Introduction
Adrenomedullin (ADM) was originally discovered in
1993 by Kitamura et al (1)
following its isolation from human pheochromocytoma tissue. It has
since been demonstrated that ADM can be synthesized by numerous
mammalian tissues, including the adrenal medulla, endothelial and
vascular smooth muscle cells, myocardium and central nervous system
(2,3). ADM has diverse and profound effects on
cellular proliferation, contractility, migration and interaction
with other neurohormonal factors, including atrial and brain
natriuretic peptide (ANP and BNP, respectively) (4). The most important and known
pathophysiological role of ADM is that it is a potent vasorelaxant
and natriuretic peptide (5–7). ADM administered through intravenous
infusion has been shown to lower blood pressure (BP) and increase
the heart rate and cardiac output (8). A direct cardiostimulatory effect has
also been found in the isolated perfused rat heart (9). The natriuretic peptide system consists
of a group of neurohormones, including ANP and BNP (10). ANP and BNP are cardiac hormones
believed to act against the elevation of blood pressure and
retention of body fluid in cardiovascular diseases, such as
hypertension and heart failure, through their vasorelaxant and
natriuretic effects (5). ADM exerts
its chief biological actions via an accumulation of intracellular
cyclic adenosine monophosphate (11), while several of the actions of ANP
and BNP are mediated by cyclic guanosine monophosphate (10). Since the actions of ADM are similar
to those of ANP and BNP, despite differences in their intracellular
signaling systems, we hypothesized that ADM functions along with
ANP and BNP to act against a further elevation of blood pressure in
hypertensive patients.
In the present study, the pathophysiological roles
of ADM, ANP and BNP in essential hypertension (EH) were
investigated by monitoring any changes in the plasma concentrations
of these peptides in untreated hypertensive patients whose disease
was classified into one of three stages. These results were then
compared with those of the control subjects, and comparisons were
additionally made among the three disease stage groups. After 4
weeks of effective antihypertensive therapy for the hypertensive
patients, the ADM, ANP and BNP levels were re-measured. These
results were then compared with the pretherapy results and those of
the control subjects.
Materials and methods
Study subjects
Between August 2010 and December 2012, 64 patients
with established EH and 35 normotensive control subjects were
recruited for this study in the Renmin Hospital of Wuhan University
(Wuhan, China). All subjects agreed with the aim of the study and
gave their informed consent prior to their inclusion in the study.
The study was approved by the Ethics Committee of Renmin Hospital
of Wuhan University.
Routine laboratory studies of the patients included
routine blood tests; urinalysis; prothrombin time, activated
partial thromboplastin time, fibrinogen and D-Dimer concentrations;
serum electrolytes, serum creatinine (Scr), blood urea nitrogen
(BUN) and fasting blood glucose levels; liver function tests;
plasma renin activity, aldosterone, catecholamine, cortisol and
thyroid hormone levels; a chest roentgenogram; an
electrocardiogram; B-scan ultrasonography of the liver, cholecyst,
pancreas, spleen, bilateral kidneys and adrenal glands; and an
excretory urogram or renal arteriogram. The glomerular filtration
rate (GFR) was calculated by endogenous cystatin C clearance, as
previously described (12). On the
basis of all results and guidelines from the World Health
Organization (2003) (13), subjects
were placed into a diagnostic category. Normal BP was defined as a
systolic pressure <140 mmHg and a diastolic pressure <90
mmHg. Stage I hypertension was defined as a systolic pressure ≥140
mmHg but <160 mmHg or a diastolic pressure ≥90 mmHg but <100
mmHg, or both. Stage II hypertension was defined as a systolic
pressure ≥160 mmHg but <180 mmHg or a diastolic pressure ≥100
mmHg but <110 mmHg, or both. Stage III hypertension was defined
as a systolic pressure ≥180 mmHg or a diastolic pressure ≥110 mmHg,
or both. Secondary hypertension was excluded by clinical history,
physical examination, routine laboratory tests and imaging
examinations. None of the patients had clinical evidence of cardiac
or hepatic failure, diabetes, pulmonary disease, angina pectoris,
myocardial infarction or other diseases that could cause secondary
hypertension. The hypertensive patients either had no history of
antihypertensive treatment or had ceased their antihypertensive
treatment ≥4 weeks previously. The normotensive controls were age-
and gender-matched healthy subjects who had been hospitalized for a
healthy checkup.
Following the initial evaluation, the plasma
concentrations of ADM, ANP and BNP were determined. A total of 64
patients with EH then commenced antihypertensive therapy with one
or more of the following drugs: Nifedipine, metoprolol or
benazepril. After 4 weeks of effective antihypertensive therapy,
the BP of the hypertensive patients was normalized. The plasma
levels of the three peptides were then re-measured.
