Introduction
Abortion is defined as termination of pregnancy when
the gestation period is <28 weeks and the fetal weight is
<1,000 g. An abortion that occurs before 12 weeks of gestation
is termed early abortion, accounting for >80% of cases of
spontaneous abortion (SA). There are numerous causes of abortion,
including fetal chromosomal abnormality, maternal systemic disease,
genital abnormality, endocrine abnormality, immune dysfunction and
environmental factors. In addition, certain unknown reasons for
abortion exist (1).
Pregnancy is a successful semi-allograft phenomenon,
and embryo implantation is a very complex and sophisticated
biological process, which is affected and regulated by a variety of
cytokines. Under normal circumstances, the embryo implantation
process is similar to the infiltration of tumor cells; the basic
biological process is that extravillous cytotrophoblast cells
migrate and invade the endometrium (1). In recent years, a number of studies
have found that matrix metalloproteinases (MMPs) are associated
with invasive diseases and angiogenesis (2–4), and a
considerable amount of research has been directed towards the
correlations of MMPs with cancer, endometriosis and trophoblastic
diseases. MMP family members are inhibited by endogenous tissue
inhibitors, namely tissue inhibitors of matrix metalloproteinases
(TIMPs). The balance between MMPs and TIMPs determines the
invasiveness of cells, and the homeostasis of the two plays a very
important role in numerous in vivo physiological and
pathological processes. There have been an increasing number of
studies concerning the roles of MMPs and TIMPs in the abortion
process, some of which have confirmed that in the MMP family, MMP-9
is the major protease involved in the invasion of human
cytotrophoblast cells into the endometrium, and plays a major role
in the process of embryo implantation and development (5,6). Among
the four TIMPs, TIMP-3 is the major tissue inhibitor of MMP-9,
which regulates the invasion process of cytotrophoblast cells
(5,6). In the present study, the reverse
transcription-polymerase chain reaction (RT-PCR) method was used to
detect the expression of MMP-9 and TIMP-3 mRNA in the villous
tissues of SA patients, with the aim of exploring their correlation
with SA.
Materials and methods
Subjects
A total of 30 SA cases, admitted into the Department
of Obstetrics and Gynecology (Xinxiang Central Hospital, Xinxiang,
China) from July to December 2013, were selected and defined as the
SA group. The patients in this group were aged 23–38 years, with a
median age of 29 years. The gestational periods were 56–72 days,
with an average of 61 days, and all were confirmed by B-mode
ultrasound to have no fetal heart beat. Another 20 patients, who
were in normal early pregnancy while requesting an abortion in the
same period, were selected as the control [normal abortion (NA)]
group. The patients in this group were aged 22–44 years, with a
median age of 32 years. The gestational periods were 48–68 days,
with an average of 58 days; B-mode ultrasound confirmed
intrauterine pregnancy and the fetal heart beat could be observed.
The two groups were free from genital malformations and infectious
diseases, and were not accompanied by surgical and medical
complications, genetic diseases and pregnancy complications. There
was no statistical significance between the age and gestational age
of the two groups (P>0.05); thus, they were comparable. The
present study was approved by the Central Hospital of Xinxiang
(Xinxiang, China). Informed consent was obtained from the patients
or patients' families prior to their participation.
Methods: Tissue sampling and
processing
Approximately 0.2 g villi were obtained during
uterine curettage, washed with saline, placed into a
diethylpyrocarbonate (DEPC)-treated frozen tube, and then stored in
liquid nitrogen immediately for further uniform RNA extraction when
the specimen collection was completed.
