Introduction
Osteoarthritis (OA) is a metabolically active,
dynamic process that affects all joint tissues. The major clinical
signs of the disease include destruction of the articular cartilage
and changes to the underlying subchondral bone. However, the
etiology of the disease remains poorly understood. Several
biochemical and biomechanical factors are considered to play a role
in the pathogenesis. Osteopontin (OPN), also known as early T cell
activation gene-1 (Eta-1) is abundant in bone tissue and may be
secreted by a number of different cell types including chondrocytes
and synoviocytes (1–4). OPN is upregulated in human chondrocytes
(5). A previous study found that the
level of OPN mRNA isolated from human OA cartilage was increased as
compared with that in normal cartilage (5). Furthermore, the expression level of OPN
in the plasma, synovial fluid and articular cartilage is associated
with progressive joint damage and may be a useful biomarker for
determining the severity and progression of disease in knee OA
(6,7). OPN interacts with a variety of cell
surface receptors, including integrin and CD44 (8). The receptor CD44 has been implicated in
the development and progression of OA, and CD44 present in
articular cartilage has been associated with progressive knee OA
joint damage (9,10). However, the role of OPN in the
pathological changes in knee OA remains unknown.
Articular cartilage is an avascular tissue that
derives its nutritional and oxygen supply by a diffusion process
from the synovial fluid and subchondral bone. Thus, articular
cartilage is maintained in a low oxygen environment in the body
(11). Chondrocytes are therefore
adapted to these hypoxic conditions. A number of previous studies
have shown that hypoxia triggers essential positive signals for the
chondrocyte phenotype (12–14). Adaptation to this avascular
environment is mediated by hypoxia-inducible factor (HIF)-1 and
HIF-2 (12). The HIF protein family
consists of α and β subunit members that function by forming
heterodimers (12). Two HIF isoforms
(HIF-1α and HIF-2α) mediate the response of cells to hypoxia
(13,14). In a previous study, HIF-2α was
demonstrated to be essential for the endochondral ossification of
cultured chondrocytes and embryonic skeletal growth in mice
(15). Furthermore, HIF-2α
expression has been found to be higher in osteoarthritic cartilage
than in non-diseased cartilage in mice and humans (15). Another study observed that HIF-2α
increased the expression levels of genes encoding catabolic
factors, including matrix metalloproteinases (MMPs) −1, 3, 9, 12
and 13, aggrecanase-1, nitric oxide synthase-2 and
prostaglandin-endoperoxide synthase-2 in chondrocytes (16). Thus, HIF-2α is an important catabolic
transcription factor in the process of OA development and may be
considered as a therapeutic target for OA.
The association between HIF-2α and OPN in
chondrocytes remains unclear. The aim of the current in
vitro study was to investigate the effect of OPN on HIF-2α mRNA
expression in chondrocytes from patients with OA of the knee in
order to reveal the role of OPN in OA.
Materials and methods
Chondrocyte culture
The present study protocol was approved by the
Institutional Review Board of the Xiangya Hospital Central South
University (Changsha, China). The articular hyaline cartilage was
removed from the tibial surfaces of 6 patients with OA of the knee
who had undergone a total knee replacement. Written informed
consent was obtained from the patients. After washing twice with
phosphate-buffered saline (PBS), the cartilage was ground with a
scalpel blade into 1–5-mm3 sections. The cartilage
sections were subsequently digested with 5–8 ml 0.2% collagenase II
(Sigma-Aldrich, St. Louis, MO, USA) for 12–16 h at 37°C with 5%
CO2. The digestion was terminated with 8–10 ml
Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Hyclone, Logan,
UT, USA). The released chondrocyte pellets at the bottom of the
centrifuge tube were suctioned and transferred to a culture flask
following centrifugation at 150 × g for 6 min. The cells were
subsequently counted using a hemocytometer (Beckman Coulter, Brea,
CA, USA) and cell viability was determined using trypan blue
exclusion. Cell pellets were resuspended in 5 ml DMEM/F12
containing 15% fetal bovine serum (FBS; Gibco, Grand Island, NY,
USA) and 1% penicillin/streptomycin solution (Gibco), and incubated
for 24 h at 37°C with 5% CO2 in a plastic culture flask.
The non-adherent cells were subsequently washed out. The remaining
adherent cells were cultured for an additional 2 weeks in a flask,
while the growth medium was changed every 3 days prior to
trypsinization, and then transferred to new culture flasks.
Transfection of OPN small interfering
RNA (siRNA) into chondrocytes
The siRNAs specific to OPN were designed and
synthesized by Invitrogen Trading (Shanghai) Co., Ltd (Shanghai,
China) with reference to the coding sequence for human OPN. The
siRNA sequences were as follows: sense, 5′-CCU GUG CCA UAC CAG UUA
ATT-3′ and antisense, 5′-UUA ACU GGU AUG GCA CAG GTT-3′.