Arterial BP was measured by a mercury
sphygmomanometer once the patients had rested in a seated position
for ≥30 min in a quiet and warm room without smoking or drinking
coffee. The mean of three BP measurements at 5-min intervals was
used to classify the subjects.
Preparations of human ADM (hADM), ANP
and BNP
Blood samples were drawn from an antecubital vein
early in the morning following an overnight fast and were
transferred to ice-chilled tubes containing aprotinin (500 KIU/ml)
and EDTA (1 g/l). Plasma was separated by centrifugation at 2,000 ×
g for 10 min at 4°C and immediately frozen and stored at −70°C
until radioimmunoassay (RIA).
Assay procedure
The plasma ADM concentrations were measured with a
specific RIA following the extraction of plasma, as described
previously (14). Briefly, 2 ml
plasma was loaded onto a Sep-Pak C18 cartridge (Waters Corp.,
Milford, MA, USA) equilibrated with 5 ml saline. Following the
washing of the cartridge with 5 ml saline and 10% acetonitrile in
0.1% trifluoroacetic acid (TFA), the absorbed peptides were eluted
with 4 ml 50% acetonitrile in 0.1% TFA, lyophilized, and then
stored at −70°C until RIA. The plasma extract was dissolved in 250
µl RIA buffer (Phoenix Pharmaceuticals, Inc., Mountain View, CA,
USA) and 50 mmol/l sodium phosphate buffer (pH 7.4) containing 0.5%
bovine serum albumin, 0.5% Triton X-100, 80 mmol/1 sodium chloride,
25 mmol/l EDTA, 0.05% sodium azide and 500 KIU/ml aprotinin. A
total of 100 µl dissolved plasma extract was used for an
hADM-specific RIA, as reported previously (14). The rabbit polyclonal anti-hADM
antibody (1:20,000; cat. no. RK-010-01; Phoenix Pharmaceuticals,
Inc.) used in this RIA did not show any cross-reactivity with
hADM-(13–52), rat ADM-(1–50), human calcitonin gene-related peptide
(CGRP), endothelin-1, α-human ANP-(1–28), BNP-32 or C-type
natriuretic peptide-22. The interassay coefficient of variation was
8%, and the intra-assay coefficient of variation was 6%. The
concentration of ADM was expressed in ng/l.
The plasma ANP concentration was determined by a
similar specific immunoradiometric assay for human ANP (ShionoRIA
ANP kit; Shionogi & Co., Ltd., Osaka, Japan). The plasma BNP
concentration was measured by a similar method developed by the
same company (ShionoRIA BNP kit; Shionogi & Co., Ltd.). The
accuracies and the detailed methods of these assays have been
described previously (15).
Statistical analysis
All continuous data are expressed as the mean ±
standard deviation. Statistical analysis was performed through
linear regression analysis, which was further confirmed by
Kendall's method or an analysis of variance test for multiple
comparisons, which was further examined using the
Student-Newman-Keuls's method. Comparisons between two variables
were conducted using an unpaired t-test, and comparisons between
the paired values were analyzed with a paired t-test. Categorical
variables were assessed using the χ2 or Fisher's exact
test. Non-normally distributed data were analyzed through the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
The clinical profiles of the control subjects and
hypertensive patients in each stage are summarized in Table I. No significant differences were
found in the age and gender distribution among the four groups. The
mean arterial pressure (MAP), BUN and Scr levels of the
hypertensive patients were significantly higher than those of the
control subjects (P<0.05); these increases additionally
correlated with the severity of the hypertension stage (P<0.05).
The GFRs of the hypertensive patients exhibited the opposite
changes (P<0.05).
 | Table I.Characteristics of the study
subjects. |
Table I.
Characteristics of the study
subjects.
