Introduction
Severe acute pancreatitis (SAP) is a serious
systemic disease with high rates of mortality. Intraperitoneal
lavage is a routine therapeutic regimen for SAP (1). Intraperitoneal lavage therapy with
added protease inhibitors, such as camostate or
glutaryl-trialanin-ethylamide, has been proved to improve survival
in experimental SAP in several species (2–4).
Ulinastatin is a purified glycoprotein from the urine of healthy
adult males, which has a potent inhibitory effect on the activities
of trypsin. Ulinastatin has been tested in numerous forms of acute
pancreatitis and has been shown to exert significant therapeutic
effects (5–7). Injection of ulinastatin, immediately
after establishment of an SAP rat model, led to a significant
decline in serum amylase levels, and a significant reduction in
pathological changes in the pancreas (8). Ulinastatin may also efficiently
interfere with SAP-associated acute lung injury through
anti-inflammatory functions (9).
Ulinastatin has a good effect on the recovery of blood pressure,
heart rate, respiratory rate, bowel sound, serum amylase and urine
amylase in patients with acute pancreatitis, which can prevent
acute pancreatitis from worsening and reduce complications
(10). However, peritoneal lavage
with the addition of ulinastatin to lavage fluid has not been
studied in SAP models. The theoretical advantage of ulinastatin is
its antiprotease effects, which may protect the functions and the
histopathology of the pancreas and other organs (8). The effect of intraperitoneal lavage
with ulinastatin added to the lavage fluid on the outcome of
taurocholate-induced SAP in rats was evaluated in the present study
to provide a new experimental and theoretical basis for the
treatment of SAP in the clinical setting.
Materials and methods
Experimental animals
A total of 110 healthy male Wistar rats that weighed
300±15 g were obtained from the Experimental Animal Center of the
General Hospital of the PLA (Beijing, China). The experimental
protocol was approved by the Ethics Committee for Animal Research
from the General Hospital of the PLA and all experimental rats
received humane care.
Reagents
Reagents were purchased from the following
companies: Chloral hydrate (Shanghai Yingxin Industrial Co. Ltd.,
Shanghai, China); sodium taurocholate (Shanghai Hufeng
Biotechnology Co. Ltd., Shanghai, China); ulinastatin (Guangdong
Tianpu Biochemical Pharmaceutical Co. Ltd., Guangzhou, China);
amylase and lipase assay kit (Shanghai Shifeng Biotechnology Co.
Ltd., Shanghai, China).
Experimental groups
Rats were randomly divided into eight groups: i)
Control (n=18, group C), sham-operated without the induction of
SAP, peritoneal lavage or intravenous injection, but with a
catheter inserted; ii) SAP model (n=18, group SAP), induction of
SAP without peritoneal lavage or intravenous injection but with a
catheter inserted; iii) saline lavage (n=18, group SL), induction
of SAP with saline lavage; iv) low-dose ulinastatin (n=10, group
LUL), induction of SAP with 25 U/ml ulinastatin lavage; v)
medium-dose ulinastatin (n=18, group MUL), induction of SAP with
62.5 U/ml ulinastatin lavage; vi) high-dose ulinastatin (n=10,
group HUL), induction of SAP with 125 U/ml ulinastatin lavage; vii)
ultrahigh-dose ulinastatin lavage (n=10, group UHUL), induction of
SAP with 250 U/ml ulinastatin lavage; viii) intravenous ulinastatin
(n=18, group IU), induction of SAP with 2,500 U/100 g intravenous
ulinastatin and a catheter inserted but without peritoneal
lavage.
Animal model
The rats were fasted for 12 h and had no access to
water for 4 h prior to undergoing surgery. Rats were anesthetized
with intraperitoneal injections of 10% chloral hydrate (3 ml/kg).
