Introduction
Hepatoid adenocarcinoma (HAC) is a type of gastric
cancer with adenoid and hepatocyte differentiation. In 1985,
Ishikura et al (1) first
presented and stated that HAC was a cancer with hepatocyte
characteristics: hepatoid adenocarcinoma of the stomach (HAS). The
levels of extrahepatic α-fetoprotein (AFP) in the serum and tumor
tissue are high in this form of gastric cancer, similarly to
hepatocellular carcinoma (HCC). Since the study by Ishikura et
al, there have been subsequent studies published on the
clinical manifestation and pathological features of HAC (2–4). This
tumor type has poor prognosis and therefore accurate diagnosis of
HAC is extremely important. The first description of HAC was in the
stomach, which remains the most common tumor location; however, HAC
has been reported to develop in a variety of organs including the
gallbladder, lungs, bladder, esophagus, pancreas, peritoneum,
jejunum, colon, rectum, renal pelvis, ureter, ovaries, uterus and
papilla of Vater (5–8). HAS is a relatively rare gastric
carcinoma and has an extremely poor diagnosis. Only a few cases
have been previously reported. The present case report describes a
patient who experienced acid regurgitation, belching, nausea and
occasional vomiting of acid/water, which was more notable on an
empty stomach. No diarrhea or abdominal distension was observed but
the patient's stools were black.
Case report
A 47-year-old male presented with repetitive upper
abdominal ache, without obvious inducement, that had lasted three
and a half months. The ache was accompanied with acid regurgitation