|
| Control, n=35 | Stage I EH, n=20 | Stage II EH,
n=25 | Stage III EH,
n=19 |
|---|
| Age (years) | 43±4 | 41±6 | 44±5 | 45±7 |
| Male:female ratio
(n:n) | 16:19 | 9:11 | 11:14 | 8:11 |
| MAP (mmHg) | 90±8 | 112±5a | 124±8a,b | 138±10a,b,c |
| BUN (mg/dl) | 17±3 | 19±2a | 22±3a,b | 26±6a,b,c |
| Scr (mg/dl) | 1.0±0.2 | 1.2±0.3a | 1.5±0.4a,b | 1.8±0.5a,b,c |
| GFR (ml/min) | 95±9 | 87±9a | 79±10a,b | 72±11a,b,c |
Pretherapy peptide levels
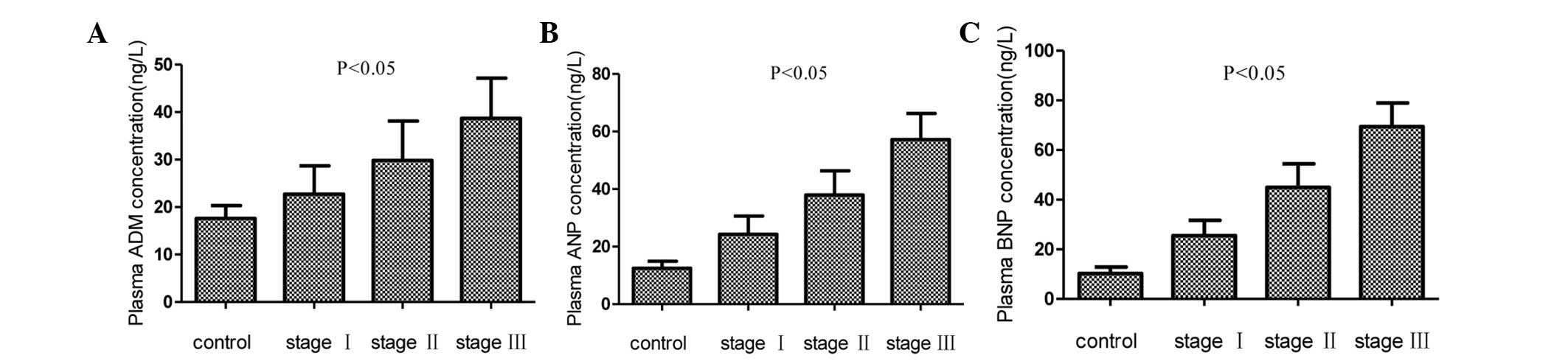
Fig. 1 shows the
plasma concentrations of ADM, ANP and BNP in the control and three
hypertension groups. The mean concentrations of ADM, ANP and BNP in
the hypertension groups were significantly higher than those in the
control group (P<0.05); significant increases were additionally
found with increases in the hypertension grade (P<0.05).
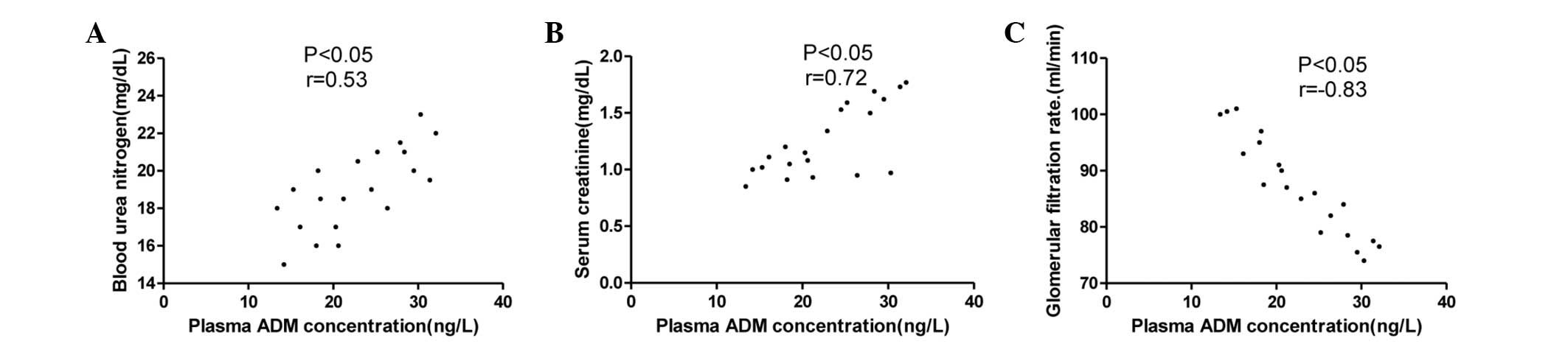
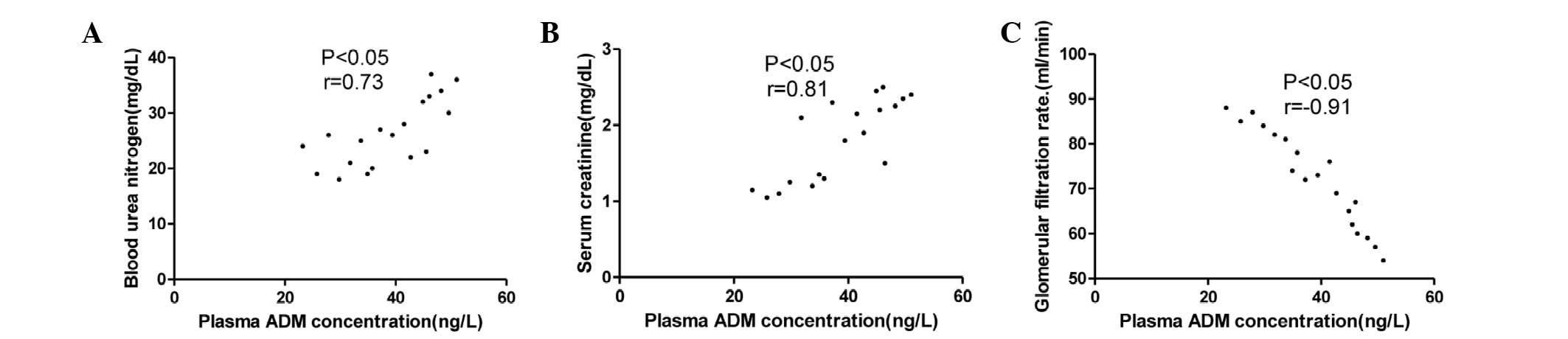
The associations between the plasma ADM
concentration and the BUN level, Scr level and GFR in the stage I,
II and III hypertension groups are summarized in Figs. 2–4.