Total RNA extraction
From each sample of villi, 50–80 mg tissue was
placed into 1 ml TRIzol RNA reagent (Takara Biotechnology Co., Ltd,
Dalian, China) for full homogenization, followed by the addition of
0.2 ml chloroform and centrifugation at 12,000 × g for 15 min. The
supernatant was then evenly mixed with an equal volume of
isopropanol prior to 10 min centrifugation at 12,000 × g. The
precipitated RNA was then washed with 75% iced ethanol, and
centrifuged at 8,000 × g for 5 min twice. The supernatant was
discarded, while the precipitate was dissolved in 30–100 µl 1% DEPC
solution. A UV spectrophotometer (U-2001; Perkin Elmer, Waltham,
MA, USA) was then used to determine the RNA purity and
concentration, which was used to control the sample concentration
within the range 0.5 to 1.4 g/l and the absorbance ratio within the
range 1.8 to 2.0.
RT reaction
From each sample, 2 µg RNA was extracted, combined
with 2 µl oligo(dT)15 primer, 4 µl 5-fold RT buffer, 1
µl Moloney murine leukemia virus reverse transcriptase (M-MLV), 0.5
µl RNA enzyme inhibition agent and 0.5 µl deoxynucleotide
triphosphate (dNTP; Shanghai Promega, Shanghai, China), and DEPC
was added to provide a final volume of 20 µl. The reaction
conditions were as follows: 70°C for 5 min, placement in an
ice-water mixture for 5 min, 37°C for 5 min, 42°C for 60 min and
70°C for 15 min. The RT products were then reserved at −20°C.
PCR
All primers were synthesized by Beijing Huada Jierui
Biotechnology Co., Ltd. (Beijing, China) and β-actin was used as an
internal reference. The primer sequences of β-actin and MMP-9 genes
were as described in the literature (3). The TIMP-3 gene primer sequences were
automatically designed using Primer Express 3.0 software (Applied
Biosystems Life Technologies, Foster City, CA, USA). β-actin,
forward primer: 5-ACACTGTGCCCATCTATGAGG-3, reverse primer:
5-GGAGGGGCCGGACTCGTCATACT-3; MMP-9 gene, forward primer:
5-GGTGGACCGGATGTTCCC-3, reverse primer: 5-GCCCACCTCCACTCCTCC-3;
TIMP-3 gene, forward primer: 5-TCCCTTGGACACTAACTCTTCC-3, reverse
primer: 5-CCTCCCTCACTCTTACATG-3. The reaction volume was 25 µl: 2.5
µl template-complementary DNA (reverse transcription products), 2.5
µl l0-fold PCR buffer, 0.5 µl dNTP, 1 µl each primer, 0.5 IU Taq
DNA polymerase (Shanghai Promega) and DEPC (remainder). The
reaction conditions for MMP-9 were initial denaturation at 94°C for
5 min, followed by 94°C denaturation for 50 sec, 60°C annealing for
50 sec and 72°C extension for 50 sec, for a total of 30 cycles, and
a final extension step at 72°C for 5 min. The reaction conditions
for TIMP-3 were initial denaturation at 94°C for 5 min, followed by
94°C denaturation for 50 sec, 49°C annealing for 5 sec and 72°C
extension for 5 sec, for a total of 30 cycles, and a final
extension step at 72°C for 5 min.
Electrophoresis and semi-quantitative
analysis of PCR products
A mixture of 5 µl MMP-9 PCR product and 1 µl 6-fold
sample buffer was subjected to 1.5% agarose gel (Shanghai Aybio,
Shanghai, China) electrophoresis (80 V) for 30 min. The 5 ul TIMP-3
primer amplification products were added to 1 ul 6X loading buffer
(Beijing GenStar Biosolutions Co., Ltd., Beijing, China), the DNA
ladder was set as the reference and 2% agarose gel electrophoresis
(60 V) was carried out for 60 min. The electrophoresis results were
then examined using the Gene Genius gel image analysis system
(Syngene Co., Cambridge, UK), with the clear visualization of 28S,
18S and 5S electrophoresis bands set as the standard, to calculate
the levels of target genes. The ratio of the absorbance of the PCR
product to that of β-actin was set as the relative expression level
of the target gene mRNA, and the ratio of MMP-9/TIMP-3 mRNAs was
calculated.
Statistical analysis
SPSS software, version 10.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. A t-test of unitizing
design was used to compare the results between groups. The
measurement data are expressed as the full range (median), and
comparisons were conducted using the rank sum test.
