Introduction
Osteosarcoma is a highly malignant primary tumor
occurring in the bone and associated tissues, characterized by a
bone-like tissue consisting of tumor cells (1). As the disease progresses, osteosarcoma
can progressively harm the health of the patients by causing lump
formation, organ dysfunction and fractures (2–4). In the
late stages, the majority of patients would develop metastases,
resulting in systemic failure. At present, the exact mechanism of
osteosarcoma pathogenesis is unclear. Certain studies have
suggested that osteosarcoma can be induced by other types of
cancer, as well as environmental stimuli to bone cells, such as
toxins, viruses and radiation (5–7). In
addition, disturbed hormone levels can increase the incidence of
osteosarcoma, particularly in teenagers with excess hormone
secretion (8).
A recent study found that cyclooxygenase (COX)-2
expression gradually increased in the development of osteosarcoma,
suggesting its involvement in the progression of the disease
(9). COX-2 has also been shown to
play an important role in the pathogenesis of several other
diseases, including gastric, pancreatic and bladder cancers, which
makes COX-2 a promising biomarker in disease diagnosis (10–12).
Additionally, microRNA (miRNA)-143 has been shown to influence the
expression of COX-2 and affect disease progression; however, the
expression of COX-2 in osteosarcoma, particularly concerning its
association with miRNA-143, has not yet been fully elucidated.
The aim of the present study was to examine the
expression profiles of COX-2 and miRNA-143 in tumor tissues and
blood samples from patients with osteosarcoma and to discuss the
association between the expression profiles and the disease
severity. The findings could provide novel insights into the early
diagnosis and clinical treatment of osteosarcoma.
Materials and methods
Patients and tissue samples
A total of 46 patients diagnosed with osteosarcoma
who had been admitted to Jinling Hospital (Southern Medical
University, Nanjing, China) between December 2011 and December 2013
were enrolled in the present study, including 22 stage I patients,
18 stage II patients and 6 stage III patients (Table I). In addition, 26 normal subjects
were used as controls. A total of 20 osteosarcoma samples were
obtained from the patients (9 from stage I patients, 8 from stage
II patients and 3 from stage III patients), with adjacent tissues
used as negative controls (Table
II). Prior written and informed consent was obtained from every
patient and the study was approved by the Ethics Review Board of
Jinling Hospital.
 | Table I.Basic patient information. |
Table I.
Basic patient information.
| Group | Total (n) | Men (n) | Women (n) | Age range
(years) | Mean age (years) |
|---|
| Control | 26 | 16 | 10 | 10–28 | 18.8 |
| Osteosarcoma I | 22 | 16 | 6 | 12–26 | 18.6 |
| Osteosarcoma II | 18 | 12 | 6 | 11–27 | 19.8 |
| Osteosarcoma III | 6 | 1 | 5 | 15–23 | 18.1 |
 | Table II.Information for osteosarcoma
samples. |
Table II.
Information for osteosarcoma
samples.
| Group | Total (n) | Men (n) | Women (n) | Age range
(years) | Mean age (years) |
|---|
| Osteosarcoma I | 9 | 6 | 3 | 13–25 | 17.6 |
| Osteosarcoma II | 8 | 2 | 6 | 11–20 | 16.4 |
| Osteosarcoma III | 3 | 2 | 1 | 15–20 | 17.3 |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The miRcute
miRNA cDNA First Strand Synthesis kit (Tiangen Biotech Co., Ltd.,
Beijing, China) was used to perform the RT according to the
manufacturer's instructions. qPCR was then performed with the
miRcute miRNA qPCR detection kit (SYBR Green) (Tiangen Biotech Co.,
Ltd.). The primer sets used were synthesized by TIANGEN Biotech
Co., Ltd., Beijing, China (Table
III). The amplification conditions were as follows: 95°C for 5
min; then 95°C for 50 sec and 60°C for 30 sec, for 40 cycles.
β-actin and U6 served as the internal controls for COX-2 and
miRNA-143, respectively. The relative expression levels were
calculated using the 2−ΔΔCt method.
 | Table III.Primer sets for the reverse
transcription-quantitative polymerase chain reaction. |
Table III.