The plasma ADM concentration was correlated with BUN and a stronger
correlation with Scr was found. The strongest correlation was
observed between the plasma ADM concentration and the GFR but this
correlation was negative.
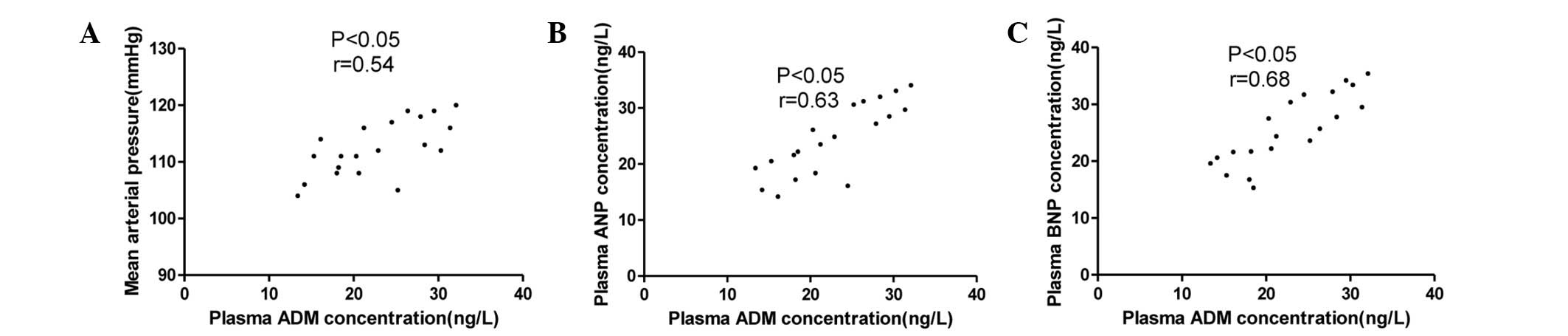
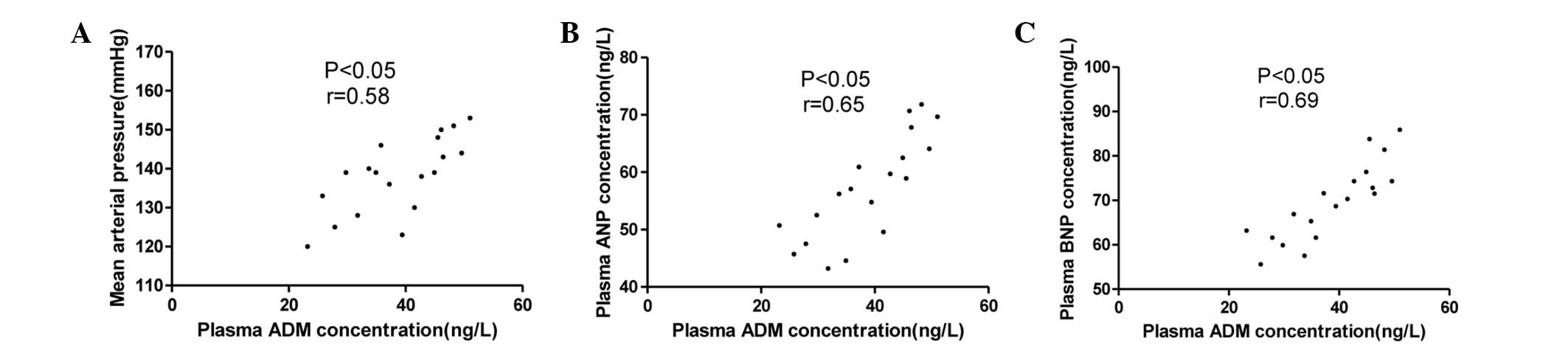
The associations between the plasma ADM
concentration and the MAP, ANP and BNP levels in the stage I, II
and III hypertension groups are summarized in Figs. 5–7.