Transfection of siRNAs to the cells was performed with
Lipofectamine™ 2000 reagent (Invitrogen Life
Technologies, San Diego, CA, USA) according to the manufacturer's
instructions. Briefly, on the day prior to transfection,
exponentially growing cells were seeded onto six-well plates at a
density of 1.5×105 cells/well in the DMEM without
antibiotics. Upon reaching 70% confluence, the cells were
transfected with 50 nmol siRNA using Lipofectamine™
2000. After 24 h, total RNA was isolated and the expression levels
of the relative transcripts were detected by reverse transcription
(RT)-quantitative polymerase chain reaction (qPCR).
Cell treatment
The chondrocytes were plated in 6-well culture
plates and serum starved for 24 h in DMEM/F12 medium containing 1%
FBS to synchronize cells in a non-activating and non-proliferating
phase. The chondrocytes were subsequently cultured in DMEM/F12
containing 15% FBS. Three groups were established. The control
group comprised chondrocytes that were unstimulated and untreated.
The recombinant human OPN (rhOPN) group comprised chondrocytes that
were treated with 1 µg/ml rhOPN (1433-OP; R&D Systems,
Minneapolis, MN, USA) for 24 h, and the third group was the OPN
siRNA group comprising chondrocytes transfected with OPN siRNA. In
blocking experiments carried out to determine the possible
involvement of CD44, chondrocytes were incubated with a mouse
anti-CD44 monoclonal antibody (20 µg/ml; LS-C87848; LifeSpan
Biosciences, Seattle, WA, USA) or isotype control IgG1 (10 µg/ml;
Abcam, Cambridge, UK) 1 h prior to rhOPN treatment for 24 h.
Cell viability assay
Cell viability following treatment with rhOPN or
siRNA for 24 h was determined using a colourimetric MTT assay. One
day prior to the rhOPN or siRNA treatment, the cells were seeded
into 96-well plates. After 24 h of rhOPN or siRNA treatment,
culture medium was removed and 20 µl MTT solution (5 mg/ml in PBS)
was added into each well and incubated at 37°C with 5%
CO2 for 4 h. The supernatant was then aspirated and the
formazan reaction products were dissolved using dimethyl sulphoxide
(Sigma-Aldrich) solution and agitated for 15 min.
Spectrophotometric absorbance was measured at 570 nm using a
Multiskan MK3 ELISA plate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
RNA isolation, quantification and
RT
Following treatment, the chondrocytes were lysed and
total RNA was extracted with TRIzol® reagent (Invitrogen Life
Technologies, Rockville MD, USA) according to the manufacturer's
instructions. Total RNA was quantified using a Biochrom Libra S60
spectrophotometer (Biochrom Ltd, Cambridge, UK). A total of 1 µg
RNA was converted to cDNA using a RevertAid™ First
Strand cDNA Synthesis kit (Fermentas, Thermo Fisher Scientific,
Inc.). First, all components were mixed, including the template RNA
(1 µg), oligo (dT)18 primer (1 µl) and RNase-free water
(to a total volume of 12 µl). The mixture was produced by gentle
mixing, brief centrifugation at 8,000 × g for 15 sec and incubation
at 65°C for 5 min. It was subsequently chilled on ice, centrifuged
at 1,000 × g for 5 sec and the vial was placed back on ice. The
following components were added: 5X Reaction buffer (4 µl),
RiboLock™ RNase inhibitor (20 u/µl) (1 µl), 10 mM dNTP
mix (2 µl) and RevertAid™ M-MuLV Reverse transcriptase
(200 U/µl; 2 µl) to a final volume of 20 µl. This reaction mixture
was incubated for 60 min at 42°C and terminated by heating at 70°C
for 5 min. The cDNA products were stored in aliquots at −80°C until
required.
qPCR assays
Primers were synthesized by Shanghai Genechem Co.,
Ltd (Shanghai, China). The sequences of the primers are as follows:
OPN forward: 5′-GTGGGA AGG ACA GTT ATG AA-3′ and reverse: 5′-CTG
ACT TTG GAA AGT TCC TG-3′; HIF-2α forward: 5′-GTG ACA TGA TCT TTC
TGT CGG AA-3′ and reverse: 5′-CGC AAG GAT GAG TGA AGT CAAA-3′;
GAPDH forward: 5′-TGA CTT CAA CAG CGA CAC CCA-3′ and reverse:
5′-CAC CCT GTT GCT GTA GCC AAA-3′. The components used for qPCR
were as follows: 12.5 µl Maxima® SYBR Green/ROX qPCR Master mix
(2X; Thermo Fisher Scientific, Inc.), 2.5 µl forward primer (0.3
µM), 2.5 µl reverse primer (0.3 µM), 2 µl template cDNA and 5.5 µl
RNase free water at a volume of 25 µl. The TP800 Thermal Cycler
Dice Real Time system (Takara Bio, Inc., Otsu, Japan) was used for
all qPCR. The PCR thermal conditions were as follows: 50°C
uracil-DNA glycosylase (UDG; Roche Diagnostics, Basel, Switzerland)
pretreatment for 2 min, 1 cycle at 95°C for 10 min for initial
denaturation, 40 repeats of a 15 sec 95°C denaturation step, a 30
sec 30°C annealing step and a 30 sec extension step at 72°C. A
melting curve was constructed following the final amplification
period via a temperature gradient from 95°C for 15 sec, 55°C for 30
sec and 95°C for 15 sec. The GAPDH gene was used as an endogenous
control. Relative expression levels of the genes of interest were
calculated and expressed as 2−ΔΔCt. All quantities were
expressed as n-fold relative to a calibrator.
