Introduction
Lung cancer is a common clinical malignancy. In
recent years, the incidence rate of the disease has shown a marked
increasing trend, and the condition has become a major threat to
human health (1). Approximately 85%
of patients with lung cancer suffer from non-small cell lung
cancer; therefore, the treatment of non-small cell lung cancer has
become a particular research focus (2,3).
Chemotherapy, including gemcitabine plus cisplatin (GP) and
paclitaxel plus cisplatin regimens, is the most commonly used
treatment for non-small cell lung cancer (4,5);
however, the prognosis of patients receiving pure chemotherapy is
worse compared with the prognosis following a combined chemotherapy
regimen such as GP. In recent years, with the development of
molecular targeted therapy, the combined utilization of
antineoplastic therapy with chemotherapy, taking tumor vessels as
the main treatment target, has had a significant effect in the
clinic (6,7). Angiogenesis is the process of new blood
vessel formation, which is an essential process during wound
healing. In addition, angiogenesis is regulated by the extremely
sensitive interaction of certain growth factors and inhibitors.
Avastin®, a recombinant humanized monoclonal antibody, is the first
inhibitor of tumor angiogenesis to be approved in America. Several
clinical studies have shown that Avastin exhibits good efficacy in
the treatment of non-small cell lung cancer (8,9). Our
clinical experience has indicated that the curative effect of a
combinatorial Avastin and GP treatment regimen is superior to the
separate curative effects of the drugs; however, the mechanism of
this phenomenon is not fully understood. The aim of the present
study, therefore, was to analyze the mechanism of Avastin combined
with a GP regimen in the treatment of non-small cell lung cancer in
an animal model, in order to provide a theoretical basis for
clinical practice.
Materials and methods
Model construction and drug
treatment
Balb/c nude mice were purchased from the Beijing
Academy of Military Medical Sciences (Beijing, China). The mice
were 7 weeks old, weighed 19–20 g, and were raised in a specific
pathogen free animal house. This study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of the Second Affiliated Hospital of
Zhengzhou University (Zhengzhou, China).
A549 cells (The Type Culture Collection of the
Chinese Academy of Sciences, Beijing, China) were grown in
Dulbecco's modified Eagle's medium with 10% fetal bovine serum
(Invitrogen Life Technologies, Carlsbad, CA, USA). Subsequent to
reaching the exponential growth phase, the cells were digested
using trypsin (Gibco-BRL, Grand Island, NY, USA) and washed twice
with phosphate-buffered saline (PBS). The A549 cells were then
resuspended in PBS buffer solution and the cell concentration was
adjusted to 2×107/ml. Following the resuspension and
concentration adjustment, the cells (2×106/100 µl) were
inoculated in the fat pad close the armpit in the left rib of each
mouse. The tumors were left to grow for 7–9 days to reach a volume
of 100–150 mm3, and the tumor-bearing nude mice were
then randomly divided into three groups: Avastin, chemotherapy and
combined treatment (n=10/group). The mice in the Avastin group were
administered an intraperitoneal injection of 5 mg/kg Avastin
(Roche, Basel, Switzerland) every other day, 10 times in total. The
mice in the chemotherapy group were administered an intraperitoneal
injection of 4 mg/kg gemcitabine and 4 mg/kg cisplatin (Jiangsu
Hansoh Pharmaceutical Inc., Lianyungang, China) every other day, 10
times in total. The mice in the combined treatment group were
administered an intraperitoneal injection of 5 mg/kg Avastin and
then, on the next day, an intraperitoneal injection of GP. The
treatments were administered 10 times in total. During the course
of each treatment, the tumor volume and weight were measured every
two days, and the mortality rates of the mice were recorded.
Analysis of the level of vascular
endothelial growth factor (VEGF) in the tumor tissue
The mice in the three groups were sacrificed
following treatment for 10 days. A total of 0.1 g tumor tissue was
obtained for tissue homogenization and cell lysis. This sample was
then stood on ice and, after 30 min, centrifuged at 15,000 × g for
10 min. The supernatant was transferred to a new centrifuge tube
and the concentration of total protein was determined. The
expression level of VEGF in the tumor tissue was subsequently
analyzed in accordance with the ELISA kit instructions (R&D
Systems Inc., Minneapolis, MN, USA).
