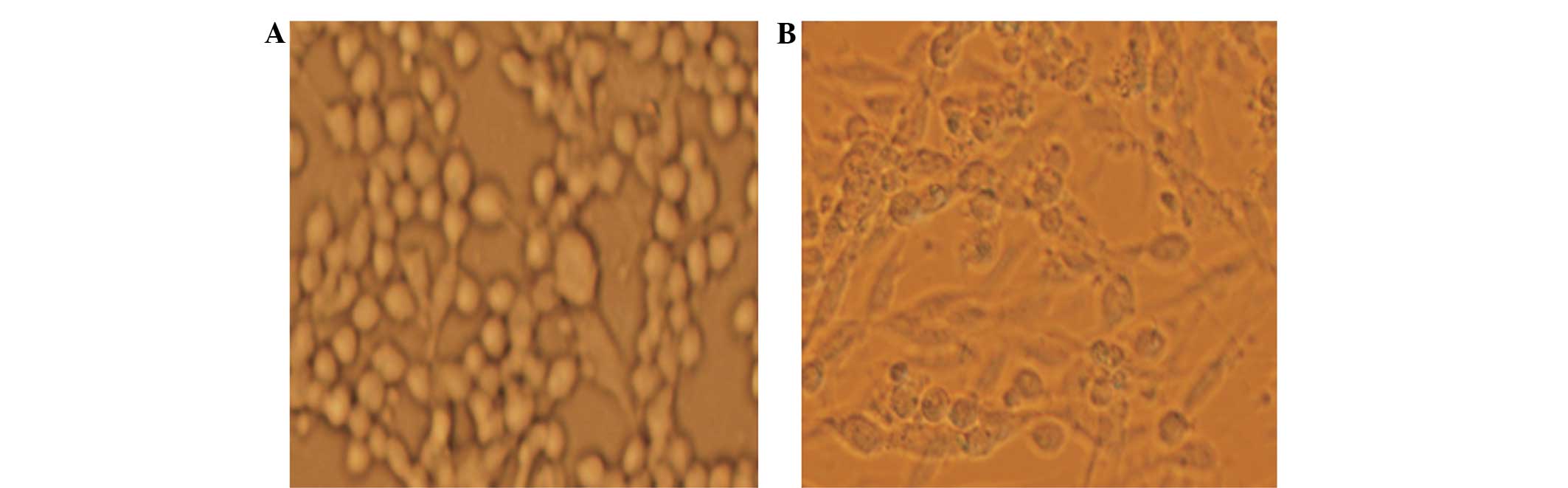
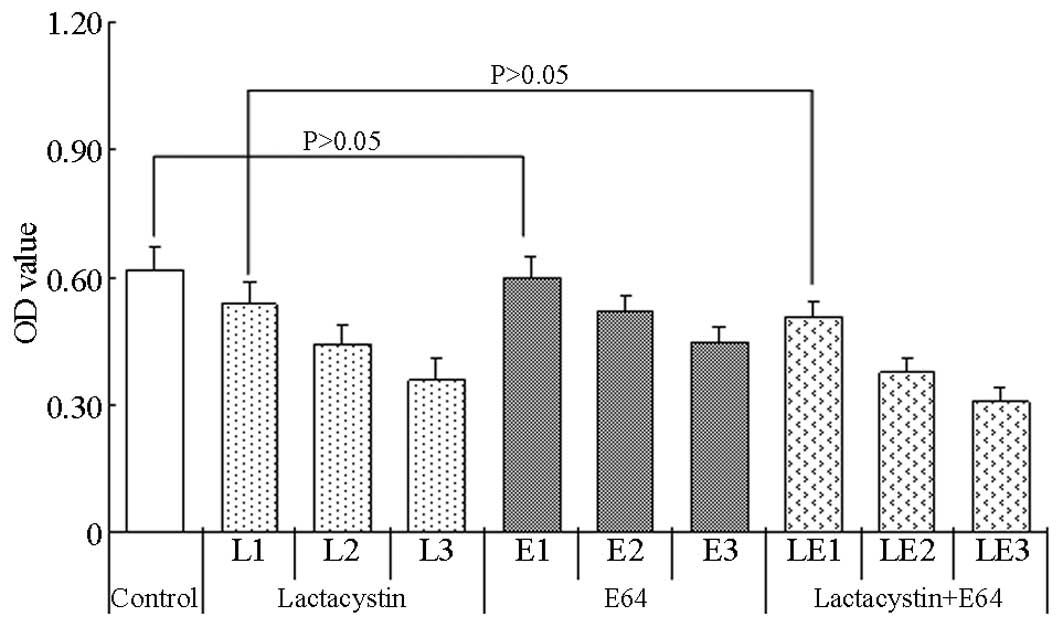
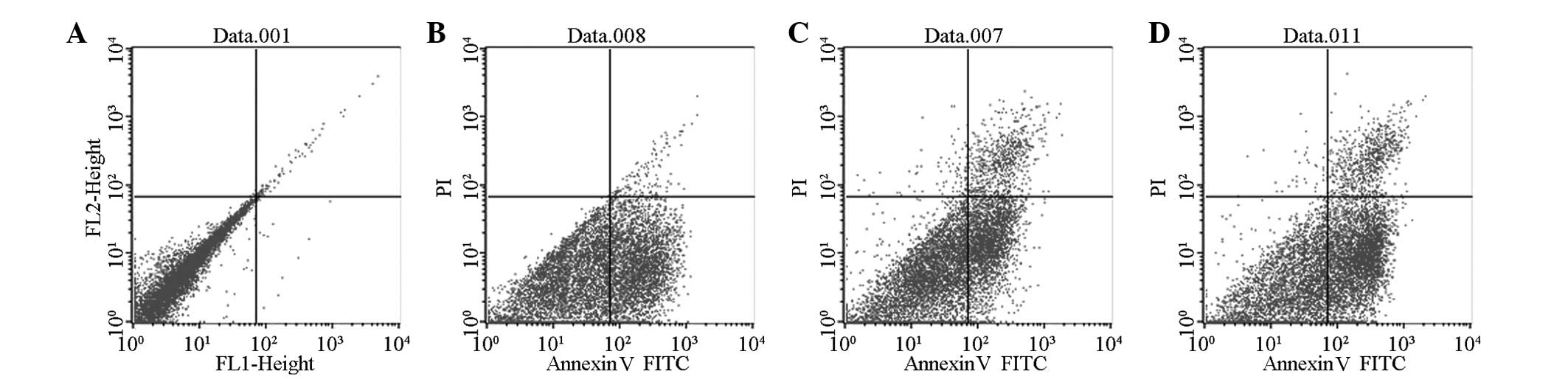
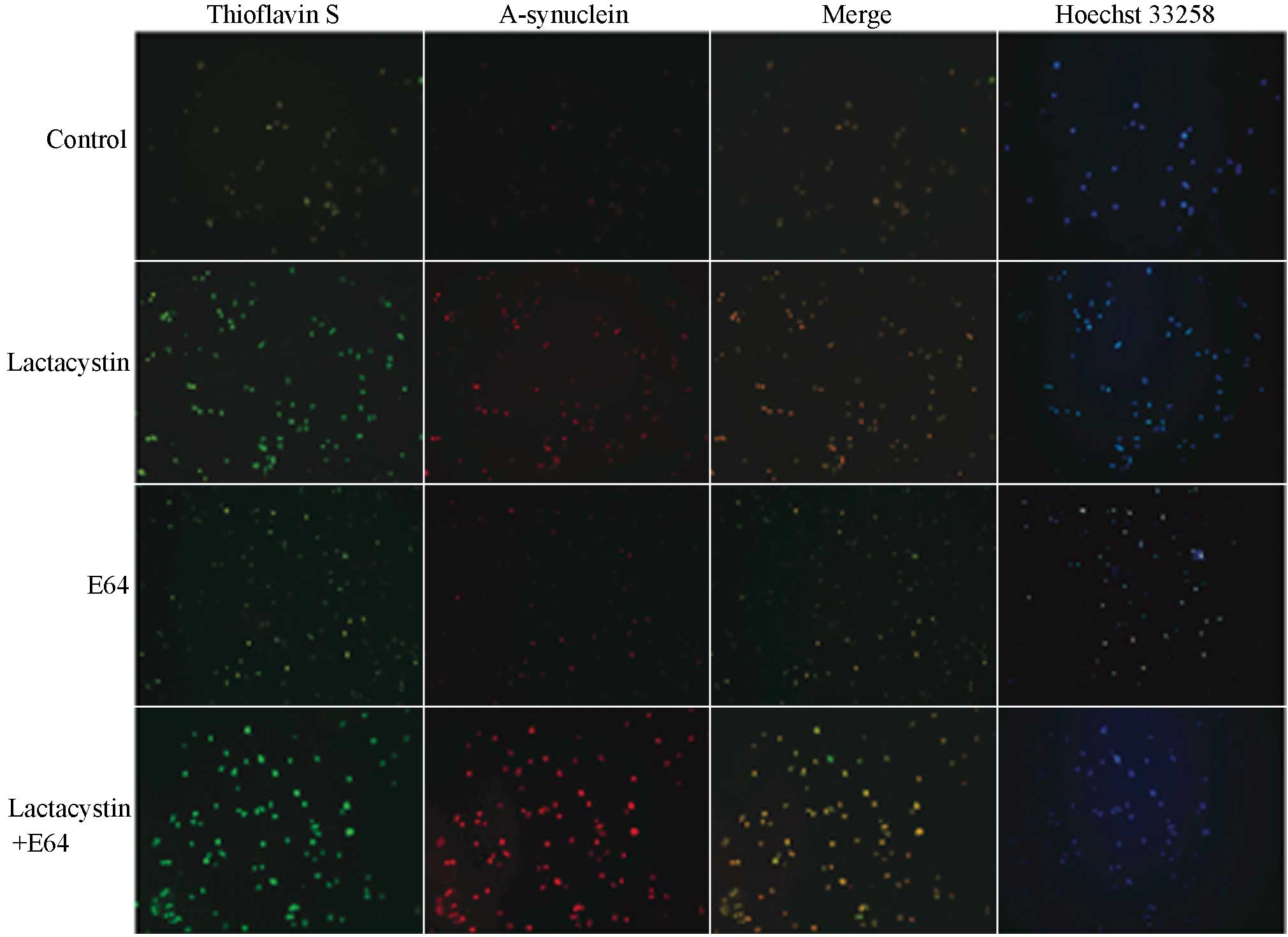
|
1
|
Ghosh A, Roy A, Liu X, et al: Selective
inhibition of NF-kappaB activation prevents dopaminergic neuronal
loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci
USA. 104:18754–18759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennett MC: The role of alpha-synuclein in
neurodegenerative diseases. Pharmaco1 Ther. 105:311–331. 2005.
View Article : Google Scholar
|
|
3
|
Bisaglia M, Mammi S and Bubacco L:
Structural insights on physiological functions and pathological
effects and alpha-synuclein. FASEB J. 23:329–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemani VM, Lu W, Berge V, et al: Increased
expression of alpha-synuclein reduces neurotransmitter release by