and belching, which was more notable on an empty stomach. The
patient had nausea and occasional acid vomiting but no diarrhea or
abdominal distension. The patient's stools were black. Following
gastroscopy and pathological examination, the patient was diagnosed
with gastric signet-ring cell carcinoma and a total gastrectomy was
performed on the operable lesion. Abdominal ultrasonography showed
normal results in the liver, gallbladder, pancreas, spleen and
kidney, and CEA levels were normal. The patient did not have lymph
node metastasis or other organ metastasis. Thus, 2 weeks following
surgery the patient left hospital without undergoing chemotherapy
or radical therapy. He is currently being followed up for 12
months. Informed consent was obtained from the patient and the
patient's family prior to participation in the present study.
Pathological examination
Naked eye
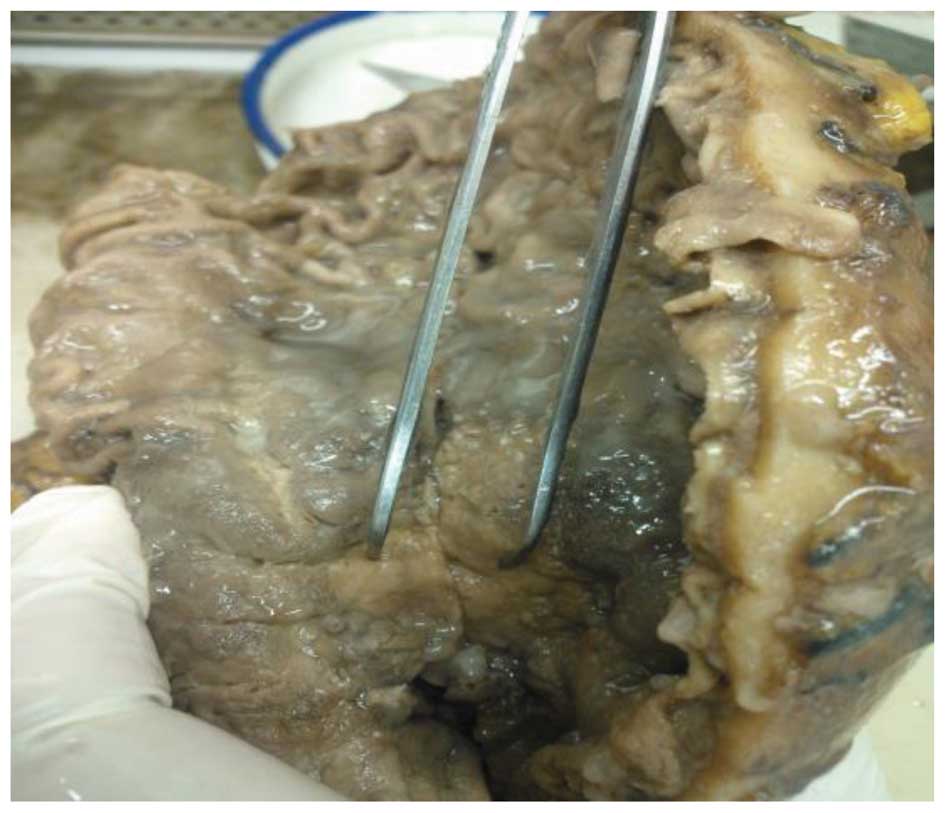
The whole stomach was inspected: The greater gastric
curvature measured 26 cm and the lesser gastric curvature measured
14 cm. A 2.3 × 2.2 × 2.0-cm nodule was identified on the serosal
surface. On incision along the greater curvature side, a 4.5 × 3.5
× 0.4 cm ulcerative neoplasm was found 4.5 cm from the pylorus and
6.0 cm from the gastric cardia, on the lesser gastric curvature
side (Fig. 1). Following incision
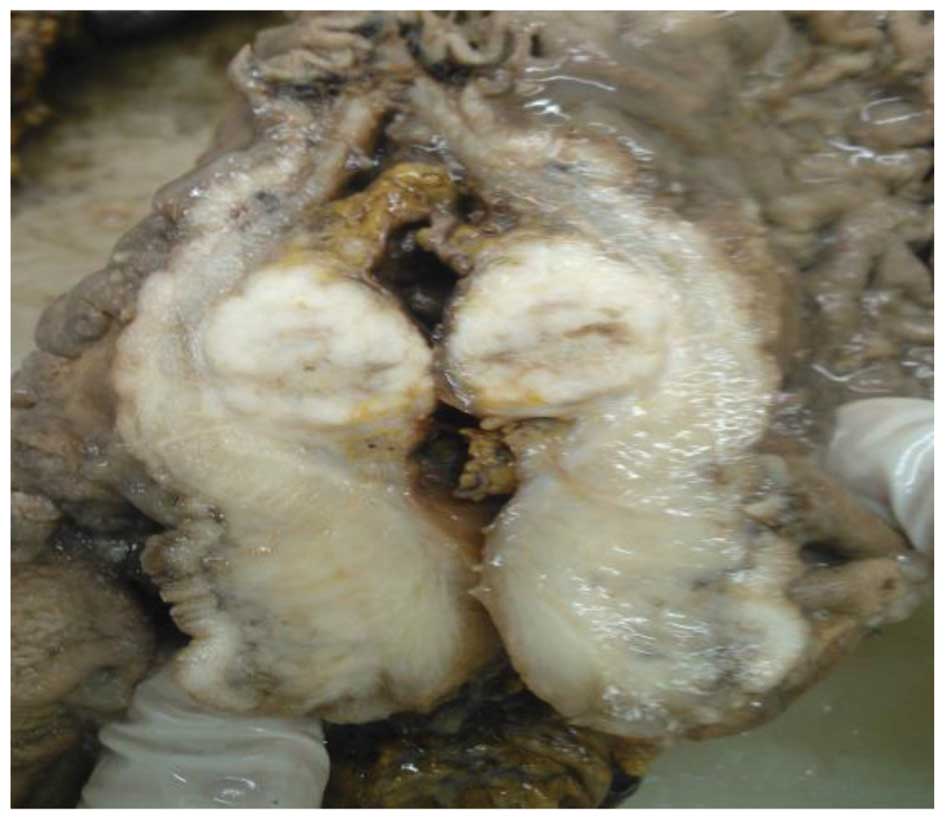
along the ulcerative neoplasm, it was observed that the section was
brown and gray, hard and had invaded the shallow muscularis. An
isolated circular nodule, which was 2.0 cm in diameter, gray and
hard, with clear boundaries (Fig.
2).