Statistical analysis
Data were analyzed using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA) for statistical evaluation. Data are
expressed as the mean ± standard error of the mean. The statistical
analysis of the differences between experimental groups was
performed by the Student's t-test. One-way analysis of variance
followed by the Student-Newman-Keuls test were used to analyze the
differences among the three experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
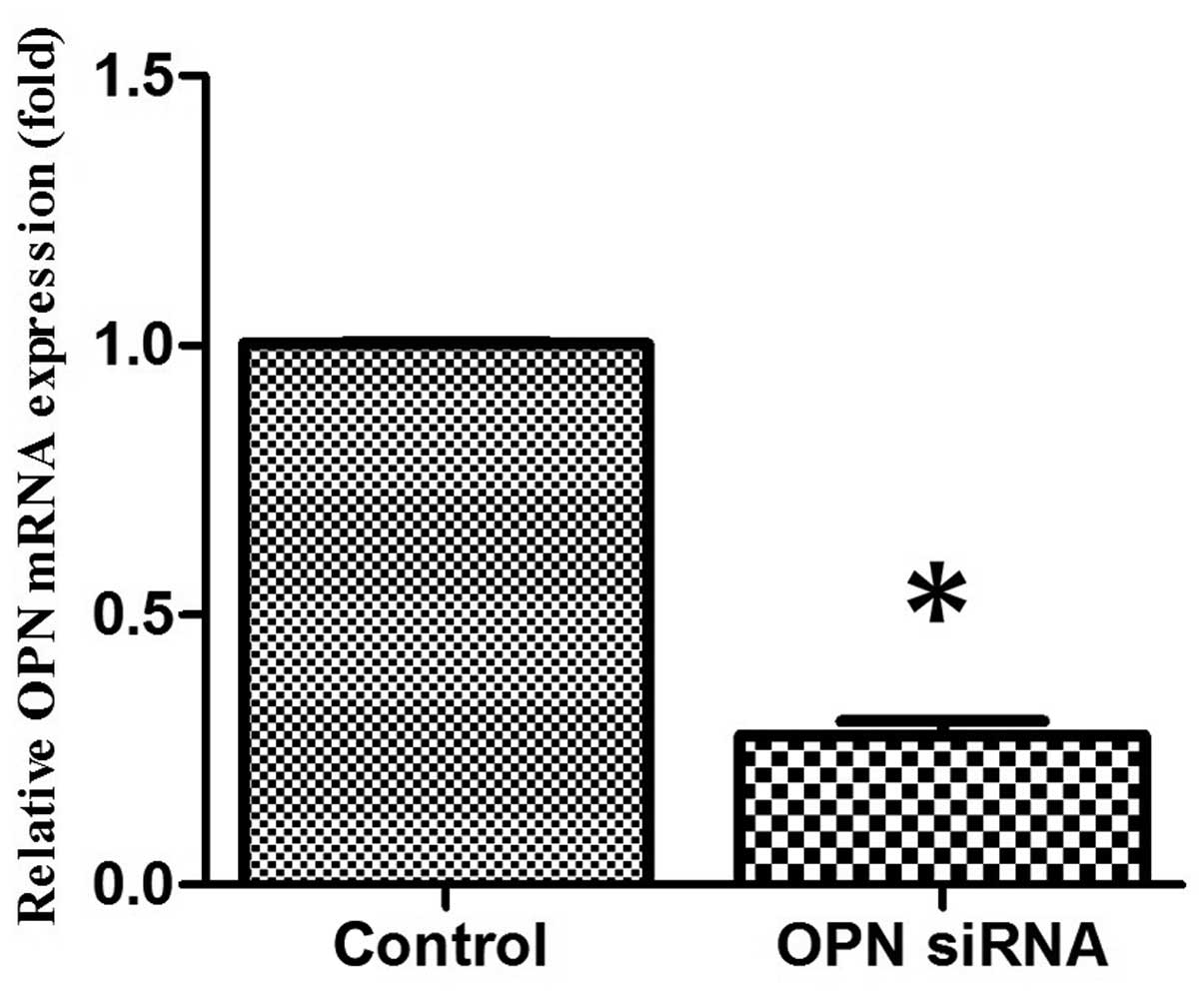
OPN siRNA effectively inhibits
endogenous chondrocyte OPN expression in vitro
Chondrocytes were transfected with OPN siRNA
oligonucleotides using Lipofectamine™ 2000. After 24 h
of culture in vitro, OPN gene expression was analyzed by
RT-qPCR. The OPN siRNA oligonucleotide was able to effectively
suppress OPN gene expression in vitro when compared with the
control group (Fig. 1). The
OPN-specific siRNAs did not affect the expression of the
housekeeping gene GAPDH (data not shown).