Analysis of tumor vessel density
Tumor tissue was embedded in paraffin and sliced
into 6-µm sections. The sections were stuck on the slides. Each
slide was dewaxed and washed with PBS three times, followed by
treatment with a hydrogen peroxide scavenger, 3% catalase, and
sodium citrate for antigen retrieval. The sample was subsequently
blocked for 30 min using 1% goat serum (Gibco-BRL) at room
temperature and excess liquid was sucked away. The monoclonal rat
anti-mouse cluster of differentiation 31 (CD31) antibody (1:200;
#MAB3420; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was
dripped onto the surface of the slide and incubated at 4°C
overnight. The next day, the sample was placed at room temperature
for 30 min and then washed with PBS three times (5 min/wash). The
diluted secondary antibody (Santa Cruz Biotechnology, Inc.) was
dripped onto the slide, incubated for 1 h at room temperature,
washed three times with PBS and colored using 3,3′-diaminobenzidine
for 5–10 min. The staining was observed under the microscope. The
slides were rinsed with tap water for 5 min. Hematoxylin was used
to the stain the nuclei and hydrochloric acid alcohol was used for
differentiation. The samples were then dehydrated and clarified in
75, 80, 90, 95 and 100% ethanol and xylene successively. Finally,
the slides were sealed with neutral resin. The vessel density was
observed under an optical microscope (Leica Microsystems,
Heidelberg, Germany).
Analysis of the morphological
structure of tumor vessels
The mice in the three groups were sacrificed
following 10 days of treatment. The tumor tissues were sliced into
blocks measuring 3×3 µm and placed in 3% glutaraldehyde to fix for