Following an incision into the abdomen, the distal region of the
duodenal bile duct was clamped with injury-free metal clips, a
syringe needle was inserted into the opening of the duodenal bile
duct. Then, 5% sodium taurocholate (freshly prepared in saline
solution, 0.6 ml) was retrogradely injected into the bile duct at a
constant rate of 0.2 ml/min using an infusion pump (Zhejiang
University Medical Instrument Co., Ltd., Hangzhou, China). After 5
min, the needle and metal clips were removed. A consistently high
mortality rate was produced (>80% within 12 h). Group C was
sham-operated without the induction of SAP.
Prior to closure of the abdomen, a silicon catheter
(Catheter A; Shandong Weigao Group Medical Polymer Co., Ltd.,
Weihai, China) with five lateral outlets was placed adjacent to the
pancreas and another silicon catheter (Catheter B; Shandong Weigao
Group Medical Polymer Co., Ltd.) with five lateral outlets was
placed in the pelvic cavity. The groups all accepted peritoneal
catheter insertion.
Peritoneal lavage
Intraperitoneal lavage was performed immediately
subsequent to the establishment of the SAP model in the SL, LUL,
MUL, HUL and UHUL groups. Warmed (37°C) lavage fluid was injected
from catheter A at 80 ml/h for 15 min and catheter B was blocked.
Catheter A was then blocked and fluid was allowed to flow out for
15 min from catheter B. Thus, each lavage procedure lasted 30 min,
and lavage was performed six times, for 3 h in total. The input and
output volumes were monitored. The lavage fluid consisted of saline
solution with or without ulinastatin added at four concentrations:
25, 62.5, 125 and 250 U/ml. Following lavage, catheters A and B
were blocked and the rats were kept in single cages with free
access to water but no solid food.
Intravenous ulinastatin
To compare the additional effects of peritoneal
lavage with those of the intravenous administration of ulinastatin,
the IU group received intravenous ulinastatin 2,500 U/100 g
(freshly prepared in 0.15 ml saline solution, approximately equal
to the total dose of the MUL group) from the caudal vein
immediately following SAP induction, but no lavage.
Assays and calculations
Survival time was recorded for 12 h and the median
survival time for each group was calculated. Animals surviving to
12 h were anesthetized and sacrificed.
Rats in groups C, SAP, SL, MUL and IU (n=8 for each
group) were sacrificed for histology and amylase and lipase
measurements 3 h after the establishment of each model. Arterial
blood was also collected into heparinized syringes from the
abdominal aorta following general anesthesia and a second
laparotomy. Blood pH, lactic acid and base excess were detected
using a blood gas analyzer (GEM 3000; Instrumentation Laboratory
Company, Bedford, MA, USA). The activity of plasma amylase and
lipase was determined using an automatic biochemical analyzer
(Beckman Coulter-AU5800; Beckman Coulter, Indianapolis, IN,
USA).
Pancreatic tissue for histology was fixed in
formalin, subjected to conventional dehydration, embedded in
paraffin and then sectioned into 5-µm sections, for subsequent
staining by hematoxylin and eosin. Examination by light microscopy
was performed by two professional pathologists using a double-blind
method as to whether the section was from the treatment or the
control groups. Three slices were randomly selected from each
group; 10 high-power fields of vision were randomly selected for
each slice and the extent of pancreatic tissue damage due to edema,
inflammation and necrosis was evaluated as the histological
parameters for grading the specific tissue damage to the pancreas.
The pathological score for pancreatic tissue was calculated
according to Rongione's standards as a reference with a minimum
score of 0 and the highest score of 4 (11).
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical analyses were performed using the SPSS software package
(version 19.0; IBM SPSS, Armonk, NY, USA). In the survival
experiments, the survival time after the 12-h observation period
was conducted by the Kaplan-Meier or Kruskal-Wallis H test.