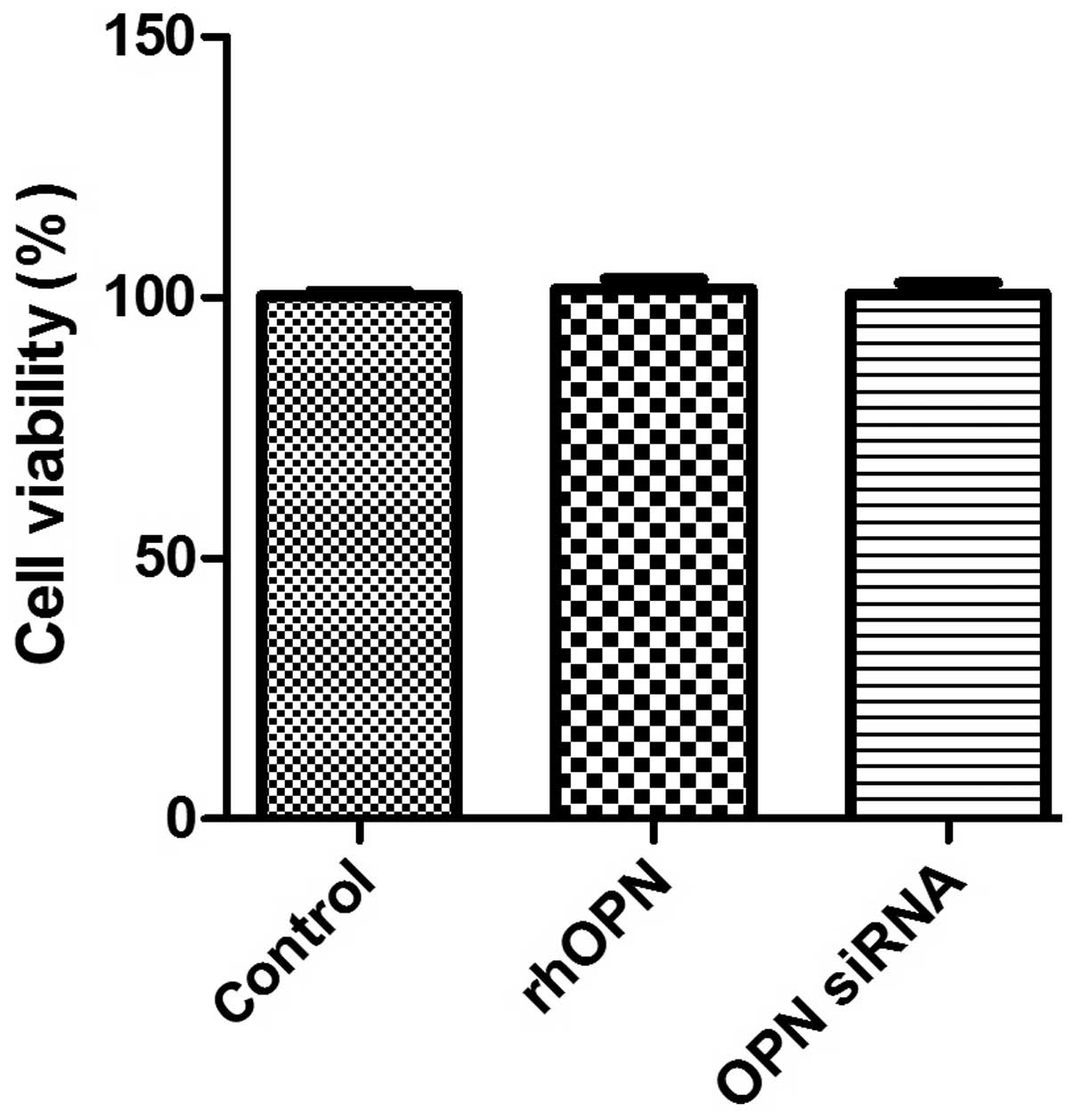
Cell viability
Fig. 2 shows the MTT
data as the percentage of cell viability compared with that of the
control. The results revealed that rhOPN and OPN siRNA,
respectively, did not suppress human chondrocyte survival in
vitro following incubation for 24 h (P>0.05).