>2 h. Each sample was then washed with 0.1 mol/l phosphate
buffer solution three times (5 min/wash) and dehydrated with a
graded tert-butyl alcohol series (50, 70, 80, 90, 95 and 100%) for
15 min each time. The sample was subsequently dried using the
critical drying method. The dry sample was attached to the slides
using a conductive adhesive in preparation for the metal plating of
the sample. The vascular morphology was directly observed under a
scanning electron microscope (Agilent Technologies, Beijing,
China).
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS Inc., Chicago, IL, USA). The measurement data are presented
as the mean ± standard error. The χ2 test was used to
compare the count data. Multiple groups of measurement data were
compared using analysis of variance. Paired comparisons between two
groups were performed using the Least Significant Difference
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
Combined treatment significantly
inhibits tumor growth and improves the survival rate of mice
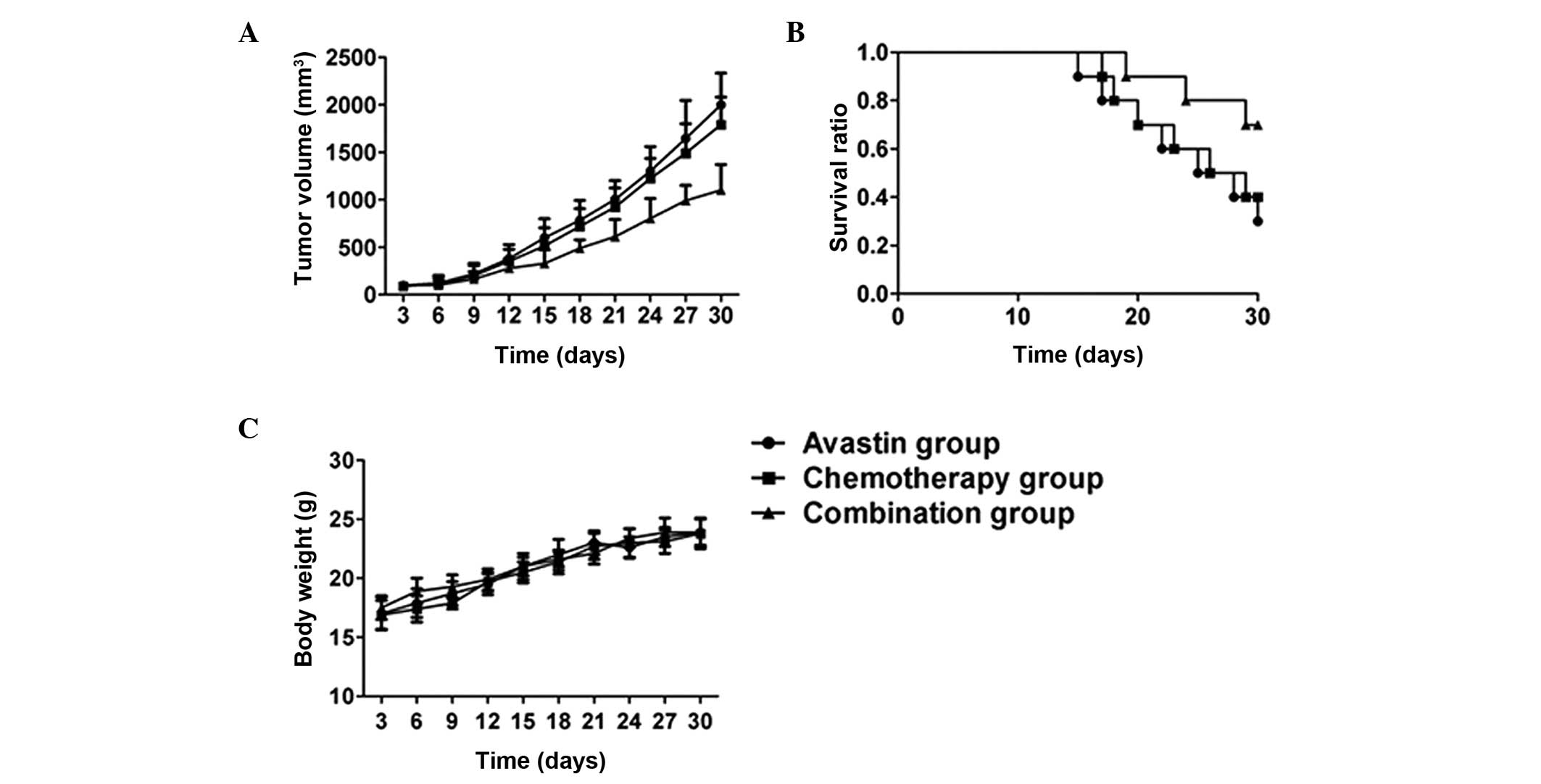
As shown in Fig. 1,
no statistical difference was observed in the tumor growth curve
between the Avastin and chemotherapy groups (P>0.05). The tumor
growth curve in the combined treatment group was significantly
lower than that in the Avastin and chemotherapy groups (P<0.05).
The survival rate of the mice in the combined treatment group was
significantly higher than that of the mice in the Avastin and
chemotherapy groups (P<0.05). No significant difference in mouse
bodyweight was found among the three groups during the treatment
(P>0.05).
Avastin reduces the level of VEGF in
tumor tissue