Results
Analysis of MMP-9 and TIMP-3 mRNA
expression levels and their ratio in the two groups
The relative expression levels of MMP-9 and TIMP-3
mRNA in the villi of the two groups were determined and the
MMP-9/TIMP-3 mRNA ratios were calculated. Intergroup analysis by
t-test revealed significant differences in this ratio (P<0.001).
The MMP-9 expression level and MMP-9/TIMP-3 ratio in the SA group
were significantly higher than those in the NA group (Table I; Fig.
1). There was no significant difference between the expression
level of TIMP-3 in the normal abortion and natural abortion
groups.
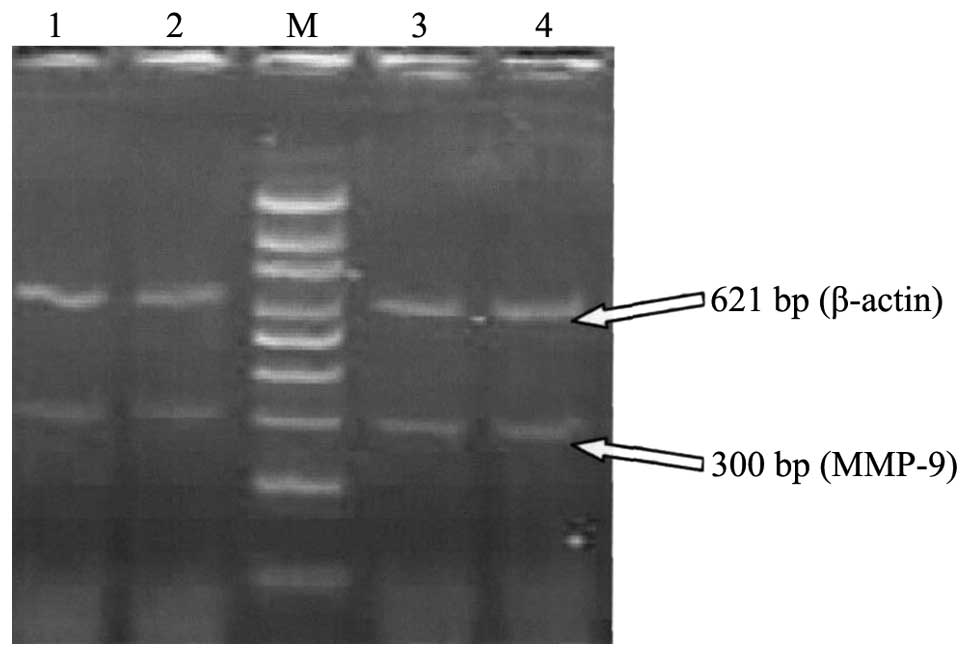 | Figure 1.Agarose gel electrophoresis of MMP-9
mRNA expression of the two groups. Lanes 1 and 2, normal early
pregnancy; lanes 3 and 4, spontaneous abortion in early pregnancy,
Lane M, 100 bp DNA ladder. The samples of villi in lanes 1, 2, 3
and 4 were obtained on the 58th, 60th, 59th and 58th days of
pregnancy, respectively. The MMP-9 bands of lanes 3 and 4 were
brighter than those of lanes 1 and 2. MMP, matrix
metalloproteinase. |
 | Table I.Comparison of the relative expression
of MMP-9 and TIMP-3 mRNA in the villi of the two groups. |
Table I.
Comparison of the relative expression
of MMP-9 and TIMP-3 mRNA in the villi of the two groups.
| Group | No. of cases | MMP-9 | TIMP-3 | MMP-9/TIMP-3 |
|---|
| SA | 30 | 0.35–0.74
(0.55)a | 0.80–1.00 (0.90) | 0.35–0.80
(0.54)a |
| NA | 20 | 0.11–0.39 (0.25) | 0.76–0.99 (0.87) | 0.11–0.30 (0.19) |
Agarose gel electrophoresis of MMP-9
and TIMP-3 mRNA expression in the villi of the two groups
The stable expression of β-actin was detected in all
the tissue specimens, confirming the integrity of the cDNA products
and the success of the PCR. The fragment length of the β-actin mRNA
RT-PCR product was 621 bp, while those of target gene MMP-9 and
TIMP-3 mRNAs were 300 bp and 593 bp, respectively (Fig. 2).