The ADM concentration was not only correlated with MAP but also
with the plasma concentrations of ANP and BNP.
The plasma concentrations of ADM, ANP and BNP in the
stage I, II and III hypertension groups with or without renal
dysfunction are summarized in Tables
II–IV. The patients with
increased Scr (≥1.5 mg/dl) or decreased GFR (≤80 ml/min) had
markedly higher plasma concentrations of ADM, ANP and BNP than
those of the patients with normal Scr and GFR. The plasma ADM, ANP
and BNP levels of the patients with or without renal dysfunction
were also higher than those of control subjects.
 | Table II.Plasma concentrations of ADM, ANP and
BNP in stage I hypertensive patients with or without renal
dysfunction. |
Table II.
Plasma concentrations of ADM, ANP and
BNP in stage I hypertensive patients with or without renal
dysfunction.
| Characteristics | n | ADM (ng/l) | ANP (ng/l) | BNP (ng/l) |
|---|
| Scr ≥1.5 mg/dl | 7 | 29.4±2.0a,b | 31.3±1.9a,b | 32.4±2.1a,b |
| Scr <1.5
mg/dl | 13 |
19.1±3.8b |
20.5±4.2b |
21.9±3.9b |
| GFR ≤80 ml/min | 6 |
29.9±1.7b,c |
31.8±1.6b,c |
32.9±1.8b,c |
| GFR >80
ml/min | 14 |
19.6±4.0b |
21.1±4.6b |
22.4±4.3b |
| Control
subjects | 35 | 17.6±2.7 | 12.5±2.4 | 10.3±2.6 |
 | Table IV.Plasma concentrations of ADM, ANP and
BNP in stage III hypertensive patients with or without renal
dysfunction. |
Table IV.
Plasma concentrations of ADM, ANP and
BNP in stage III hypertensive patients with or without renal
dysfunction.
|
Characteristics | n | ADM (ng/l) | ANP (ng/l) | BNP (ng/l) |
|---|
| Scr ≥1.5 mg/dl | 12 |
44.0±4.8a,b |
62.9±5.9a,b |
75.3±6.1a,b |
| Scr <1.5
mg/dl | 7 |
29.6±4.2b |
47.7±3.4b |
59.5±4.3b |
| GFR ≤80 ml/min | 13 |
43.3±5.2b,c |
62.1±6.4b,c |
74.5±6.5b,c |
| GFR >80
ml/min | 6 |
28.7±3.9b |
46.9±2.9b |
58.5±3.8b |
| Control
subjects | 35 | 17.6±2.7 | 12.5±2.4 | 10.3±2.6 |
Post-therapy results
Table V shows the
clinical parameters of the three groups of hypertensive patients
initially and 4 weeks after effective antihypertensive therapy.
Following treatment, the MAP of the patients was normal, while the
BUN and Scr levels were decreased compared with the pretherapy
values (P<0.05). By contrast, the GFR was increased compared
with the pretherapy values (P<0.05).
 | Table V.Hemodynamic parameters of the
hypertensive subjects. |
Table V.
Hemodynamic parameters of the
hypertensive subjects.