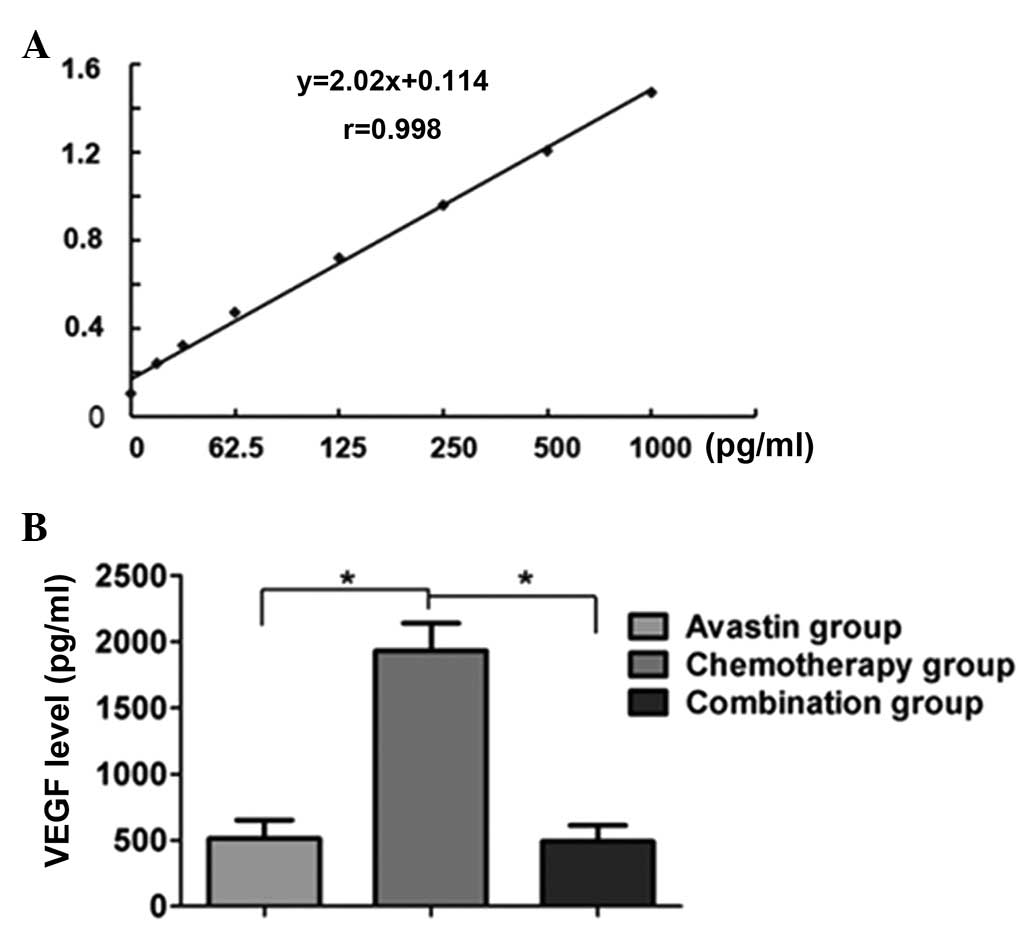
The standard curve of VEGF was established according
to the instructions of the ELISA kit (R&D Systems Inc.). The
standard curve was observed to have good linearity (r=0.998).
Comparison of the level of VEGF in the tumor tissue showed that the
levels in the Avastin and combined treatment groups were
significantly lower than those in the separate chemotherapy group
(P<0.05); however, no statistical difference in VEGF levels was
observed between the Avastin and combined treatment groups
(P>0.05) (Fig. 2).
Avastin significantly reduces the
tumor vascular density
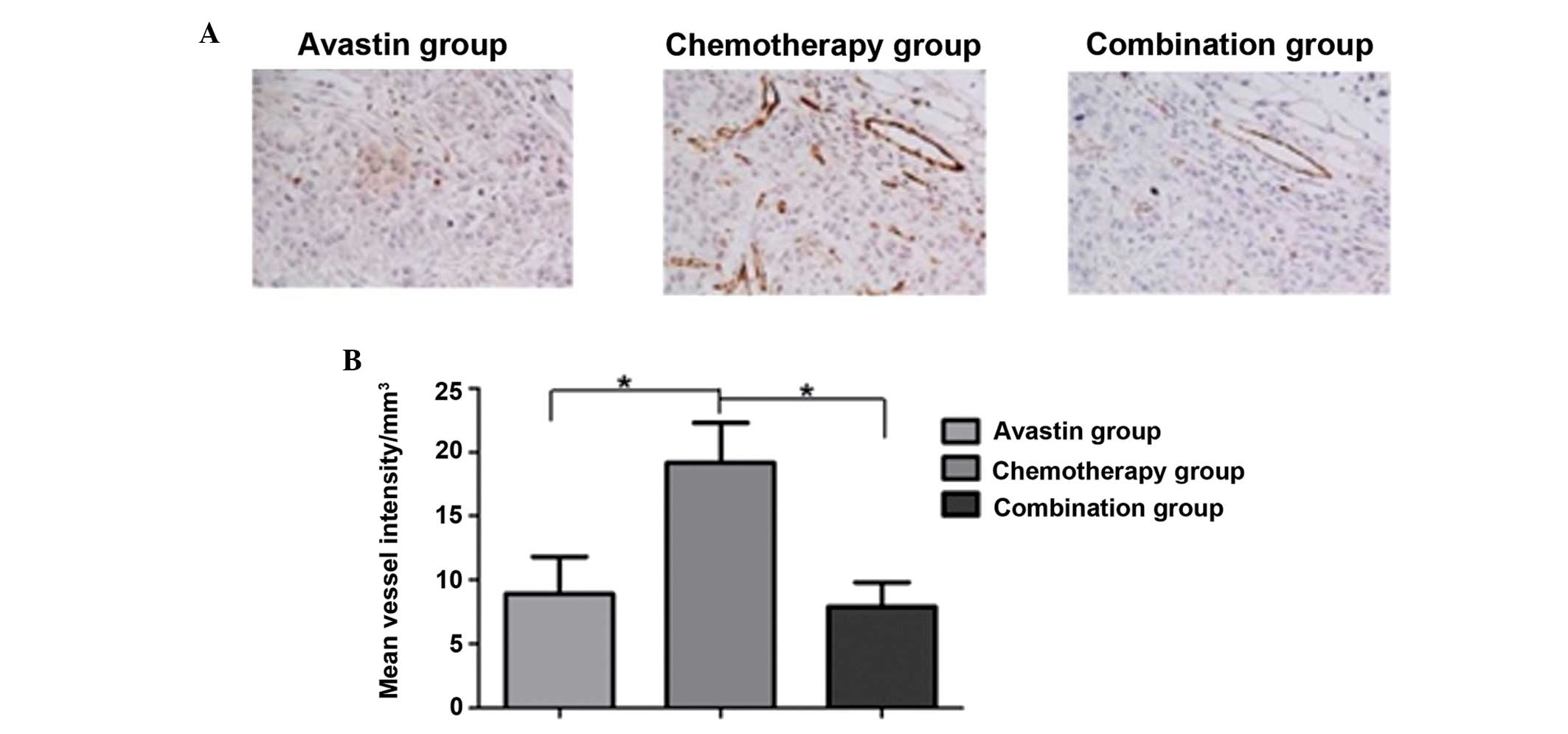
Immunohistochemical staining showed that the number
of CD31-positive vessels in the Avastin and combined treatment
groups was lower than that in the chemotherapy group; additionally,
the tumor vessel density was higher in the chemotherapy group. The
quantitative analysis showed that the number of vessels in the
Avastin and combined treatment groups was significantly lower than
that in the chemotherapy group (P<0.05); however, no statistical
difference in vessel density was observed between the Avastin and
combined treatment groups (P>0.05) (Fig. 3).
Avastin can promote the normalization
of tumor vascular structure
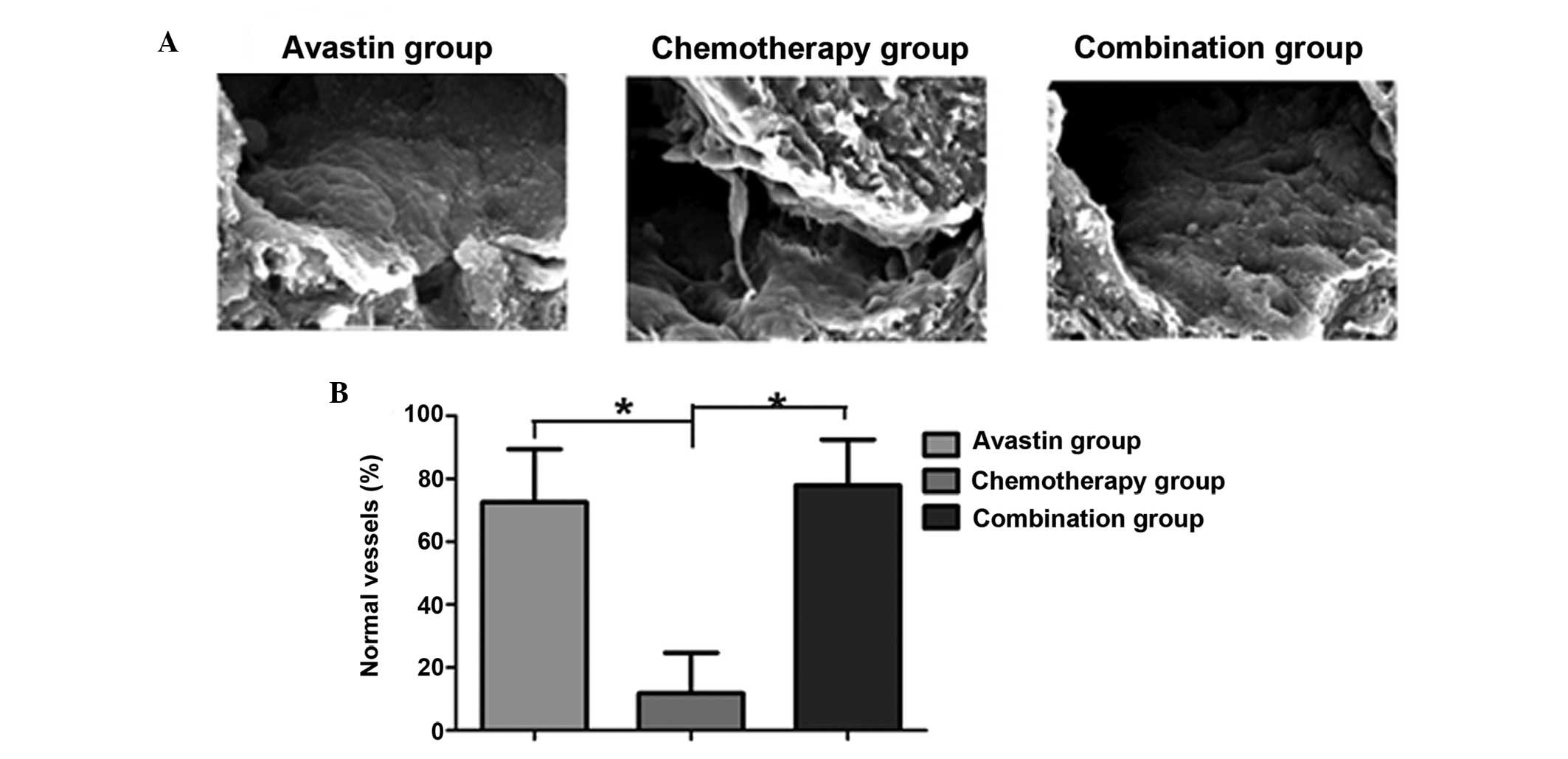
The microstructure of the tumor vessels was analyzed
through scanning electron microscopy. It was observed that the
tumor tissue in the Avastin and combined treatment groups exhibited
an integrated tumor vascular morphology; furthermore, the
endothelial cells were noted to be tightly associated with the
vessel wall. By contrast, the chemotherapy group exhibited an