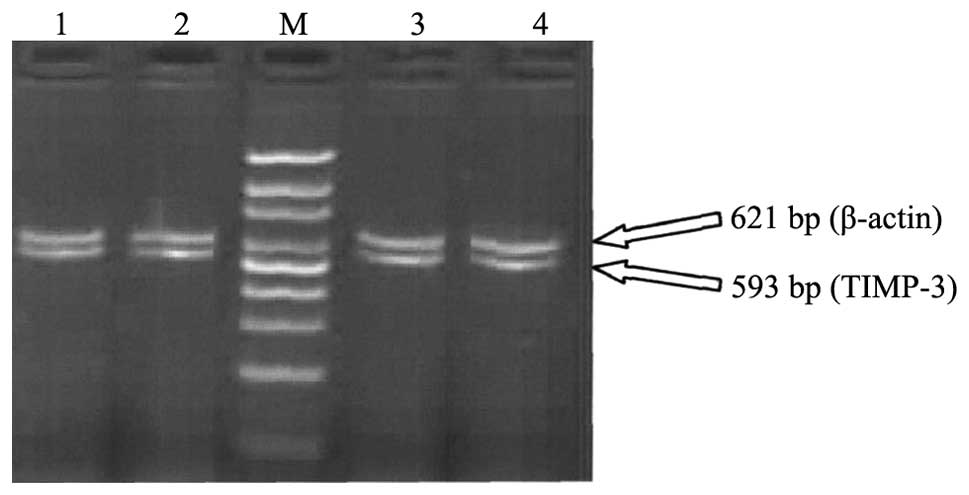 | Figure 2.Agarose gel electrophoresis of TIMP-3
mRNA expression of the two groups. Lanes 1 and 2, normal early
pregnancy; lanes 3 and 4, spontaneous abortion in early pregnancy,
Lane M, 100 bp DNA ladder. The samples of villi in lanes 1, 2, 3
and 4 were obtained on the 58th, 60th, 59th and 58th days of
pregnancy, respectively. The brightness of the TIMP-3 bands in
lanes 1–4 were similar to each other. TIMP, tissue inhibitor of
metalloproteinase. |
Discussion
The maintenance of normal pregnancy is a synergic
process between the embryo and the mother. Cytotrophoblast cells in
normal early pregnancy have similar characteristics to malignant
cells. They secrete a large amount of MMPs, thus selectively
hydrolyzing the endometrial stromal components and basement
membrane, while simultaneously generating TIMPs to inhibit MMP
generation. This maintains the dynamic balance of MMPs/TIMPs within
a certain range, and subjects the invasion of cytotrophoblast cells
into the maternal body to certain restrictions (5,6,7). The majority of non-Chinese studies have
shown that MMP-9 and TIMP-3 play particularly important roles in
early pregnancy; the MMP-9/TIMP-3 balance is significant in
regulating the depth of invasion of cytotrophoblast cells into the
uterus and embryo implantation (8,9). There
have also been certain Chinese studies indicating that the high
expression levels of TIMP-3 during the endometrial implantation
window may avoid the excessive degradation of endometrial
extracellular matrix (ECM) by MMP-9, prevent the excessive
infiltration of cytotrophoblast cells, and regulate the activity of
MMP-9. An imbalance in the expression of MMP-9 and TIMP-3 would
affect fetal growth and development, and excessive invasion by
cytotrophoblast cells is likely to result in miscarriage in early
pregnancy (7).