|
| Stage I EH,
n=20 | Stage II EH,
n=25 | Stage III EH,
n=19 |
|---|
|
|
|
|
|
|---|
| Parameters | At diagnosis | After drugs | At diagnosis | After drugs | At diagnosis | After drugs |
|---|
| MAP (mmHg) | 112±5 | 92±3a | 124±8 | 94±4a | 138±10 | 95±5a |
| BUN (mg/dl) | 19±2 | 17±2a | 22±3 | 19±2a | 26±6 | 22±3a |
| Scr (mg/dl) | 1.2±0.3 |
1.0±0.2a | 1.5±0.4 |
1.2±0.2a | 1.8±0.5 |
1.4±0.3a |
| GFR (ml/min) | 87±9 | 93±7a | 79±10 | 88±9a | 72±11 | 84±9a |
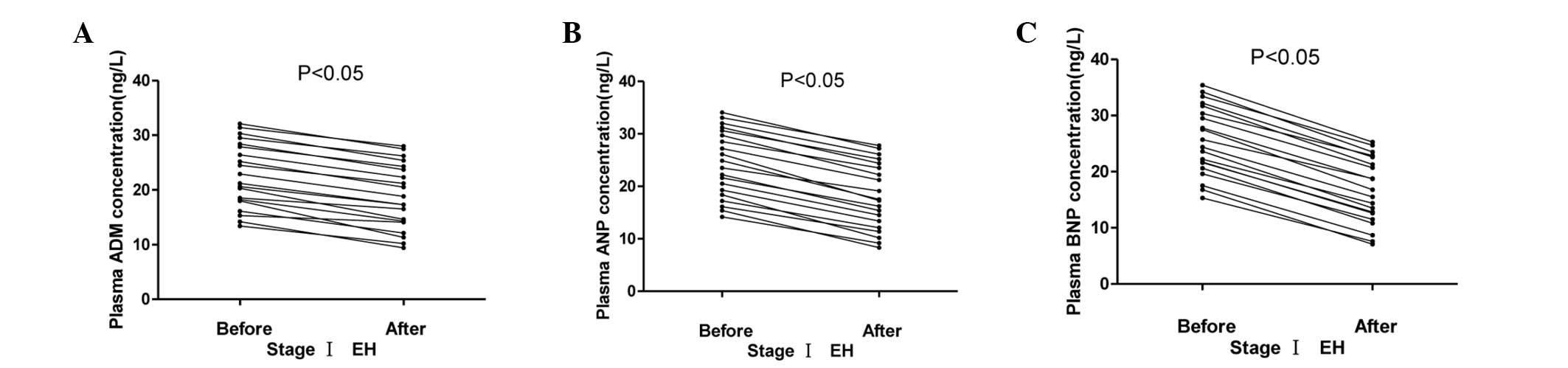
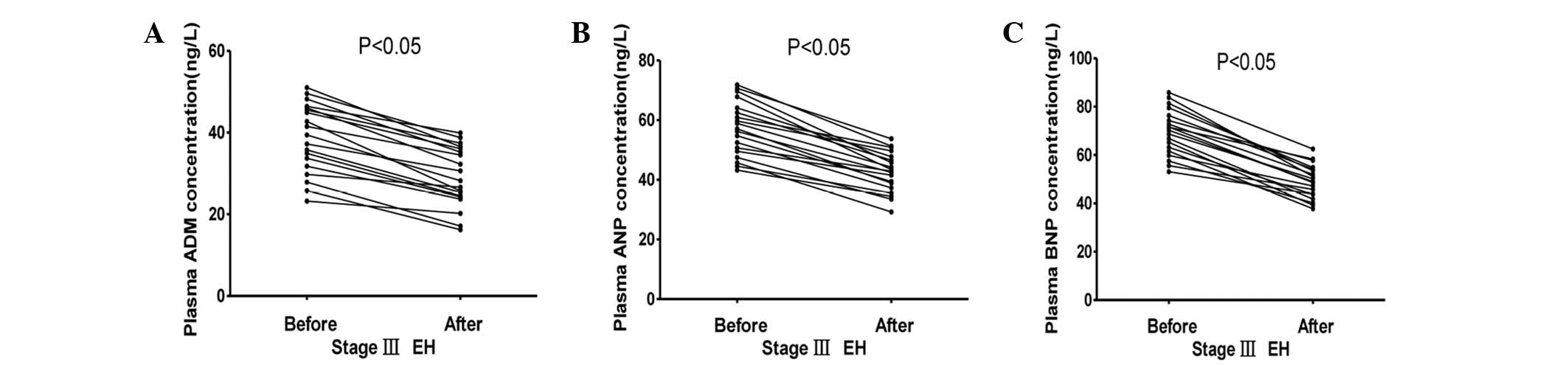
The plasma ADM, ANP and BNP concentrations in the
stage I, II and III hypertension groups initially and 4 weeks after
effective antihypertensive therapy are summarized in Figs. 8–10.
The plasma concentrations of ADM, ANP and BNP fell significantly
following antihypertensive treatment (P<0.05).
Discussion
As a 52-amino acid vasodilatory peptide, ADM has a
ring structure formed by a disulfide bond and an amidated carboxyl
terminus and shows homology with CGRP and amylin, which are other
members of this family of peptides (16). It has been demonstrated that this
peptide is present not only in a variety of organs and cells, but
also in human plasma (1). Factors of
both the physical, such as shear and ventricular wall stress and
hypoxia, and humoral, such as cytokines and endocrine and paracrine
hormones, variety are known to affect the synthesis of ADM
(16). Compared with control
subjects, it has been found that the plasma levels of ADM are
increased in patients with hypertension, heart failure or
arteriosclerosis (17,18). Results from genetic manipulation and
prolonged low-dose infusion experiments have indicated that ADM
acts to suppress increases in blood pressure and limit the
progression of hypertensive organ damage through its protective
effects on cardiovascular tissues (19,20). ANP
and BNP exert similar cardiovascular effects to ADM, including
natriuresis, diuresis and vasodilatation (21).