irregular tumor vascular morphology, and the endothelial cells were
dissociated from the vessel wall. Quantitative analysis of the
vessels in the tumor tissue in the three groups showed that the
number of normal vessels in the tumor tissue in the Avastin and
combined treatment groups was significantly higher than that in the
chemotherapy group. The difference was statistically significant
(P<0.05) (Fig. 4).
Discussion
In 1971, Folkman (10) found that, following the growth of the
solid tumor volume to 1–2 mm3, tumor growth was
dependent on ongoing oxygen and nutrients supplied by
neovascularization. The time quantum of tumor metastasis was also
closely associated with tumor angiogenesis; therefore, tumor
vessels laid the material foundation for the tumor growth, invasion
and metastasis (10–12). Antineoplastic therapy, taking tumor
the vasculature as a target, was soon applied to clinical therapy.
VEGF is the growth factor of vascular endothelial cells and plays
an important role in mediating angiogenesis and formation (13). Effective inhibition of the activation
of the VEGF signaling pathway can significantly inhibit the
formation of angiogenesis (14).
Based on this theory, Avastin has been the first used clinical
humanized antibody drug targeting VEGF, and has shown a gratifying
effect in numerous tumors when used separately to or combined with
chemo- or radiotherapy (15).
Our clinical experience has indicated that the
curative effect of Avastin in combination with chemotherapy is
superior to that of Avastin or chemotherapy administered
separately. Furthermore, this increase in curative effect was not a
simple superposition of effect. The aim of the present study,