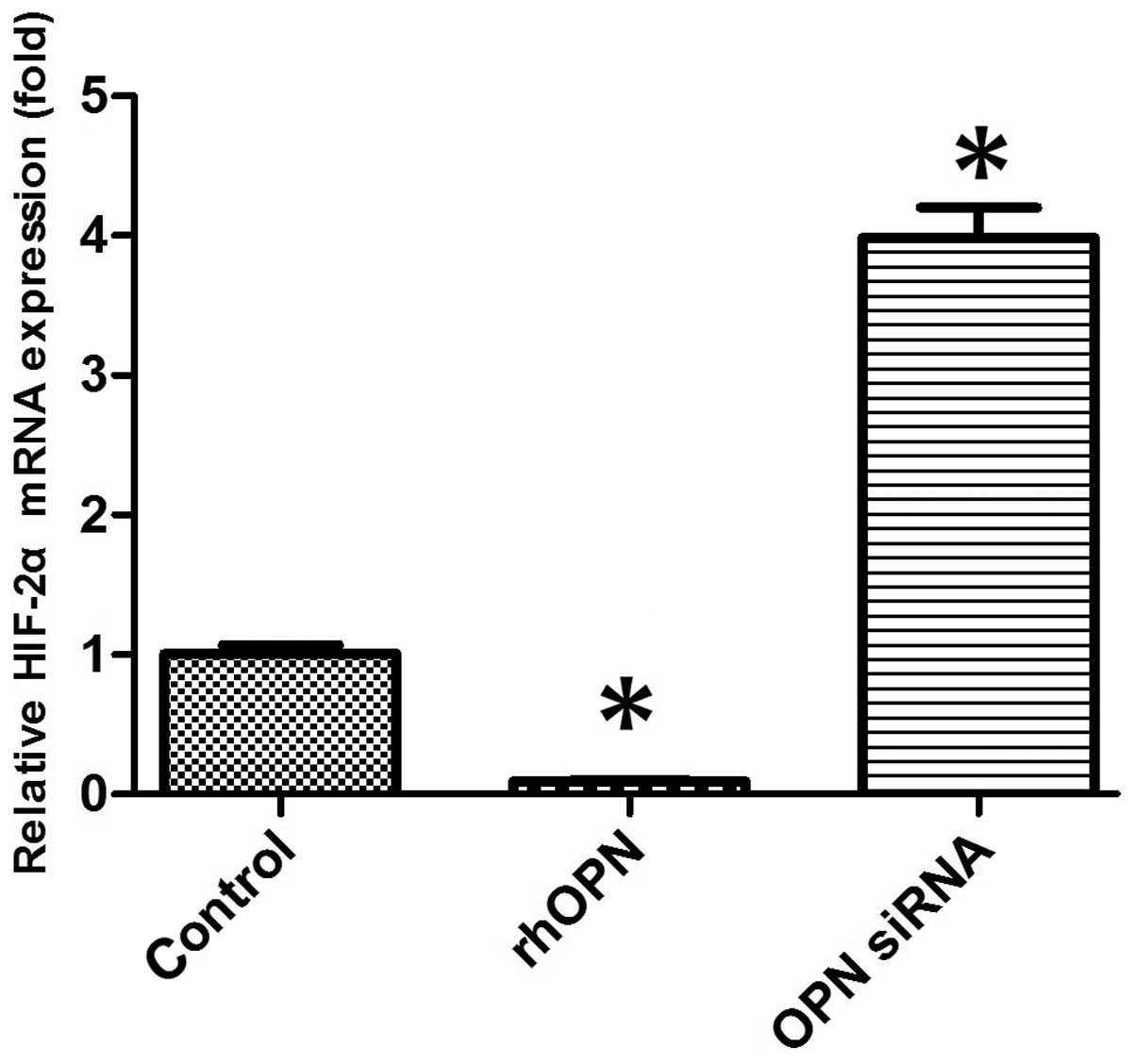
HIF-2α mRNA expression of chondrocytes
in vitro
The mRNA expression level of HIF-2α was markedly
decreased in the rhOPN group compared with the control group
following 24 h of treatment. OPN siRNA, however, increased the
HIF-2α mRNA expression level compared with that in the other groups
(P<0.05; Fig. 3).