Primer sets for the reverse
transcription-quantitative polymerase chain reaction.
| Primers | Sequences |
|---|
| COX-2 |
|
|
Forward |
5′-CAGCCATACAGCAAATCCTTG-3′ |
|
Reverse |
5′-CAAATGTGATCTGGATGTCAAC-3′ |
| β-actin |
|
|
Forward |
5′-CACCAGGGCGTGATGGT-3′ |
|
Reverse |
5′-CTCAAACATGATCTGGGTCAT-3′ |
| miRNA-143 |
|
|
Forward |
5′-TGTAGTTCGGAGTTAGTGTCGCGC-3′ |
|
Reverse | 5′-CCTACGATCGAA
AACGACGCGAACG-3′ |
| U6 |
|
|
Foward |
5′-GTTTTGTAGTTTTTGGAGTTAGTGTTGTGT-3′ |
|
Reverse | 5′-CTCAACCTACAATCA
AAAACAACACAAACA-3′ |
Western blot analysis
Proteins were extracted from the tumor tissue and
blood samples. A total of 20 µg protein was subjected to 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto a polyvinylidene difluoride membrane. The membrane was
incubated with rabbit anti-human anti-COX-2 polyclonal antibody
(1:1,000 dilution; cat. no. ab15191; Abcam, Boston, MA, USA) and
rabbit anti-human anti-β-actin polyclonal antibody (1:5,000
dilution; cat. no. ab8227; Abcam), respectively, at 4°C overnight.
Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(1:3,000 dilution; cat. no. ab6721; Abcam) was then added and the
membrane was incubated at room temperature for another hour.
Coloration was performed using the Electrochemiluminescence Western
Blot Substrate kit (Abcam), and the relative intensity was analyzed
by Image Lab™ version 3.0 software (Bio-Rad Laboratories, Hercules,
CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The SPSS software package (version 18.0; SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. Normality tests and one-way
analysis of variance were performed for the comparison. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of COX-2 in tumor tissues
from patients with osteosarcoma
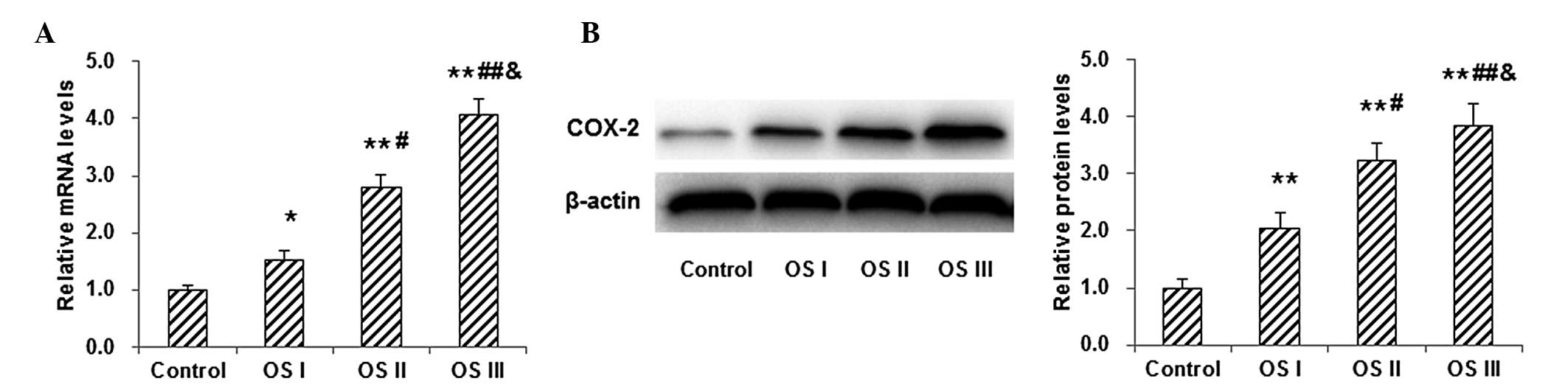
To investigate the mRNA and protein expression
levels of COX-2 in osteosarcoma tumor tissue, RT-qPCR and western
blot analysis were performed, respectively, in normal control
subjects and patients with stages I–III osteosarcoma. Results from
the RT-qPCR demonstrated that the mRNA expression levels of COX-2
in patients with osteosarcoma were significantly higher than those
in the normal control subjects (P<0.05; Fig. 1A). Furthermore, the mRNA expression
level of COX-2 in patients with stage III osteosarcoma was
significantly higher than that in patients with stage II
osteosarcoma, which was significantly higher than that in patients
with stage I osteosarcoma, indicating that the COX-2 mRNA
expression level was increasing along with the disease severity. In
addition, western blot analysis showed that the protein expression
levels of COX-2 exhibited similar trends to COX-2 mRNA (Fig. 1B). These results suggested that the
expression of COX-2 increased as the disease progressed, implying
the involvement of COX-2 in the disease pathogenesis.