therefore, was to study the cause of this phenomenon using an
animal model. Similar findings to those obtained through clinical
practice were observed in the animal experiments. One explanation
is that Avastin significantly decreased the level of VEGF in the
tumor tissue and downregulated the vascular density of the tumor
tissue, while chemotherapy had a synergistic effect. As such, the
combination of Avastin and chemotherapy significantly inhibited the
growth of the tumor.
In the present study it was additionally observed
that vessels in the tumor tissue exhibited marked abnormalities,
with endothelial cell damage, exfoliation, an incomplete vascular
wall and a loss of pericytes, resulting in a fall in the tumor
vascular pressure and a retardation of blood flow. These
characteristics led to the ineffective transportation of the
chemotherapeutic drugs to the tumor tissue and reduced the
tumoricidal effect (16–18). Jain proposed a vascular normalization
theory, in which the normalization of the tumor vasculature was
considered to be beneficial to enhance the antitumor effect
(19). Based on this theory, the
vascular morphology in the tumor tissue from mice subjected to
different treatments was observed in the present study. It was
found that the tumor vascular morphology underwent marked changes
following treatment for 7 days with Avastin, and tended towards
normalization. The normalization of tumor vessels can enable
chemotherapeutic drugs to effectively reach the tumor tissue and
kill the tumor cells (20–22). This phenomenon may explain the
enhanced curative effect of the combinatorial Avastin and GP
regimen in the treatment of tumors.