Among the various MMPs that are secreted by the
maternal endometrium and cytotrophoblast cells during the processes
of endometrial degeneration and blastocyst implantation, MMP-9 is
considered to be the main proteolytic enzyme (10). MMP-9 is a major proteolytic enzyme,
the activity of which is dependent on Zn2+ and
Ca2+, and it is the main enzyme that degrades the ECM.
During the invasion of placental cytotrophoblast cells into the
maternal uterine stroma, type IV collagen is the main component of
the ECM between the fetus and the mother, and the substrate that
MMP-9 decomposes is type IV collagen. Following implantation, MMP-9
decomposes the ECM, and initiates cytotrophoblast adhesion,
migration and differentiation; thus, cytotrophoblast cells are able
to penetrate the basement membrane and enter the maternal
circulation. During the in vitro cultivation of
cytotrophoblast cells, Whiteside et al (5) found that the cells secreted a large
amount of MMP-9 and that MMP-9 expression was significantly
increased subsequent to embryo implantation, which would be
beneficial towards embryo implantation and placental formation.
Jeziorska et al (11)
measured MMP-9 and MMP-3 secretion every day of the menstrual
cycle, and revealed that MMP-9 expression was regulated precisely
and according to the time within the cycle; MMP-9 was not expressed
in endometrial tissues in the early proliferation stage, began to
exhibit glandular epithelial expression on day 7 of the cycle, with
extracellular secretion into the glandular lumen appearing from day
15. On days 21–23, the expression reached a peak and was also
observed in the uterine cavity, which lasted until menstruation.
MMP-9 is richly expressed in the post-ovulation implantation
window, suggesting that it is conducive to blastocyst implantation.
Xu et al (12) performed
in vitro culture of human cytotrophoblast cells for 6–11
weeks. At the end of week 6, no MMP-9 secretion was detected, while
secretion started and gradually increased from weeks 7–11, and
reached a level in week 11 that was 10-fold greater than that in
week 7. By contrast, the expression of MMP-2 decreased from week 6
to week 11, and the amount expressed in week 11 was only one-sixth
of that in week 6. The expression levels of MMP-2 and MMP-9 mRNA
were consistent with their protein secretion, suggesting that MMP-2
plays an important role in the human embryo peri-implantation
period. After that, the invasive ability of cytotrophoblast cells
is likely to be determined by MMP-9 production. MMP-9 was also
detected in the chorionic villi in the present study, suggesting
that MMP-9 plays an important role in the process of
cytotrophoblast invasion. Further experiments found that MMP-9 mRNA
expression levels in the SA patients were significantly higher than
those of normal early pregnancy (P<0.01), which may be due to
the cytotrophoblast cells of SA patients producing excessive
quantities of MMP-9, thus reinforcing the hydrolysis of basement
membrane proteins, and resulting in the excessive invasion,
infiltration and metastasis of cytotrophoblast cells.
In the TIMP family of proteins, TIMP-3 has been
shown to be the main inhibitor of MMP-9. A study by Whiteside et
al (5) found that MMP-9 was
expressed by embryonic cytotrophoblast cells, and that when an
MMP-9 antisense oligonucleotide was used to treat mouse blastocysts
cultivated on an ECM gel, ECM degradation by the mouse blastocysts
was significantly reduced due to restricted MMP-9 protein
secretion. In TIMP-3-enriched ECM, the degradation of ECM by
growing blastocysts was also reduced compared with that of normal
ECM, in a dose-dependent manner; this decline was due to the
inhibition of MMP-9 activity by TIMP-3. This suggests that
interaction between MMP-9 and TIMP-3 is the key mechanism by which
cytotrophoblast cells implement an appropriate invasive force, and
explains the ability of cytotrophoblast cells to avoid excessive
invasion towards the endometrium. The present study detected the
expression of TIMP-3 mRNA in the villi of normal early pregnancy,
and the results revealed that the TIMP-3 mRNA expression levels in
the SA patients and patients with normal pregnancy were equivalent
(P>0.05).