Expression of COX-2 in blood samples
from patients with osteosarcoma
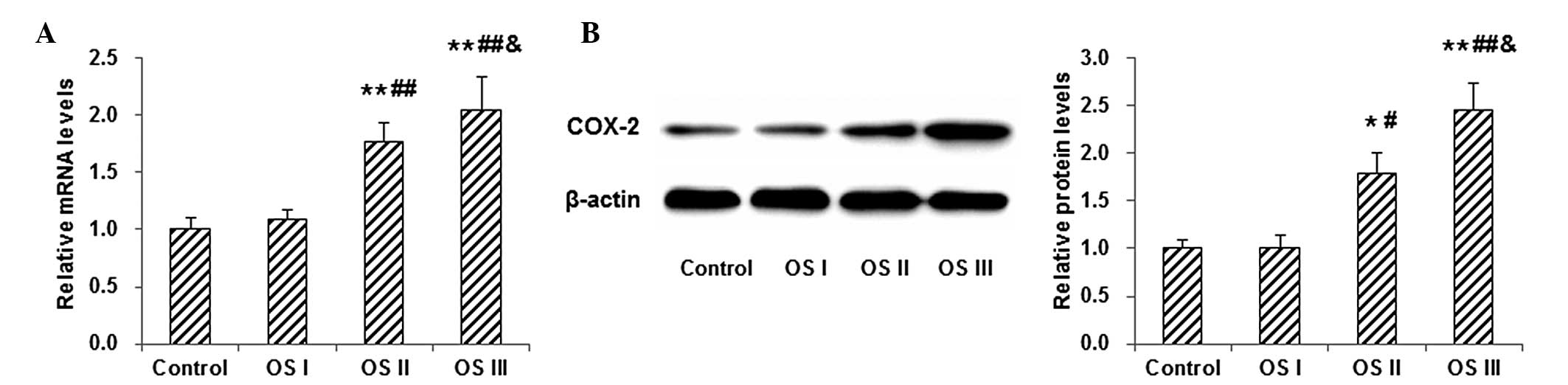
The mRNA and protein levels of COX-2 in blood
samples from patients with osteosarcoma were measured by RT-qPCR
and western blot analysis. The results showed that, compared with
normal control subjects, the mRNA level of COX-2 was slightly
elevated in the blood samples from patients with stage I
osteosarcoma; however, the difference was not statistically
significant (P>0.01; Fig. 2A). In
patients with stages II and III osteosarcoma, the COX-2 mRNA
content in the blood samples was significantly higher than that in
the normal controls (P<0.01), and the mRNA level was found to
increase as the disease progressed (Fig.
2A). Similar results were observed with the western blot
analysis of COX-2 protein expression in the blood samples from
patients with osteosarcoma (Fig.
2B). These results indicated that the mRNA and protein levels
of COX-2 in the blood were increased in patients with osteosarcoma,
despite the fact that no significant difference was measured
between patients with early-stage osteosarcoma and normal control
subjects.
Expression of miRNA-143 in tumor
tissue and blood samples from patients with osteosarcoma
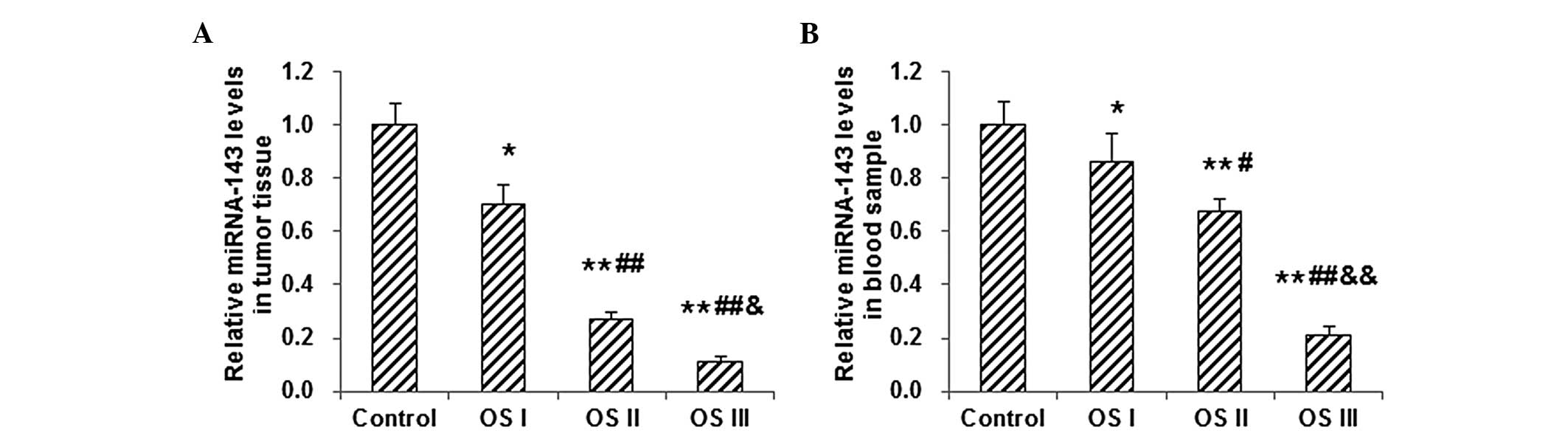
To evaluate the role of miRNA-143 in osteosarcoma
pathogenesis, its expression in osteosarcoma tumor tissue and blood
samples was detected by RT-qPCR. The results showed that the level
of miRNA-143 in osteosarcoma tumor tissue was significantly
decreased in patients with stages I–III osteosarcoma, as compared
with the level in the normal control subjects (P<0.05; Fig. 3A). Among those patients with
osteosarcoma, the miRNA-143 level in the tumor tissue declined
along with the disease severity, i.e. the miRNA-143 levels in the
tumor tissue from patients with osteosarcoma at stage III were
significantly higher than those in tissue from patients at stages
I–II, and the miRNA-143 level in stage II osteosarcoma was
significantly higher than that in stage I osteosarcoma (P<0.05;
Fig. 3A). Similar results were found
with the measurement of miRNA-143 in the blood samples from
patients with stages I–III osteosarcoma (Fig. 3B). These results indicated that the
expression of miRNA-143 notably decreased along with the increasing
severity of the osteosarcoma, which is the opposite trend to the
COX-2 expression, suggesting that miRNA-143 and COX-2 may play
different roles in the disease pathogenesis.
Discussion
Osteosarcoma is a highly malignant primary bone