Analysis of variance or the Kruskal-Wallis H test was used for
comparisons of biochemical data among all groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
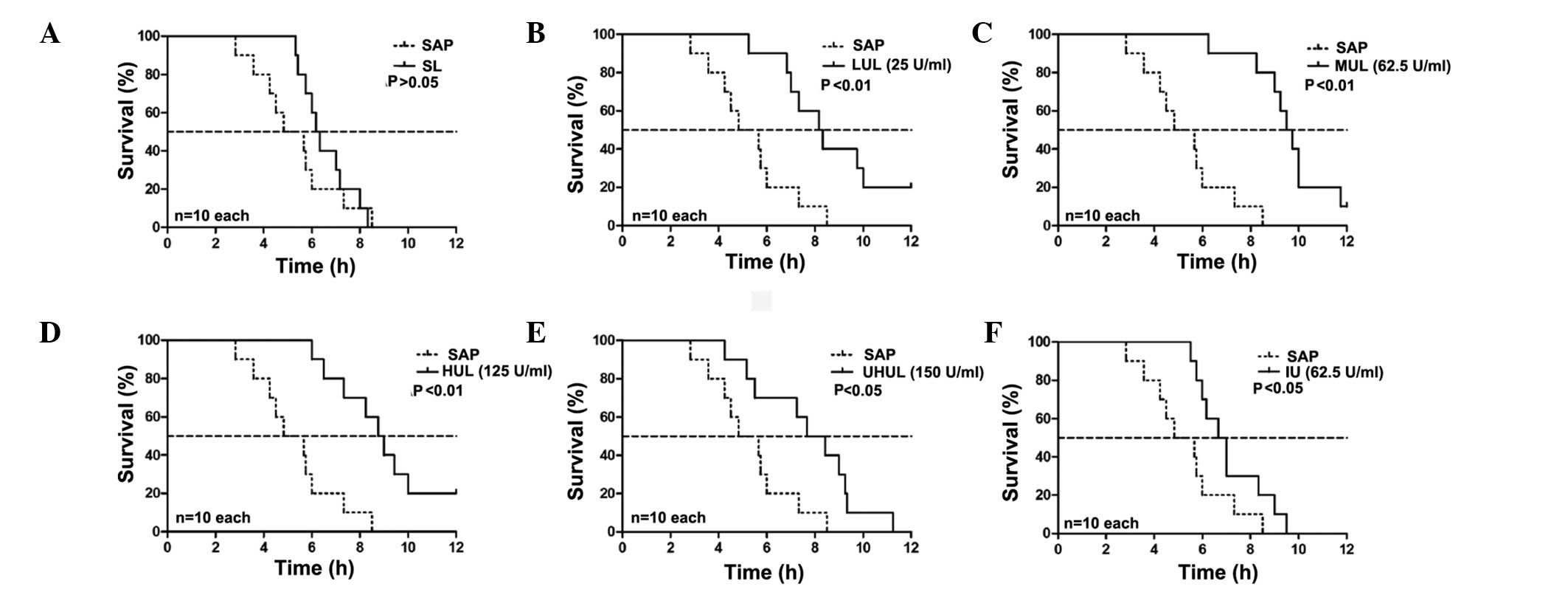
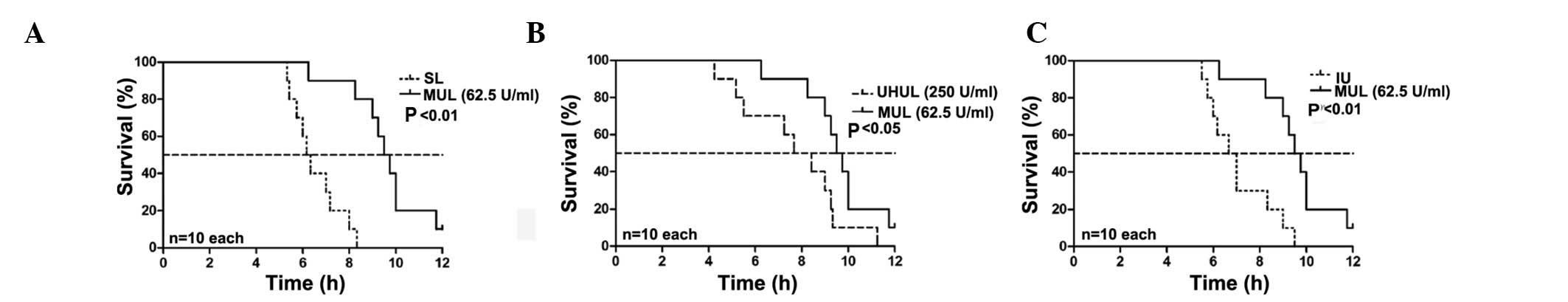
Effect of intraperitoneal lavage with
different ulinastatin concentrations on the survival time
The rats in group C remained alive after 12 h. The
median survival time of the SAP group was 4.83 h. The SL group did
not show a prolonged median survival time compared with the SAP
group (6.17 vs. 4.83 h; P>0.05, Fig.