Microscopic evaluation
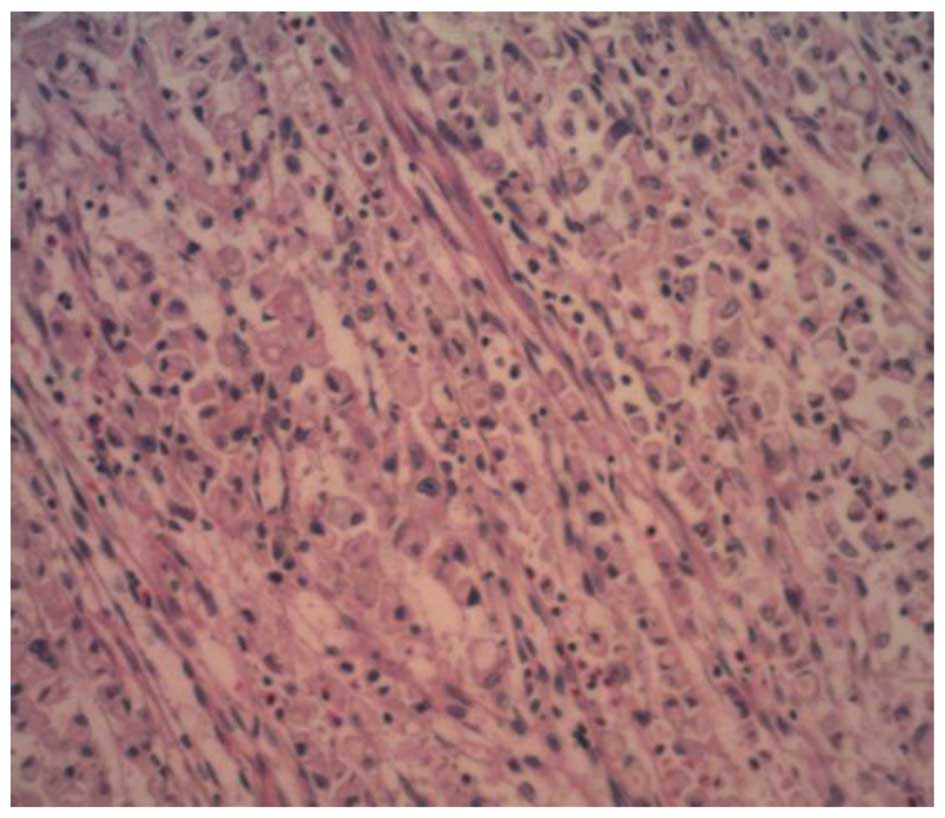
The mucosa of the neoplasm showed features
consistent with signet-ring cell carcinoma (Fig. 3); however, large cells arranged in a
solid, clumped shape were observed on the serosa of the neoplasm.
The nuclei were large and centrally located. Nucleolus and nuclear
fission were observed. The cytoplasm was abundant, acidophilic and
transparent. A small amount of fibrous tissue, capillaries and a
Periodic-Acid Schiff (PAS)-positive corpuscle with necrosis were
also observed (Fig. 4).
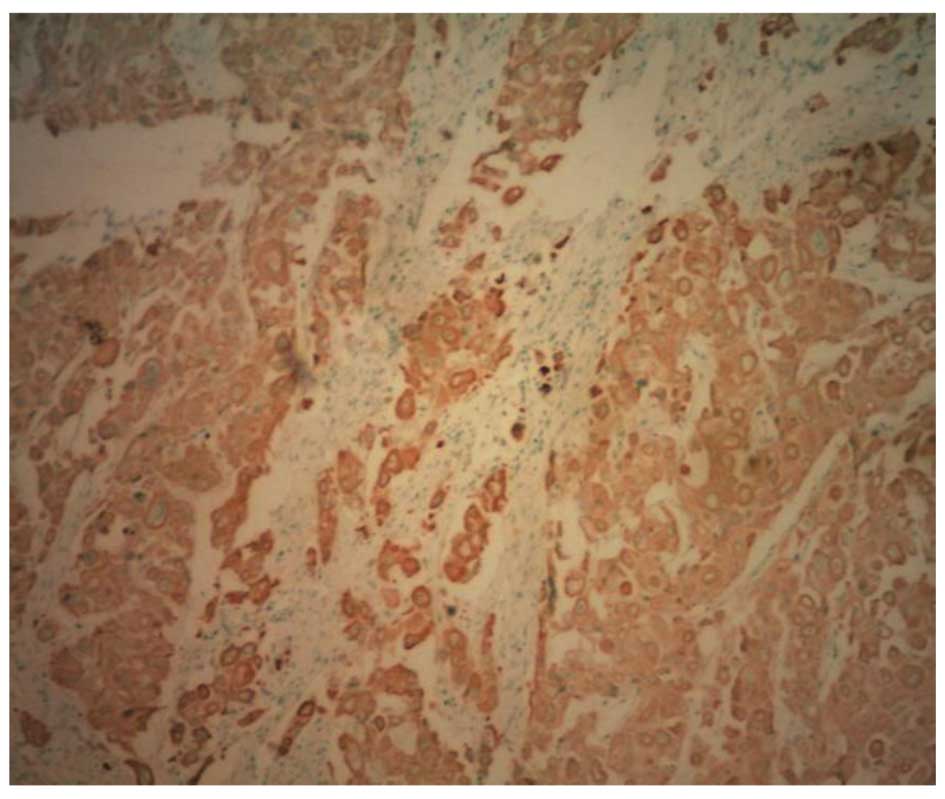
Immunohistochemistry (IHC)
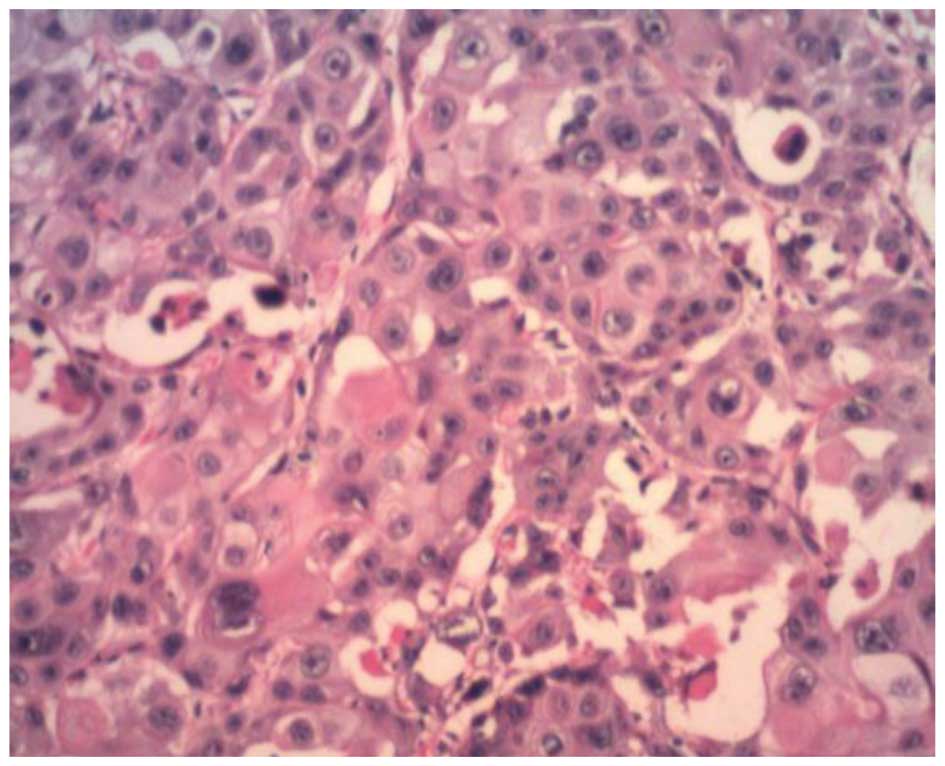
Positive results for cytokeratin low/high molecular
weight (AE1/AE3), Cam5.2, cytokeratin 7, p53, epithelial membrane
antigen (EMA) and carcinoembryonic antigen (CEA) were found on both
sides of the neoplasm. Staining for AFP was strongly positive in
the neoplasm at the serosa (Fig. 5).
Tests for neuroendocrine markers (synaptophysin, chromogranin A and
cluster of differentiation 56), mesothelioma markers (calretinin,
D240 and mesothelial cells), markers of the gastric choriocarcinoma
(β-human chorionic gonadotrophin and inhibin) and a marker of
squamous cell carcinoma (p63) were negative.
Pathological diagnosis
The patient was diagnosed with gastric
adenocarcinoma combined with HAC and signet-ring cell carcinoma
differentiation. The signet-ring cell carcinoma invaded the middle
muscularis. Three out of the 19 lymph nodes on the greater gastric
curvature were identified as metastases and one of the 10 lymph
nodes on the lesser gastric curvature was a metastasis. All
metastases were signet-ring cell carcinoma.
Discussion
HAC is a type of gastric cancer with adenoid and
hepatocyte differentiation. In 1985, Ishikura et al
(1) first presented and stated
unequivocally that HAC was a cancer with hepatocyte
characteristics. Levels of AFP in the serum and tumor tissue are
significantly high in this form of gastric cancer; however, HAC is
rare, accounting for 1.3–15% of gastric cancers (9). Although there have been some previous
reports about this disease (10,11),
there remains a lack of understanding. HAC is discussed here with
reference to the present case and published literature.