In the present study, consistent with a previous
investigation by Kohno et al (22), the MAP, BUN and Scr levels were
elevated in patients with EH and increased with the severity of the
hypertension stage; by contrast, GFR exhibited the opposite
changes. The elevated MAP can be simply explained, and the changes
in the BUN and Scr levels and GFR may have resulted from the renal
damage caused by the hypertension, with more severe changes
occurring with a higher disease stage.
It has been demonstrated that ADM has a broad
spectrum of biological actions, including potent vasodilatation,
natriuresis, inhibition of renin and aldosterone secretion, and
inhibition of vascular smooth muscle cell proliferation and
migration (23). ANP and BNP, which
are predominantly secreted from the atrium and ventricle in the
heart, respectively, function as hormones acting against a further
elevation in BP in hypertensive patients through their natriuretic
and vasodilatory effects (24).
In the present study, the plasma concentrations of
ADM, ANP and BNP increased with increasing disease severity in the
patients with EH, a finding consistent with that in a previous
study by Kato et al (5). It
appears, therefore, that high BP is an important factor involved in
the observed elevation of the three peptides in the plasma; as
previously mentioned, the renal damage was more serious with higher
BP. The metabolism of ADM primarily occurs in the kidney, and ADM
concentration was positively correlated with the BUN and Scr levels
but negatively correlated with the GFR in three groups of
hypertensive patients. It is possible, therefore, that the reduced
renal clearance of peptides accounted for the elevations in the
levels of these bioactive peptides in patients with EH. This was
further confirmed by the fact that the plasma ADM, ANP and BNP
concentrations of the hypertensive patients with renal dysfunction
were significantly higher than the values of the control subjects
and hypertensive subjects without renal dysfunction. Furthermore,
the plasma ADM level was strongly correlated with the level of ANP
and BNP. It can be inferred that ADM, along with ANP and BNP, may
be involved in the mechanisms counteracting a further elevation in
BP due to their similar physiological roles, and that this is a
protective and compensatory mechanism in the cardiovascular system.
This theory was supported by the association between ADM and MAP
and higher plasma ADM, ANP and BNP levels in hypertensive patients
without renal dysfunction compared with control subjects. In
addition, it was noted that the plasma levels of ADM, ANP and BNP
were significantly, but not sharply, decreased due to the
improvement in MAP and renal function following antihypertensive
treatment for 4 weeks; therefore, the delivery of ADM, ANP and BNP
to the tissue either through exogenous administration or the
augmentation of endogenous production should be considered as a
potential therapeutic strategy for a number of disorders,
particularly hypertension. It was reported by Kato et al
(5), however, that although the
plasma ADM level was significantly decreased following urgent
antihypertensive treatment in malignant hypertensive patients, the
values of ANP and BNP were not. In addition, Kohno et al
(22) found that the plasma ADM and
Scr levels were not significantly changed following
antihypertensive treatment for 4 weeks. The most likely
explanations may be that i) a number of elderly hypertensive
patients, whose renal function may not have easily improved if
impaired by hypertension, were included in the study by Kohno et
al (22); ii) the duration of
the hypertension was different; and iii) the statistical analysis
was different.
Numerous studies have found increased plasma ADM,
ANP and BNP levels in patients with EH or secondary hypertension
(21,25); however, there are few reports on the
concentrations of these values in cases of hypertension that has
been classified into stages. The present study indicated that the
correlation between the ADM and BUN levels became stronger as the
severity of the hypertension increased. Similar correlations
between ADM and Scr levels and between the ADM level and GFR were
observed. It can be inferred that the ADM level is associated with
renal function and that the lower renal clearance of ADM results
from the increasingly serious renal damage caused by
hypertension.
With regard to the physiological roles of ADM, ANP
and BNP, the results of the present study suggest that ADM may be
involved, along with ANP and BNP, in mechanisms counteracting a
further elevation in BP and may be useful biomarkers for the
diagnosis and treatment of patients with EH. Furthermore, the
plasma concentrations of ADM, ANP and BNP are strongly associated
with renal function. Further studies are necessary to clarify the
specific physiological significance of ADM, ANP and BNP in EH and
to elucidate the exact pharmacokinetics of these peptides in
hypertensive patients with renal functional damage.
Acknowledgements
This study was supported by grants from the National
Science Fund Project of China (no. 81200501) and the Doctor
Research Fund Project of Wuhan University of China (no.
2012302020203). The authors would like to thank the Department of
Urology and Cardiology in Renmin Hospital of Wuhan University.