In conclusion, Avastin combined with chemotherapy
has a good application prospect in the treatment of lung cancer.
The clinical effect of the combinatorial strategy is significant
and the mechanism of action is clear. The synergistic effect of the
combined treatments makes the approach worthy of clinical
application.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naim Younes R, Gross JL, Abrao FG and
Rodrigues Pereira J: Impact of adjuvant chemotherapy in completely
resected stage IIIA non-small cell lung cancer. Minerva Chir.
68:169–174. 2013.PubMed/NCBI
|
|
4
|
Reaume MN, Leighl NB, Mittmann N, et al:
Economic analysis of a randomized phase III trial of gemcitabine
plus vinorelbine compared with cisplatin plus vinorelbine or
cisplatin plus gemcitabine for advanced non-small-cell lung cancer
(Italian GEMVIN3/NCIC CTG BR14 trial). Lung Cancer. 82:115–120.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arrieta O, Michel Ortega RM,
Villanueva-Rodríguez G, et al: Association of nutritional status
and serum albumin levels with development of toxicity in patients
with advanced non-small cell lung cancer treated with
paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer.
10:502010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Falco S: Antiangiogenesis therapy: An
update after the first decade. Korean J Intern Med. 29:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dings RP, Loren M, Heun H, et al:
Scheduling of radiation with angiogenesis inhibitors anginex and
Avastin improves therapeutic outcome via vessel normalization. Clin
Cancer Res. 13:3395–3402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki H, Hirashima T, Kobayashi M, et al:
Carboplatin plus paclitaxel in combination with bevacizumab for the
treatment of adenocarcinoma with interstitial lung diseases. Mol
Clin Oncol. 1:480–482. 2013.PubMed/NCBI
|
|
9
|
Tsimberidou AM, Adamopoulos AM, Ye Y, et
al: Phase I clinical trial of bendamustine and bevacizumab for
patients with advanced cancer. J Natl Compr Canc Netw. 12:194–203.
2014.PubMed/NCBI
|
|
10
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimova I, Popivanov G and Djonov V:
Angiogenesis in cancer - general pathways and their therapeutic
implications. J BUON. 19:15–21. 2014.PubMed/NCBI
|
|
12
|
Harper J and Moses MA: Molecular
regulation of tumor angiogenesis: Mechanisms and therapeutic
implications. EXS. 96:223–268. 2006.PubMed/NCBI
|
|
13
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tunik S, Nergiz Y, Keklikci U and Akkus M:
The subconjunctival use of cetuximab and bevacizumab in inhibition
of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol.
250:1161–1167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keunen O, Johansson M, Oudin A, et al:
Anti-VEGF treatment reduces blood supply and increases tumor cell
invasion in glioblastoma. Proc Natl Acad Sci USA. 108:3749–3754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teicher BA: A systems approach to cancer
therapy. (Antioncogenics + standard cytotoxics → mechanism(s) of
interaction). Cancer Metastasis Rev. 15:247–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wouters BG and Brown JM: Cells at
intermediate oxygen levels can be more important than the ‘hypoxic
fraction’ in determining tumor response to fractionated
radiotherapy. Radiat Res. 147:541–550. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Powathil GG, Adamson DJ and Chaplain MA:
Towards predicting the response of a solid tumour to chemotherapy
and radiotherapy treatments: Clinical insights from a computational
model. PLoS Comput Biol. 9:e10031202013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batchelor TT, Sorensen AG, di Tomaso E, et
al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hormigo A, Gutin PH and Rafii S: Tracking
normalization of brain tumor vasculature by magnetic imaging and
proangiogenic biomarkers. Cancer Cell. 11:6–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sorensen AG, Batchelor TT, Zhang WT, et
al: A ‘vascular normalization index’ as potential mechanistic
biomarker to predict survival after a single dose of cediranib in
recurrent glioblastoma patients. Cancer Res. 69:5296–5300. 2009.
View Article : Google Scholar : PubMed/NCBI
|