HAC, an aggressive and highly malignant tumor, is a
type of adenocarcinoma with the structural and cytological
characteristics of HCC that occurs in extrahepatic organs or
tissues. In cases of HAC, high levels of AFP can be detected in the
serum and tumor tissue. HAC usually occurs in the stomach; however,
the tumors may also be observed in the duodenum, colon, rectum,
esophagus, gallbladder, pancreas, lung, bladder, kidney, ureter,
ovaries, uterus, retroperitoneum and local soft tissue (12). HAS occurs more frequently than the
other types of HAC, generally occurs in middle-aged patients and is
more commonly found in males than females. It is likely that it
originates in the gastric antrum and is associated with
infiltration and ulceration. The tumor locates itself in the
submucosa and infiltrates to the muscularis layer. HAS generally
has no specific clinical symptoms, and the digestive tract
symptoms, such as abdominal pain, abdominal distension and black
stools, are often apparent in the middle and late periods of the
disease. HAC is frequently associated with an elevated serum AFP
and positive staining for AFP in IHC tests. Postoperative serum AFP
levels may be used as a predictive index for tumor recurrence or
metastasis (13). HAC is
additionally prone to spread to the liver and lymph nodes, since it
is similar to liver cancer cells in histology. A thrombus is easily
formed and can invade the vascular system early on due to a rich
blood supply. In certain studies, the blood and lymphatic vessel
invasion by HAS and the level of surrounding lymph node metastasis
have been observed to be evidently higher than those associated
with a less-differentiated gastric adenocarcinoma (14,15);
however, in the present case, the lymph node metastasis was
signet-ring cell carcinoma rather than HAC, which was not in
conformity with the literature.
Pathologically, HAC comprises primary tumors in the
gastric mucosa gland. The tumor cells generally form two different
but closely associated areas: Adenocarcinoma and hepatocellular
cancer. The former is a poorly differentiated, visible tubular and
papillary adenocarcinoma (5,16). The latter exhibits HCC
differentiation. The pathology and IHC of HAC show numerous
features. Under a microscope, the cancer cells are large with
abundant cytoplasm, and the nuclei are round or ovoid. The
nucleolus and mitotic spindle can be observed and the cytoplasm
exhibits pale eosin staining (17).
A small number of cancer cells (transparent and acidophilic
polygonal cells) form the medullary or cable structure. These cells
are separated by a few fibrous tissues and a rich blood supply, and
exhibit transitional or overlapping processes. Visible eosinophilic
hyaline PAS-positive corpuscles lie between the cells. The cancer
cells may show different degrees of liver cell differentiation,
characterized by fatty degeneration or the secretion of bile. Under
the electron microscope, a capillary bile duct structure may be
observed between tumor cells. The two areas of HAC both exhibit
intestinal epithelial microvilli, which come from the
gastrointestinal epithelium. In IHC, tests for AFP are strongly
positive or positive, tests for CEA may be positive or negative and
tests for α1-antitrypsin and α1-antichymotrypsin are positive in
the liver cancer region. AFP expression is strongly positive on the
serosal surface. According to the results of AFP IHC staining, HAC
can be divided into AFP-positive and AFP-negative types (18); however based on the characteristics
of structural organization, HAC can be divided into simple (only
the structure of liver cancer) and mixed (adenocarcinoma and HCC)
types. In the current case, the HAC was an AFP-positive, mixed
type.
It is important to monitor the increase in serum AFP
level and the expression of AFP in tumor tissues when studying HAC.
Patients with HAC have higher serum AFP levels. It is generally
believed that this may be due the following factors: i) The
liver-differentiated area of the HAC produces AFP; ii) the gastric
cancer itself produces AFP instead of the liver-differentiated
area; iii) the gastric cancer metastasizes to the liver and AFP is
then produced by the new or proliferating hepatocytes (19). Inagawa et al (2) reported that the serum AFP level was
associated with the differentiation degree, and Chang et al
(20) found that a higher serum AFP
level resulted in a poorer prognosis than that for AFP-negative
gastric cancer. Nagai et al (21) suggested that patients with HAC with a
high or normal AFP had the same prognosis.
Gastric cancer is complex and highly heterogeneous.
There have been few studies regarding the differences between the
signet-ring cell carcinoma and HAC. Akiyama et al (22) found that tubular adenocarcinoma and
HAC had the same origin following the technical application of
molecular pathology. Both carcinomas had X chromosome and p53 loss;
therefore we hypothesize that they have the same origin. Tumors
from the same origin can have different biological behavior,
morphology and IHC profiles, which fully reflect the heterogeneous
characteristics of tumor growth. This feature makes the treatment
of such tumors difficult, due to their particularly complex
biological behavior and poor prognosis.