tumor, with bone-like tissue formed by tumor cells (1). As one of the early symptoms of
osteosarcoma, patients experience intermittent pain, even prior to
tumor formation. This intermittent pain can gradually transform
into persistent, severe pain, particularly at night. Osteosarcoma
can be caused by other types of cancer, including Li-Fraumeni
syndrome (resulting from a p53 mutation), hereditary retinoblastoma
(RB) and associated diseases caused by an RB mutation (13). Other internal or external factors can
also contribute to the disease development (6,14). In
the present study, the pathogenesis of osteosarcoma, with regard to
the expression of COX-2 and miRNA-143 and the association between
them, was investigated.
COX-2 is the rate-limiting enzyme for the synthesis
of prostaglandins, which are critically important for inflammation
(15). Recent studies have indicated
that COX-2 is also closely associated with the progression of
various types of tumor (16,17). The current results showed that the
mRNA and protein expression levels of COX-2 became markedly
upregulated as the disease severity increased. These results
suggested that COX-2 may be involved in the development of
osteosarcoma. The blood expression level of COX-2 may reflect the
degree of malignancy and metastasis of osteosarcoma cells, since
tumor metastasis largely relies on blood and tissue fluid flow. The
present results showed that the mRNA and protein expression levels
of COX-2 in the blood gradually increased from osteosarcoma stage I
to stage III; however, the difference in the blood COX-2 expression
level between the normal controls and patients with stage I
osteosarcoma did not reach statistical significance. This may be
due to the fact that a limited number of cells migrate in the blood
circulation from the newly formed osteosarcoma tumor, and some of
these would be phagocytosed by monocytes in the blood (18). COX-2 expression in the blood may,
therefore, not be a perfect predictor for tumor metastasis,
particularly for stage I osteosarcoma.
The present results demonstrated that the COX-2
expression levels in osteosarcoma tumor tissue increased along with
the disease severity, from the normal control to osteosarcoma
stages I, II and III, while the expression of miRNA-143
sequentially decreased as the disease progressed. We hypothesized
that the upregulation of COX-2 may have been associated with the
downregulation of miRNA-143 in the disease progression. miRNA-143
may therefore negatively regulate COX-2, which could be an
important mechanism for chronic inflammation and the development of
cancer.