1); however, 25 U/ml ulinastatin added to the lavage fluid (LUL
group) began to significantly increase the median survival time
(8.17 vs. 4.83 h; P<0.01, Fig.
1), and a maximal effect was exerted with the addition of 62.5
U/ml ulinastatin to the lavage fluid (MUL group) (MUL versus SAP,
9.50 vs. 4.83 h; P<0.01, Fig. 1)
(MUL versus SL, 9.50 vs. 6.17 h; P<0.01, Fig. 2). A concentration of 125 U/ml
ulinastatin (HUL group) caused similar results to the MUL group
when compared with the SAP group (8.75 vs. 4.83 h; P<0.01,
Fig. 1). The therapeutic effects
were attenuated at a concentration of 250 U/ml (UHUL group)
compared with the MUL group (7.67 vs. 9.50 h; P<0.05, Fig. 2) but the UHUL group also showed a
significantly increased median survival time compared with the SAP
group (7.67 vs. 4.83 h; P<0.05, Fig.
1). Intravenous ulinastatin of 2,500 U/100g (IU group) showed a
beneficial effect on the median survival time compared with the SAP
group (6.67 vs. 4.83 h; P<0.05, Fig.
1) but a diminished effect when compared with the MUL group
(6.67 vs. 9.50 h; P<0.01, Fig.
2). The results are summarized in Table I.
 | Table I.Comparison of survival time following
the induction of SAP in rats. |
Table I.
Comparison of survival time following
the induction of SAP in rats.
|
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|
|---|
| Group | n | Median survival time
(h) | Interquartile range
(h) | Compared with the SAP
group | Compared with the IU
group | Compared with the SL
group |
|---|
| SAP | 10 | 4.83 | 2.83–8.50 |
|
|
|
| IU | 10 | 6.67 | 5.50–9.50 | 0.044a |
|
|
| SL | 10 | 6.17 | 5.33–8.33 | 0.370 | 0.267 |
|
| LUL | 10 | 8.17 | 5.25–12.00 | 0.003b | 0.046a | 0.011a |
| MUL | 10 | 9.50 | 6.25–12.00 | 0.000b | 0.002b | 0.000b |
| HUL | 10 | 8.75 | 6.00–12.00 | 0.000b | 0.040a | 0.001b |
| UHUL | 10 | 7.67 | 4.25–11.25 | 0.015a | 0.352 | 0.034a |
Effect of lavage with ulinastatin on
biochemical parameters
The activity of amylase and lipase in the plasma of
the SAP group was significantly increased in comparison with that
in group C. The SL, MUL and IU groups all showed significantly
reduced amylase and lipase activity compared with the SAP group,
with the treatment in the MUL group exerting the maximal
effect.
The levels of lactic acid in the arterial blood of
the SAP group were significantly increased in comparison with those
in group C. The lactic acid levels were reduced in the SL and MUL
groups when compared with those in the SAP group but no significant
difference was observed between the IU and SAP groups. The maximal
effect was observed in the MUL group. The MUL and SL group
treatments also resulted in an improved pH and base excess, as
compared with the SAP group, but the IU group treatments did not.