During the in vitro cultivation of
cytotrophoblast cells, Whiteside et al (5) found that the cytotrophoblast cells
secreted large amounts of MMP-9 and that MMP-9 expression began to
increase significantly subsequent to implantation, indicating that
it was favorable for embryo implantation and placental formation.
Polette et al (13) also
observed that MMP-9 was secreted at the highest levels in early
pregnancy. Successful embryo implantation requires a suitable
intrauterine environment and receptivity; as it has been found that
the MMP-9 activities in the endometria of women with failed early
pregnancy are significantly increased and result in disorders of
ECM components, this suggests that MMP-9 is significant in the
failure of pregnancy (14). The
present study also detected MMP-9 in the chorionic villi of women
in the early stages of pregnancy, suggesting that MMP-9 plays an
important role in cytotrophoblast invasion. Table I demonstrates that the MMP-9
expression level of the SA group was significantly increased
compared with that in the NA group (P<0.001), indicating that
the chorionic cytotrophoblast cells of the SA group produced
excessive amounts of MMP-9. Thus, the proteolytic effects of MMP-9
towards the endometrial basement membrane were increased, and were
significant in the invasion, infiltration and metastasis of
cytotrophoblast cells. These results are consistent with those of
Iwahashi and Nakano (15).
In a study of rhesus monkeys, MMP-9 was found to be
a critical factor for cytotrophoblast invasion into the endometrium
and placenta formation; however, it was also detected that TIMP-3
mRNA was highly expressed in certain endometrial arteries,
suggesting that it exhibited an important role in preventing
decidual bleeding and abortion (6).
Chinese research has demonstrated that the synergic expression of
MMP-9 and TIMP-3 is necessary to maintain normal early pregnancy
(15,16). The synergy of MMP-9 and TIMP-3 is
very important in regulating the cytotrophoblast invasion depth in
the uterus and embryo implantation (7,8,17). Whiteside et al (5) reported that the expression levels of
embryo-derived MMP-9 and ECM-derived TIMP-3 were correlated.
Increased MMP-9 expression was accompanied by high TIMP-3
expression, which antagonized the degradation effect of MMP-9 on
the ECM, and avoided the excessive invasion of cytotrophoblast
cells into the endometrium. A stable dynamic equilibrium between
these two proteins enabled the pregnancy to proceed smoothly. Li
et al (7) reported when MMP-9
is dominant, it promotes ECM degradation, which is profitable for
embryo implantation with the continuous invasion of cytotrophoblast
cells and when TIMP-3 is dominant, it inhibits the excessive
degradation of ECM by MMP-9 and prevents the excessive invasion of
villous cytotrophoblast cells; therefore, the balance of these two
proteins may help to maintain normal pregnancy. In the present
study, the ratio of MMP-9/TIMP-3 mRNA expression in the SA group
was significantly higher than that in the NA group (P<0.01).
However, with the increased expression of MMP-9, the TIMP-3 level
of the SA group did not increase appropriately to antagonize the
ECM-degrading effect of MMP-9. This resulted in an increase in the
MMP-9/TIMP-3 mRNA ratio of the SA group. From these results, it may
be speculated that the synergic expression of MMP-9 and TIMP-3
genes has a close association with the depth of invasion of
cytotrophoblast cells. The imbalance in the levels of MMP-9 and
TIMP-3 is likely to cause excessive degradation of the ECM and the
excessive invasion of cytotrophoblast cells into the endometrium,
thus affecting embryo implantation and even resulting in
pathological SA or other pathological pregnancy.
In summary, the MMP-9 mRNA expression levels of the
patients undergoing SA were high compared with those in normal
patients, and the MMP-9/TIMP-3 mRNA ratio was also high in the SA
group. The MMP-9 mRNA expression level and MMP-9/TIMP-3 mRNA ratio
may be relevant to SA. The results of this study provide an
experimental basis for the clinical investigation of the mechanism
of abortion, and also a theoretical basis for the clinical
application of MMP-9 as a diagnostic indicator of threatened
abortion. In addition, they support the use of synthetic TIMPs to
treat threatened abortion and maintain a normal pregnancy.