inhibiting synaptic vesicle reclustering after endocytosis. Neuron.
65:66–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu J, Kao SY, Lee FJ, Song W, Jin LW and
Yankner BA: Dopamine-dependent neurotoxicity of α-synuclein: A
mechanism for selective neurodegeneration in Parkinson disease. Nat
Med. 8:600–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamanka K, Saito Y, Yamamori T, Urano Y
and Noguchi N: 24(S)-hydroxycholesterol induces neuronal cell death
through necroptosis, a form of programmed necrosis. J Biol Chem.
286:24666–34673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beyer K and Ariza A: Protein aggregation
mechanism in synucleinopathies: Commonalities and differences. J
Neuropathol Exp Neurol. 66:965–974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu X, Zhang H, Zhang Y, et al:
Differential protein profile of PC12 cells exposed to proteasomal
inhibitor lactacystin. Neurosci Lett. 575:25–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryu EJ, Angelastro JM and Greene LA:
Analysis of gene expression changes in a cellular model of
Parkinson disease. Neurobiol Dis. 18:54–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McNaught KS, Shashidharan P, Perl DP,
Jenner P and Olanow CW: Aggresome-related biogenesis of Lewy
bodies. Eur J Neurosci. 16:2136–2148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pouplana S, Espargaro A, Galdeano C, et
al: Thioflavin-S staining of bacterial inclusion bodies for the
fast, simple, and inexpensive screening of amyloid aggregation
inhibitors. Curr Med Chem. 21:1152–1159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maroteaux L, Campanelli JT and Scheller
RH: Synuclein: A neuron-specific protein localized to the nucleus
and presynaptic nerve terminal. J Neurosci. 8:2804–2815.