The IU group treatment did not affect blood pH, lactic acid or base
excess in arterial blood compared with the SAP group. The results
of the effects of lavage with ulinastatin on biochemical parameters
are summarized in Table II.
 | Table II.Effect of lavage with the addition of
62.5 U/ml ulinastatin (MUL) to lavage fluid on amylase and lipase
activity in plasma, arterial blood gases and the histological
degree of pancreatitis. |
Table II.
Effect of lavage with the addition of
62.5 U/ml ulinastatin (MUL) to lavage fluid on amylase and lipase
activity in plasma, arterial blood gases and the histological
degree of pancreatitis.
| Parameter | Control | SAP | SL | MUL | IU |
|---|
| Plasma |
|
|
|
|
|
| Amylase,
U/l | 1831.1
(437.6)a | 10172.0 (1286.0) | 5651.8
(1293.9)a | 3426.8
(484.4)a | 6275.1
(988.5)a |
| Lipase,
U/l | 42.8
(21.4)a | 3789.4 (496.5) | 2476.9
(618.5)a | 1398.8
(384.2)a | 2478.5
(385.2)a |
| Arterial blood |
|
|
|
|
|
| pH | 7.26
(0.04)a | 7.04 (0.06) | 7.07 (0.06) | 7.18
(0.06)a | 7.05 (0.06) |
| Lactic
acid, mmol/l | 3.40
(1.07)a | 5.45 (1.04) | 3.62
(0.59)a | 3.33
(0.74)a | 5.04 (1.01) |
| Base
excess, mmol/l | 6.83
(1.33)a | 18.99 (1.35) | 17.88
(1.00)b | 16.74
(1.07)a | 18.61 (1.25) |
| Histological
gradingsc |
|
|
|
|
|
|
Necrosis | 0a | 3 | 3 | 2a | 3 |
|
Edema | 0a | 3 | 2–3b | 1a | 2a |
|
Inflammation | 0a | 3 | 3 | 1–2a | 1–2a |
Effect of lavage with ulinastatin on
histopathological features of pancreatic tissue
The histological changes 3 h after the onset of SAP
were significantly reduced in the MUL group when compared with
those in the SAP group with regard to necrosis, edema and
inflammation. The IU group also showed improved edema and
inflammation scores compared with those for the SAP group but no
difference was observed in necrosis. The SL group showed improved
edema results but no effect was noted on necrosis and inflammation.
The results are also summarized in Table II.
Discussion
Peritoneal antiprotease lavage therapy has been the
subject of experimental and clinical research in pancreatitis for
>40 years. It has been reported that when pancreatic enzymes are
released into the peritoneal exudate they may lead to hypotension
and mortality so that peritoneal lavage becomes necessary, and
antiprotease therapy should be directed locally into the peritoneal
cavity for the greatest therapeutic effect (12–14).
Certain studies have shown that peritoneal lavage with the addition
of a protease inhibitor to the lavage fluid improves the mortality
in experimental SAP (1,4). Although some studies in humans have not
shown a significant improvement in survival with peritoneal
antiprotease lavage therapy, it was reported that this treatment
could counteract the development of pancreatic necrosis for
patients with SAP and reduce the need for surgical intervention
(12,15,16).
Ulinastatin is a purified glycoprotein from healthy
adult male urine, and numerous studies suggest that it has a
significant therapeutic effect on SAP (17–19). In
previous years, ulinastatin has been widely used for the treatment
of patients with SAP in Asia. The therapeutic effect is greater
using an arterial infusion catheter than that with an intravenous
injection, as only a small amount of the intravenously administered
ulinastatin reaches the pancreas (20–22). To
the best of our knowledge, the experimental study of peritoneal
lavage with the addition of ulinastatin in SAP has not been
reported, and there has been no commitment to a clinical study.