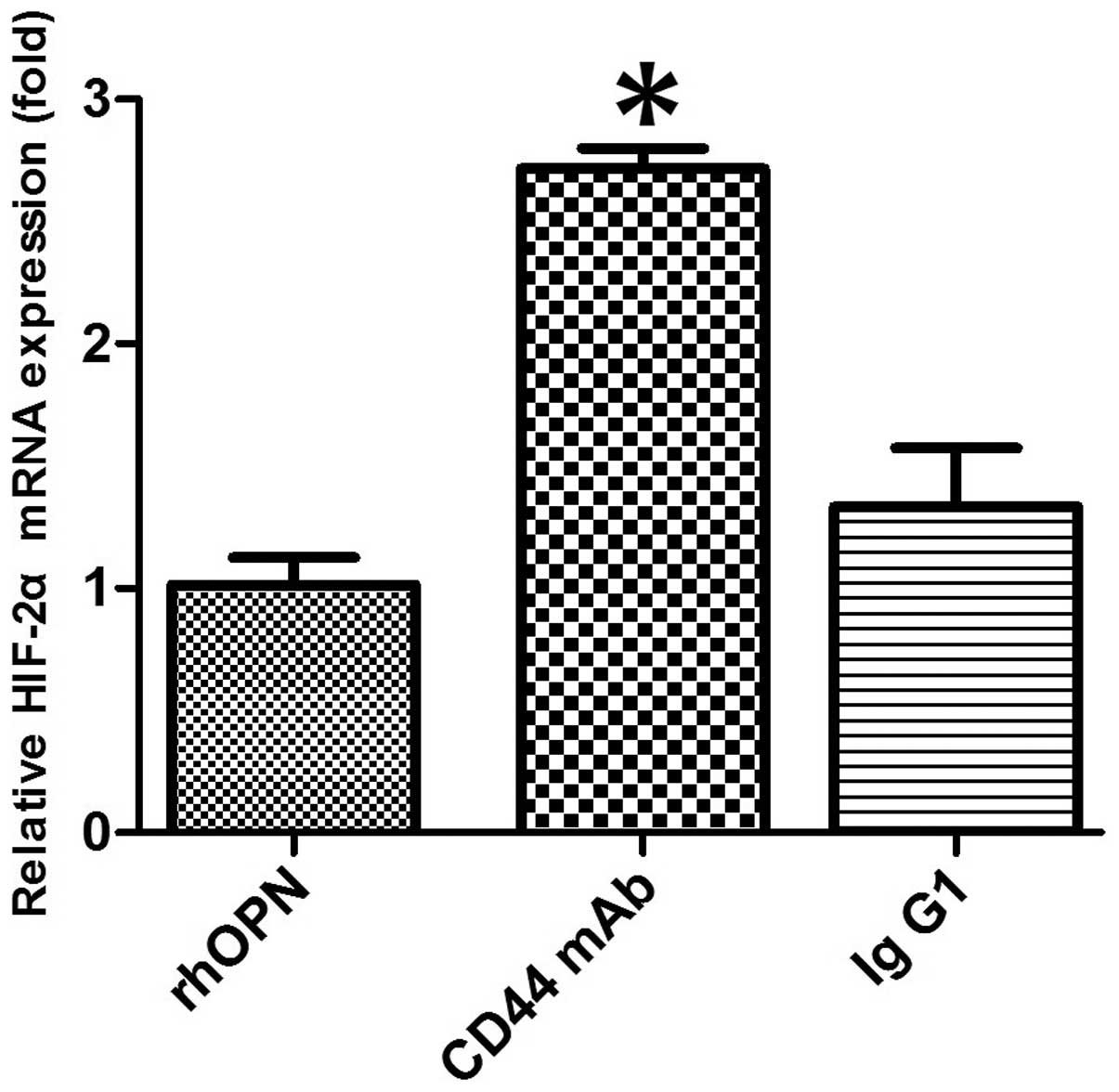
CD44-blocking mAb attenuates the
inhibitory effect of OPN on HIF-2α mRNA expression
In the chondrocytes obtained from patients with OA,
pretreatment with anti-CD44 blocking mAb caused the level of
OPN-induced HIF-2α mRNA expression to increase when compared with
that in the rhOPN group (P<0.05; Fig.
4). However, pretreatment with an isotype-matched control IgG1
had no significant effect on the HIF-2α mRNA expression level when
compared with that in the rhOPN group (P>0.05; Fig. 4).
Discussion
Cartilage damage is one of the main pathological
changes in OA. In a study of human OA cartilage samples conducted
by Attur et al (17), it was
found that the expression levels of OPN were increased in OA
cartilage, with a significant upregulation of the expression of OPN
mRNA compared with that in normal cartilage. The study also
observed that the addition of recombinant OPN to the OA cartilage
under ex vivo conditions inhibited the production of nitric
oxide and prostaglandin E2. This suggests that OPN is overexpressed
in OA cartilage and may function as an endogenous inhibitor of
inflammatory mediators in cartilage (17). In another study, OPN deficiency was
demonstrated to exacerbate aging-associated and instability-induced
OA; the structural changes and loss of proteoglycan from cartilage
tissue were shown to be greater in OPN-deficient mice than in
wild-type mice (18). The study also
found that OPN deficiency led to the induction of MMP-13. This
indicates that OPN is involved in the progression of OA; however,
the role of OPN in arthritis and joint diseases remains
incompletely understood (18).
