al: Nkt cells stimulated by long fatty acyl chain sulfatides
significantly reduce the incidence of type 1 diabetes in nonobese
diabetic mice [corrected]. PLoS One. 7:e377712012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimamura M, Huang YY, Goji H, Endo S,
Migishima R and Yokoyama M: Regulation of immunological disorders
by invariant vα19-j α33 tcr-bearing cells. Immunobiology.
216:374–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emoto M, Yoshida T, Fukuda T, et al:
Alpha-galactosylceramide promotes killing of Listeria monocytogenes
within the macrophage phagosome through invariant nkt-cell
activation. Infect Immun. 78:2667–2676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeung VP, Gieni RS, Umetsu DT and DeKruyff
RH: Heat-killed listeria monocytogenes as an adjuvant converts
established murine Th2-dominated immune responses into
th1-dominated responses. J Immunol. 161:4146–4152. 1998.PubMed/NCBI
|
|
27
|
Kukeja A, Costi G, Marker J, Zhang CH,
Sinha S and Maclaren N: NKT cell defects in NOD mice suggest
therapeutic opportunities. J Autoimmun. 19:117–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tellier J, Andrianjaka A, Vicente R, et
al: Increased thymic development of regulatory T cells in nod mice
is functionally dissociated from type I diabetes susceptibility.
Eur J Immunol. 43:1356–1362. 2013. View Article : Google Scholar : PubMed/NCBI
|