The present study is the first to evaluate the
effect of peritoneal lavage with the addition of ulinastatin to the
lavage fluid in SAP rats. The retrograde injection of 5% sodium
taurocholate (freshly prepared in saline solution, 0.6 ml) into the
pancreatic duct at the rate of 0.2 ml/min produced an SAP model
with a consistently high mortality rate (>80% within 12 h)
(4). This high-mortality SAP model
was selected in order that the study under investigation would be
comparable to a life-threatening pancreatitis in humans.
Peritoneal lavage with ulinastatin added to the
lavage fluid significantly improved the median survival time. In
the MUL group, the enzyme activity of amylase and lipase, arterial
blood lactic acid, blood pH and base excess, and the histological
degree of the pancreatitis were significantly improved compared
with the SAP group. Furthermore, the MUL group treatment exerted
superior effects to the treatments in the different concentration
groups. The therapeutic effects of intraperitoneal lavage with 62.5
U/ml ulinastatin added to the lavage fluid on the outcome of SAP
are better than those of lavage alone and intravenous
interventions. The microcirculation could also be significantly
improved by intraperitoneal lavage with ulinastatin, which resulted
in marked reductions in the lactic acid levels and base excess.
The beneficial effects of lavage with ulinastatin
are associated with a reduction in the severity of pancreatitis and
an improved circulation as estimated by histology, enzyme activity
in the blood plasma and blood gases; thus, the improvement in
survival time by the intraperitoneal lavage with ulinastatin may
stem from combinational effects, including local and systemic
effects.
In the MUL group, the survival rate at 12 h (1 out
of 10) was not significantly better than that of the SAP group (0
out of 10). The short-term effect of peritoneal lavage may be one
of the reasons. It has been reported that early and extended
peritoneal lavage may be a useful therapy in the management of SAP
(23); therefore, further studies
are required to show that the addition of ulinastatin into the
peritoneal lavage fluid further improves the survival rate and
multiple organ function in SAP when the procedure of peritoneal
lavage is prolonged. The combinational effect of intravenous
ulinastatin and peritoneal lavage with ulinastatin may have
superior therapeutic effects; this could be the subject of further
studies.
Acknowledgements
The authors are grateful to Professor Chong-Hiu Li
(Department of Hepatobiliary Surgery, General Hospital of the PLA)
for her recommendations.
References
|
1
|
Niederau C, Crass RA, Silver G, Ferrell LD
and Grendell JH: Therapeutic regimens in acute experimental
hemorrhagic pancreatitis. Effects of hydration, oxygenation,
peritoneal lavage, and a potent protease inhibitor.
Gastroenterology. Gastroenterology. 95:1648–1657. 1988.PubMed/NCBI
|
|
2
|
Wilson C and Imrie CW: Effective
intraperitoneal antiprotease therapy for taurocholate-induced
pancreatitis in rats. Br J Surg. 77:1252–1255. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fric P, Slabý J, Kasafírek E, Kocna P and
Marek J: Effective peritoneal therapy of acute pancreatitis in the
rat with glutaryl-trialanin-ethylamide: a novel inhibitor of
pancreatic elastase. Gut. 33:701–706. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leonhardt U, Seidensticker F, Fussek M,
Stöckmann F and Creutzfeldt W: Camostate (FOY-305) improves the
therapeutic effect of peritoneal lavage on taurocholate induced
pancreatitis. Gut. 31:934–937. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohnishi H, Kosuzume H, Ashida Y, Kato K
and Honjo I: Effects of urinary trypsin inhibitor on pancreatic
enzymes and experimental acute pancreatitis. Dig Dis Sci. 29:26–32.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tani S, Otsuki M, Itoh H, et al: The
protective effect of the trypsin inhibitor urinastatin on
cerulein-induced acute pancreatitis in rats. Pancreas. 3:471–476.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue K, Takano H, Shimada A, et al:
Urinary trypsin inhibitor protects against systemic inflammation
induced by lipopolysaccharide. Mol Pharmacol. 67:673–680. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Zhang H, Xu KY, Wei Q and Zhou GX:
Role of the chemokine fractalkine in a rat model of acute
necrotizing pancreatitis and the interventional effect of
ulinastatin. Arch Iran Med. 16:83–87. 2013.PubMed/NCBI
|
|
9
|
Inoue K, Takano H, Shimada A, et al:
Urinary trypsin inhibitor protects against systemic inflammation
induced by lipopolysaccharide. Mol Pharmacol. 67:673–680. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li HM, Feng QX, Dou KF, et al: Treatment
of acute pancreatitis with ulinastatin: a report of 72 cases. Xi
Bei Guo Fang Yi Xue Za Zhi. 23:183–185. 2002.[In Chinese].