1988.PubMed/NCBI
|
|
13
|
Jo E, McLaurin J, Yip CM, St George-Hyslop
P and Fraser PE: alpha-Synuclein membrane interactions and lipid
specificity. J Biol Chem. 275:34328–34334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McNaught KS, Björklund LM, Belizaire R,
Isacson O, Jenner P and Olanow CW: Proteasome inhibition causes
nigral degeneration with inclusion bodies in rats. Neuroreport.
13:1437–1441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banerjee K, Munshi S, Sen O, Pramanik V,
Roy Mukherjee T and Chakrabarti S: Dopamine cytotoxicity involves
both oxidative and nonoxidative pathways in SH-SY5Y cells:
Potential role of alpha-synuclein overexpression and proteasomal
inhibition in the etiopathogenesis of Parkinson's disease.
Parkinsons Dis. 2014:8789352014.PubMed/NCBI
|
|
16
|
Bucciantini M, Giannoni E, Chiti F, et al:
Inherent toxicity of aggregates implies a common mechanism for
protein misfolding diseases. Nature. 416:507–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ciechanover A: The ubiquitin-proteasome
pathway: On protein death and cell life. EMBO J. 17:7151–7160.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Česen MH, Pegan K, Spes A and Turk B:
Lysosomal pathways to cell death and their therapeutic
applications. Exp Cell Res. 318:1245–1251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boya P and Kroemer G: Lysosomal membrane
permeabilization in cell death. Oncogene. 27:6434–6451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Webb JL, Ravikumar B, Atkins J, Skepper JN
and Rubinsztein DC: Alpha-Synuclein is degraded by both autophagy
and the proteasome. J Biol Chem. 278:25009–25013. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin Y, Klucken J, Patterson C, Hyman BT
and McLean PJ: The co-chaperone carboxyl terminus of
Hsp70-interacting protein (CHIP) mediates alpha-synuclein
degradation decisions between proteasomal and lysosomal pathways. J
Biol Chem. 280:23727–23734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lazzeri G, Lenzi P, Busceti CL, et al:
Mechanisms involved in the formation of dopamine-induced
intracellular bodies within striatal neurons. J Neurochem.
101:1414–1427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeon SM, Cheon SM, Bae HR, Kim JW and Kim
SU: Selective susceptibility of human dopaminergic neural stem
cells to dopamine-induced apoptosis. Exp Neurobiol. 19:155–164.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ, Khoshaghideh F, Patel S and Lee
SJ: Clearance of alpha-synuclein oligomeric intermediates via the
lysosomal degradation pathway. J Neurosci. 24:1888–1896. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cuervo AM, Stefanis L, Fredenburg R,
Lansbury PT and Sulzer D: Impaired degradation of mutant
alpha-synuclein by chaperone-mediated autophagy. Science.
305:1292–1295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eskelinen EL, Illert AL, Tanaka Y,
Schwarzmann G, Blanz J, Von Figura K and Saftig P: Role of LAMP-2
in lysosome biogenesis and autophagy. Mol Biol Cell. 13:3355–3368.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu W and Silverman RB: Stereospecific
total syntheses of proteasome inhibitor omuralide and lactacystin.
J Org Chem. 76:8287–8293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wijayanti MA, Sholikhah EN, Hadanu R,
Jumina J, Supargiyono S and Mustofa M: Additive in vitro
antiplasmodial effect of N-alkyl and N-benzyl-1,10-phenanthroline
derivatives and cysteine protease inhibitor e64. Malar Res Treat.
2010:5407862010.PubMed/NCBI
|
|
29
|
Mo JS, Yoon JH, Hong JA, et al:
Phosphorylation of nicastrin by SGK1 leads to its degradation
through lysosomal and proteasomal pathways. PLoS One. 7:e371112012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rideout HJ and Stefanis L: Proteasomal
inhibition-induced inclusion formation and death in cortical
neurons require transcription and ubiquitination. Mol Cell
Neurosci. 21:223–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anglade P, Vyas S, Javoy-Agid F, et al:
Apoptosis and autophagy in nigral neurons of patients with
Parkinson's disease. Histol Histopathol. 12:25–31. 1997.PubMed/NCBI
|
|
32
|
Tain LS, Chowdhury RB, Tao RN, et al:
Drosophila HtrA2 is dispensable for apoptosis but acts downstream
of PINK 1independently from Parkin. Cell Death Differ.
16:1118–1125. 2009. View Article : Google Scholar : PubMed/NCBI
|