Previous studies have shown that the level of OPN is elevated in OA
plasma, cartilage and synovial fluid (6,7). RT-PCR
is the preferred technique for the analysis of gene expression due
to its high sensitivity. In particular, RT-qPCR has the advantages
of a wide dynamic detection range and a higher reliability of
results than conventional PCR (19).
In RT-qPCR, DNA fragment amplification may be quantified using
Taqman probes or SYBR Green fluorescence with equivalent accuracy,
however SYBR Green is less expensive compared with the Taqman
probes (20). In the current study,
RT-qPCR assays were conducted with SYBR Green dye for
quantification of the changes in the expression of different
genes.
The present study showed that OPN is able to inhibit
the expression of HIF-2α at the mRNA level in chondrocytes obtained
from patients with OA of the knee. To the best of our knowledge,
the current study is the first to report this association.
Articular cartilage is an avascular connective tissue in which the
availability of oxygen and glucose is significantly lower compared
with that in the synovial fluid and plasma (11). Oxygen and nutrient maintenance is
critical to cell fate, senescence and apoptosis (21). Previous studies have suggested that
chondrocyte death plays a key role in cartilage degeneration
(22–26). Chondrocyte cell death through
apoptosis, necrosis, chondroptosis or a combination of these
processes has been implicated in the pathogenesis of OA (26). The level of HIF-2α in human and mouse
OA chondrocytes has been observed to be markedly elevated compared
with normal chondrocytes, and to be associated with the increased
apoptosis of articular chondrocytes (27). HIF-2α increases Fas-mediated
chondrocyte apoptosis, which is associated with OA cartilage
destruction (28). In addition, a
previous study reported an enhanced expression of OPN under
hypoxia; OPN is known to confer cytoprotection against
hypoxia/reoxygenation-induced apoptosis (29). In the current study, HIF-2α mRNA
expression was decreased with OPN upregulation, whereas it was
increased with OPN downregulation. Since HIF-2α is a catabolic
regulator of osteoarthritic cartilage destruction (16), the downregulation of HIF-2α by an
elevated level of OPN may be a mechanism for the affected
chondrocytes to return to a state of homeostasis. Thus, the present
results indicate that OPN may play a protective role in OA, which
has also been speculated in certain previous studies (17,18).
OPN interacts with integrin receptors and CD44 to
initiate chemotaxis, promote cell adhesion and modulate cell
function. CD44 is noted to be an important mediator in chondrocyte
cell-matrix interactions that involve proteoglycan, hyaluronan or
link protein aggregates (29–31). A
previous study indicated that it is not necessary for each CD44
present on the chondrocyte cell to be occupied with hyaluronan in
order to maintain a cell-associated matrix (21). A previous study demonstrated the
re-expression of bovine chondrocyte cell surface CD44 following
trypsin-treatment and indicated that only 25% of normal cell
surface CD44 expression on bovine chondrocytes is required for the
assembly of a hyaluronan-anchored, cell-associated matrix (32). In the present study, pretreatment of
chondrocytes with anti-CD44 blocking mAb suppressed the inhibitory
effect of OPN on the mRNA expression of HIF-2α. The results suggest
that the inhibitory effects of OPN on HIF-2α in chondrocytes are
mediated through the interaction of CD44 with OPN. Further studies
are required to elucidate the OPN-CD44 downstream signaling pathway
in OA chondrocytes.
In conclusion, the results of the present study
indicate that OPN may play a protective role in OA by inhibiting
HIF-2α gene expression in osteoarthritic chondrocytes via CD44
interaction.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81272034), the
Hunan Provincial Innovation Foundation for Postgraduate (no.
CX2012B086) and the Fundamental Research Funds for the Central
Universities of Central South University (no. 2013zzts081).
References
|
1
|
Gravallese EM: Osteopontin: A bridge
between bone and the immune system. J Clin Invest. 112:147–149.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang FJ, Gao SG, Cheng L, et al: The
effect of hyaluronic acid on osteopontin and CD44 mRNA of
fibroblast-like synoviocytes in patients with osteoarthritis of the
knee. Rheumatol Int. 33:79–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Denhardt DT and Noda M: Osteopontin
expression and function: Role in bone remodeling. J Cell Biochem
Suppl. 30–31:92–102. 1998. View Article : Google Scholar
|
|
4
|
Lampe MA, Patarca R, Iregui MV and Cantor
H: Polyclonal B cell activation by the Eta-1 cytokine and the
development of systemic autoimmune disease. J Immunol.