|
|
11
|
Rongione AJ, Kusske AM, Kwan K, et al:
Interleukin 10 reduces the severity of acute pancreatitis in rats.
Gastroenterology. 112:960–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balldin G, Borgström A, Genell S and
Ohlsson K: The effect of peritoneal lavage and aprotinin in the
treatment of severe acute pancreatitis. Res Exp Med (Berl).
183:203–213. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohlsson K and Tegner H: Experimental
pancreatitis in the dog. Demonstration of trypsin in ascitic fluid,
lymph and plasma. Scand J Gastroenterol. 8:129–133. 1973.PubMed/NCBI
|
|
14
|
Bhatia M, Wong FL, Cao Y, et al:
Pathophysiology of acute pancreatitis. Pancreatology. 5:132–144.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berling R, Borgström A and Ohlsson K:
Peritoneal lavage with aprotinin in patients with severe acute
pancreatitis. Effects on plasma and peritoneal levels of trypsin
and leukocyte proteases and their major inhibitors. Int J
Pancreatol. Int J Pancreatol. 24:9–17. 1998.
|
|
16
|
Dong Z, Petrov MS, Xu J, et al: Peritoneal
lavage for severe acute pancreatitis: a systematic review of
randomised trials. World J Surg. 34:2103–2108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirano T and Manabe T: Human urinary
trypsin inhibitor, urinastatin, prevents pancreatic injuries
induced by pancreaticobiliary duct obstruction with cerulein
stimulation and systemic hypotension in the rat. Arch Surg. Arch
Surg. 128:1322–1329. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang G, Wen J, Wilbur RR, et al: The
effect of somatostatin, ulinastatin and Salvia miltiorrhiza on
severe acute pancreatitis treatment. Am J Med Sci. 346:371–376.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wallner G, Solecki M, Ziemiakowicz R, et
al: Morphological changes of the pancreas in course of acute
pancreatitis during treatment with Ulinastatin. Pol Przegl Chir.
85:114–122. 2013.PubMed/NCBI
|
|
20
|
Keck T, Balcom JH, Antoniu BA, et al:
Regional effects of nafamostat, a novel potent protease and
complement inhibitor, on severe necrotizing pancreatitis. Surgery.
130:175–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watanabe S: Acute pancreatitis: overview
of medical aspects. Pancreas. 16:307–311. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsukawa H, Hara A, Ito T, et al:
Continuous arterial infusion of protease inhibitor with
supplementary therapy for the patients with severe acute
pancreatitis - clinical effect of arterial injection of
ulinastatin. Nihon Shokakibyo Gakkai Zasshi. Nihon Shokakibyo
Gakkai Zasshi. 95:1229–1234. 1998.[In Japanese]. PubMed/NCBI
|
|
23
|
Botoi G and Andercou A: Early and
prolonged peritoneal lavage with laparoscopy in severe acute
pancreatitis. Chirurgia (Bucur). 104:49–53. 2009.[In Romanian].
PubMed/NCBI
|