References
|
1
|
Xie X: Abnormal pregnancy Section I:
spontaneous abortionScience of Obstetrics and Gynecology. Gou W:
8th. People's Medical Publishing House; Beijing, China: pp.
472013
|
|
2
|
Itoh T, Tanioka M, Matsuda H, et al:
Experimental metastasis is suppressed in MMP-9-deficient mice. Clin
Exp Metastasis. 17:177–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang S, Van Arsdall M, Tedjarati S, et
al: Contributions of stromal metalloproteinase-9 to angiogenesis
and growth of human ovarian carcinoma in mice. J Natl Cancer Inst.
94:1134–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamoto T, Niu R, Yamada S and Osawa M:
Reduced expression of tissue inhibitor of metalloproteinase
(TIMP)-2 in gestational trophoblastic diseases. Mol Hum Reprod.
8:392–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whiteside EJ, Jackson MM, Herington AC, et
al: Matrix metalloproteinase-9 and tissue inhibitor of
metalloproteinase-3 are key regulators of extracellular matrix
degradation by mouse embryos. Biol Reprod. 64:1331–1337. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Li Q, Shao L and Zhu C: Expression
of matrix metalloproteinase-2, -9, -14 and tissue inhibitors of
metalloproteinase-1, -2, -3, in the endometrium and placenta of
rhesus monkey (Macaca mulatta) during early pregnancy. Biol Reprod.
65:31–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Xing FQ and Chen SL: Regulation of
TNF-α towards the expression of endometrial metalloproteinase-9 and
its tissue inhibitor 3. Zhonghua Fu Chan Ke Za Zhi. 41:346–347.
2006.[In Chinese].
|
|
8
|
Walter I and Schönkypl S: Extracellular
matrix components and matrix degrading enzymes in the feline
placenta during gestation. Placenta. 27:291–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaMarca HL, Ott CM, Höner Zu, Bentrup K,
et al: Three-dimensional growth of extravillous cytotrophoblasts
promotes differentiation and invasion. P1acenta. 26:709–720.
2005.
|
|
10
|
Nuttall RK and Kennedy TG: Gelatinases A
and B and tissue inhibitors of metalloproteinases l, 2 and 3 during
in vivo and in vitro decidualization of rat endometrial stromal
cells. Biol Reprod. 60:471–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeziorska M, Nagase H, Salamonsen LA and
Woolley DE: Immunolocalization of the matrix metalloproteinase
gelatinase B and stromelysin 1 in human endomentrium throughout the
menstrual cycle. J Reprod Fertil. 107:43–51. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS and
Zhuang LZ: Expression of matrix metalloproteinase -2, -9, and-14,
tissue inhibitors of metalloproteinase-1, and matrix proteins in
human placenta during the first trimester. Biol Reprod. 62:988–994.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polette M, Nawrocki B, Pintiaux A, et al:
Expression of gelatinases A and B and their tissue inhibitors by
cells of early and term human placenta and gestational endometrium.
Lab Invest. 71:836–846. 1994.
|
|
14
|
Inagaki N, Stern C, McBain J, et al:
Analysis of intra-uterine cytokine concentration and
matrix-metalloproteinase activity in women with recurrent failed
embryo transfer. Hum Reprod. 18:608–615. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwahashi M and Nakano R: Decreased type V
collagen expression in human decidual tissues of spontaneous
abortion during early pregnancy. J Clin Pathol. 51:44–46. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bass KE, Li H, Hawkes SP, Howard E, Bullen
E, Vu TK, et al: Tissue inhibitor of metalloproteinase-3 expression
is upregulated during human cytotrophoblast invasion in vitro. Dev
Genet. 21:61–67. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gui W, Qiu Y, Zeng P, et al: Expression of
matrix metalloproteinase-9 and tissue inhibitor of
metalloproteinase-I protein in the implantation of window phase of
endometrium in ovulation normal women. Chongqing Yike Daxue Xuebao.
29:1–6. 2004.
|
