References
|
1
|
Kitamura K, Kangawa K, Kawamoto M, et al:
Adrenomedullin: A novel hypotensive peptide isolated from human
pheochromocytoma. Biochem Biophys Res Commun. 192:553–560. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hay DL, Walker CS and Poyner DR:
Adrenomedullin and calcitonin gene-related peptide receptors in
endocrine-related cancers: Opportunities and challenges. Endocr
Relat Cancer. 18:C1–C14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beltowski J and Jamroz A: Adrenomedullin -
what do we know 10 years since its discovery? Pol J Pharmacol.
56:5–27. 2004.PubMed/NCBI
|
|
4
|
Eto T: A review of the biological
properties and clinical implications of adrenomedullin and
proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and
vasodilating peptides. Peptides. 22:1693–1711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato J, Kitamura K, Matsui E, et al:
Plasma adrenomedullin and natriuretic peptides in patients with
essential or malignant hypertension. Hypertens Res. 22:61–65. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishimitsu T, Ono H, Minami J and Matsuoka
H: Pathophysiologic and therapeutic implications of adrenomedullin
in cardiovascular disorders. Pharmacol Ther. 111:909–927. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Jiang C, Wang X, Zhang Y, Shibahara
S and Takahashi K: Adrenomedullin is a novel adipokine:
Adrenomedullin in adipocytes and adipose tissues. Peptides.
28:1129–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lainchbury JG, Troughton RW, Lewis LK,
Yandle TG, Richards AM and Nicholls MG: Hemodynamic, hormonal and
renal effects of short-term adrenomedullin infusion in healthy
volunteers. J Clin Endocrinol Metab. 85:1016–1020. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szokodi I, Kinnunen P and Ruskoaho H:
Inotropic effect of adrenomedullin in the isolated perfused rat
heart. Acta Physiol Scand. 156:151–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Federico C: Natriuretic Peptide system and
cardiovascular disease. Heart Views. 11:10–15. 2010.PubMed/NCBI
|
|
11
|
Kitamura K and Eto T: Adrenomedullin -
physiological regulator of the cardiovascular system or biochemical
curiosity? Curr Opin Nephrol Hypertens. 6:80–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herget-Rosenthal S, Bökenkamp A and
Hofmann W: How to estimate GFR-serum creatinine, serum cystatin C
or equations? Clin Biochem. 40:153–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whitworth JA; World Health Organiztion, ;
International Society of Hypertension Writing Group, : 2003 World
Health Organization (WHO)/International Society of Hypertension
(ISH) statement on management of hypertension. J Hypertens.
21:1983–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitamura K, Ichiki Y, Tanaka M, et al:
Immunoreactive adrenomedullin in human plasma. FEBS Lett.
341:288–290. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Zhou Y, Meng L, Lu X, Ou N and Li
X: Inflammatory mediators in Chinese patients with congestive heart
failure. J Clin Pharmacol. 49:591–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheung BM and Tang F: Adrenomedullin:
Exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov.
6:4–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki Y, Horio T, Hayashi T, et al:
Plasma adrenomedullin concentration is increased in patients with
peripheral arterial occlusive disease associated with vascular
inflammation. Regul Pept. 118:99–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato J, Kitamura K and Eto T: Plasma
adrenomedullin level and development of hypertension. J Hum
Hypertens. 20:566–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato J, Tsuruda T, Kita T, Kitamura K and
Eto T: Adrenomedullin: A protective factor for blood vessels.
Arterioscler Thromb Vasc Biol. 25:2480–2487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuruda T, Kato J, Hatakeyama K, et al:
Antifibrotic effect of adrenomedullin on coronary adventitia in
angiotensin II-induced hypertensive rats. Cardiovasc Res.
65:921–929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irzmański R, Pawlicki L, Charłusz M and
Kowalski J: Concentration of natriuretic peptides in patients
suffering from idiopathic arterial hypertension and left
ventricular diastolic dysfunction confirmed by echocardiography.
Clin Exp Hypertens. 34:530–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kohno M, Hanehira T, Kano H, et al: Plasma
adrenomedullin concentrations in essential hypertension.
Hypertension. 27:102–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong DG, Gao H, Lu YQ, et al: Anxiety
disorders are associated with increased plasma adrenomedullin level
and left ventricular hypertrophy in patients with hypertension.
Clin Exp Hypertens. 36:27–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grzywa-Celińska A, Celiński R, Kwaśniewska
K, Dyczko M, Prystupa A and Mosiewicz J: The usefulness of
natriuretic peptides measurements in the diagnostics of chosen
cardiovascular diseases. Pol Merkur Lekarski. 34:232–234.
2013.PubMed/NCBI
|
|
25
|
Halawa B: Plasma adrenomedullin
concentration in patients with essential hypertension. Pol Merkur
Lekarski. 5:111–113. 1998.PubMed/NCBI
|