147:2902–2906. 1991.PubMed/NCBI
|
|
5
|
Pullig O, Weseloh G, Gauer S and Swoboda
B: Osteopontin is expressed by adult human osteoarthritic
chondrocytes: Protein and mRNA analysis of normal and
osteoarthritic cartilage. Matrix Biol. 19:245–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honsawek S, Tanavalee A, Sakdinakiattikoon
M, Chayanupatkul M and Yuktanandana P: Correlation of plasma and
synovial fluid osteopontin with disease severity in knee
osteoarthritis. Clin Biochem. 42:808–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao SG, Li KH, Zeng KB, Tu M, Xu M and Lei
GH: Elevated osteopontin level of synovial fluid and articular
cartilage is associated with disease severity in knee
osteoarthritis patients. Osteoarthritis Cartilage. 18:82–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weber GF, Ashkar S, Glimcher MJ and Cantor
H: Receptor-ligand interaction between CD44 and osteopontin
(Eta-1). Science. 271:509–512. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunn S, Kolomytkin OV, Waddell DD and
Marino AA: Hyaluronan-binding receptors: Possible involvement in
osteoarthritis. Mod Rheumatol. 19:151–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang FJ, Luo W, Gao SG, et al: Expression
of CD44 in articular cartilage is associated with disease severity
in knee osteoarthritis. Mod Rheumatol. 23:1186–1191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milner PI, Fairfax TP, Browning JA,
Wilkins RJ and Gibson JS: The effect of O2 tension on pH
homeostasis in equine articular chondrocytes. Arthritis Rheum.
54:3523–3532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: HIF-1 and human disease: One
highly involved factor. Genes Dev. 14:1983–1991. 2000.PubMed/NCBI
|
|
13
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith TG, Robbins PA and Ratcliffe PJ: The
human side of hypoxia-inducible factor. Br J Haematol. 141:325–334.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito T, Fukai A, Mabuchi A, et al:
Transcriptional regulation of endochondral ossification by
HIF-2alpha during skeletal growth and osteoarthritis development.
Nat Med. 16:678–686. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang S, Kim J, Ryu JH, et al:
Hypoxia-inducible factor-2alpha is a catabolic regulator of
osteoarthritic cartilage destruction. Nat Med. 16:687–693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attur MG, Dave MN, Stuchin S, et al:
Osteopontin: An intrinsic inhibitor of inflammation in cartilage.
Arthritis Rheum. 44:578–584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsui Y, Iwasaki N, Kon S, et al:
Accelerated development of aging-associated and instability-induced
osteoarthritis in osteopontin-deficient mice. Arthritis Rheum.
60:2362–2371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilhelm J and Pingoud A: Real-time
polymerase chain reaction. ChemBioChem. 4:1120–1128. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponchel F, Toomes C, Bransfield K, et al:
Real-time PCR based on SYBR-Green I fluorescence: An alternative to
the TaqMan assay for a relative quantification of gene
rearrangements, gene amplifications and micro gene deletions. BMC
Biotechnol. 3:182003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martens G, Cai Y, Hinke S, Stangé G, Van
de Casteele M and Pipeleers D: Nutrient sensing in pancreatic beta
cells suppresses mitochondrial superoxide generation and its
contribution to apoptosis. Biochem Soc Trans. 33:300–301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: Cell biology
of osteoarthritis. Arthritis Res. 3:107–13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kühn K, D'Lima DD, Hashimoto S and Lotz M:
Cell death in cartilage. Osteoarthritis Cartilage. 12:1–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zamli Z and Sharif M: Chondrocyte
apoptosis: A cause or consequence of osteoarthritis? Int J Rheum
Dis. 14:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu JH, Shin Y, Huh YH, Yang S, Chun CH
and Chun JS: Hypoxia-inducible factor-2 α regulates Fas-mediated
chondrocyte apoptosis during osteoarthritic cartilage destruction.
Cell Death Differ. 19:440–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Y, Denhardt DT, Cao H, et al: Hypoxia
upregulates osteopontin expression in NIH-3T3 cells via a
Ras-activated enhancer. Oncogene. 24:6555–6563. 2005.PubMed/NCBI
|
|
29
|
Underhill C: CD44: The hyaluronan
receptor. J Cell Sci. 103:293–298. 1992.PubMed/NCBI
|
|
30
|
Ishii S, Ford R, Thomas P, Nachman A,
Steele G Jr and Jessup JM: CD44 participates in the adhesion of
human colorectal carcinoma cells to laminin and type IV collagen.
Surg Oncol. 2:255–264. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Isacke CM: The role of the cytoplasmic
domain in regulating CD44 function. J Cell Sci. 107:2353–2359.
1994.PubMed/NCBI
|
|
32
|
Knudson CB, Nofal GA, Pamintuan L and
Aguiar DJ: The chondrocyte pericellular matrix: A model for
hyaluronan-mediated cell-matrix interactions. Biochem Soc Trans.
27:142–147. 1999.PubMed/NCBI
|