In summary, the present study described an unusual
case of HAC which had a morphological similarity to HCC. On initial
observation, the elevation in the level of AFP was caused by HCC;
however, the second spike in the level of AFP was due to HAC. Thus,
a differential diagnosis of HAC should be considered in cases
involving the elevation of the levels of serum AFP, even in
patients at a high risk of HCC.
References
|
1
|
Ishikura H, Fukasawa Y, Ogasawara K, et
al: An AFP-producing gastric carcinoma with features of hepatic
differentiation. A case report. Cancer. 56:840–848. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inagawa S, Shimazaki J, Hori M, et al:
Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 4:43–52.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Cheng Y, Sheng W, et al: Analysis
of clinicopathologic features and prognostic factors in hepatoid
adenocarcinoma of the stomach. Am J Surg Pathol. 34:1465–1471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye MF, Tao F, Liu F and Sun AJ: Hepatoid
adenocarcinoma of the stomach: a report of three cases. World J
Gastroenterol. 19:4437–4442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su JS, Chen YT, Wang RC, et al:
Clinicopathological characteristics in the differential diagnosis
of hepatoid adenocarcinoma: a literature review. World J
Gastroenterol. 19:321–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yano T, Ishikura H, Wada T, et al:
Hepatoid adenocarcinoma of the pancreas. Histopathology. 35:90–92.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikura H, Ishiguro T, Enatsu C, et al:
Hepatoid adenocarcinoma of the renal pelvis producing
alpha-fetoprotein of hepatic type and bile pigment. Cancer.
67:3051–3056. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gardiner GW, Lajoie G and Keith R:
Hepatoid adenocarcinoma of the papilla of Vater. Histopathology.
20:541–544. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang YC, Nagasue N, Kohno H, et al:
Clincicopathologic features and long-term results of
alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol.
85:1480–1485. 1990.PubMed/NCBI
|
|
10
|
Baek SK, Han SW, Oh DY, et al:
Clinicopathologic characteristics and treatment outcomes of
hepatoid adenocarcinoma of thestomach, a rare but unique subtype of
gastric cancer. BMC Gastroenterol. 11:562011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giuffrè G, Ieni A, Barresi V, Caruso RA
and Tuccari G: HER2 status in unusual histological variants of
gastric adenocarcinomas. J Clin Pathol. 65:237–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rotellini M, Messerini L, Stomaci N and
Raspollini MR: Hepatoid adenocarcinoma of the ureter: unusual case
presenting hepatic and ovarian metastases. Appl Immunohistochem Mol
Morphol. 19:478–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
deLorimier A, Park F, Aranha GV and Reyes
C: Hepatoid carcinoma of the stomach. Cancer. 71:293–296. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishikura H, Kishimoto T, Andachi H, et al:
Gastrointestinal hepatoid adenocarcinoma: venous permeation and
mimicry of hepatocellular carcinoma, a report of four cases.
Histopathology. 31:47–54. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang GH and Kim YI:
Alpha-fetoprotein-producing gastric carcinoma presenting focal
hepatoid differentiation in metastatic lymph nodes. Virchows Arch.
432:85–87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jalle T, Gérard C, Lada PE, et al:
Hepatoid adenocarcinoma of the stomach. A case report. Ann Chir.
131:213–215. 2006.[(In French)]. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cappetta A, Bergamo F, Mescoli C, Lonardi
S, Rugge M and Zagonel V: Hepatoid adenocarcinoma of the colon:
what should we target? Pathol Oncol Res. 18:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikura H, Kirimoto K, Shamoto M, et al:
Hepatoid adenocarcinoma of the stomach. An analysis of seven cases.
Cancer. 58:119–126. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rassidakis GZ, Delladetsima JK, Letsos SP,
et al: Hepatoid adenocarcinoma of the stomach with extensive
neuroendocrine differentiation and a coexisting carcinoid tumour.
Histopathology. 33:186–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang YC, Nagasue N, Abe S, et al:
Comparison between the clinicopathologic features of AFP-positive
and AFP-negative gastric cancers. Am J Gastroenterol. 87:321–325.
1992.PubMed/NCBI
|
|
21
|
Nagai E, Ueyama T, Yao T and Tsuneyoshi M:
Hepatoid adenocarcinoma of the stomach: A clinicopathologic and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akiyama S, Tamura G, Endoh Y, et al:
Histogenesis of hepatoid adenocarcinoma of the stomach: molecular
evidence of identical origin with coexistent tubular
adenocarcinoma. Int J Cancer. 106:510–515. 2003. View Article : Google Scholar : PubMed/NCBI
|