The expression tendency of miRNA-143 in the blood
was similar to that of COX-2 in the tumor tissues; it was, however,
notable that, unlike COX-2, the difference in blood miRNA-143
expression between the normal controls and the patients with stage
I osteosarcoma was statistically significant, suggesting that the
blood miRNA-143 level may be a promising early predictor and
diagnostic marker for osteosarcoma. Evidently, several potential
questions remain. The regulatory mechanisms of miRNA-143, for
example, are unclear, and there are also other factors that would
affect COX-2 expression; therefore, further studies regarding the
molecular mechanism and function of miRNA-143 in osteosarcoma are
required.
In conclusion, the results of the present study
showed that the mRNA and protein expression levels of COX-2
increased in the tumor tissue and blood samples from patients with
osteosarcoma along with the disease severity. In addition, the
expression of miRNA-143 decreased in the tumor tissue and blood
samples from patients with osteosarcoma as the disease progressed.
The opposite trends of COX-2 and miRNA-143 in the progression of
osteosarcoma indicated that they played different roles in the
disease pathogenesis. The current findings provided novel insights
into the early diagnosis and clinical treatment of
osteosarcoma.
Acknowledgements
The authors would like to thank Dr Sujia Wu, Dr
Xinguang Zhou and Dr Tao Yuan from Jinling Hospital, Southern
Medical University for their suggestions and assistance in the
study design, sample and data collection, statistical analysis and
manuscript preparation.
References
|
1
|
Lei P, Xie J, Wang L, Yang X, Dai Z and Hu
Y: microRNA-145 inhibits osteosarcoma cell proliferation and
invasion by targeting ROCK1. Mol Med Rep. 10:155–160.
2014.PubMed/NCBI
|
|
2
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai ZD and Li GD: Ezrin protein: Novel hot
point of research on mechanism of neoplasm metastasis. Zhonghua
Zhong Liu Za Zhi. 6:3223252005.(In Chinese).
|
|
5
|
Gorlick R and Khanna C: Osteosarcoma. J
Bone Miner Res. 25:683–691. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ottaviani G and Jaffe N: The etiology of
osteosarcoma. Cancer Treat Res. 152:15–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kansara M, Leong HS, Lin DM, et al: Immune
response to RB1-regulated senescence limits radiation-induced
osteosarcoma formation. J Clin Invest. 123:5351–5360. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou X, Jing J, Peng J, et al: Expression
and clinical significance of galectin-3 in osteosarcoma. Gene.
546:403–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu X, Cai M, Ji F and Lou LM: The impact
of COX-2 on invasion of osteosarcoma cell and its mechanism of
regulation. Cancer Cell Int. 14:272014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu XL, Cheng B, Li PY, et al: MicroRNA-143
suppresses gastric cancer cell growth and induces apoptosis by
targeting COX-2. World J Gastroenterol. 19:7758–7765. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pham H, Rodriguez CE, Donald GW, et al:
miR-143 decreases COX-2 mRNA stability and expression in pancreatic
cancer cells. Biochem Biophys Res Commun. 439:6–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song T, Zhang X, Wang C, et al: Expression
of miR-143 reduces growth and migration of human bladder carcinoma
cells by targeting cyclooxygenase-2. Asian Pac J Cancer Prev.
12:929–933. 2011.PubMed/NCBI
|
|
13
|
Yamasaki L: Role of RB tumor suppressor in
cancer. Cancer Treat Res. 115:209–239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jaffe N: Osteosarcoma. Pediatr Rev.
12:333–343. 1991.PubMed/NCBI
|
|
15
|
Alhouayek M and Muccioli GG: COX-2-derived
endocannabinoid metabolites as novel inflammatory mediators. Trends
Pharmacol Sci. 35:284–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Wang Z, Li J, et al: NFATc1
activation promotes the invasion of U251 human glioblastoma
multiforme cells through COX-2. Int J Mol Med. Mar 4–2015.(Epub
ahead of print). doi. View Article : Google Scholar
|
|
17
|
Wu K, Fukuda K, Xing F, et al: Roles of
the cyclooxygenase 2-matrix metalloproteinase 1 pathway in brain
metastasis of breast cancer. J Biol Chem. Feb 17–2015.(Epub ahead
of print). pii: jbc.M114.602185. View Article : Google Scholar
|
|
18
|
Toujas L, Delcros J G, Diez E, et al:
Human monocyte-derived macrophages and dendritic cells are
comparably effective in vitro in presenting HLA class I-restricted
exogenous peptides. Immunol. 91:635–642. 1997. View Article : Google Scholar
|