Introduction
Percutaneous coronary intervention (PCI) is known to
effectively improve the prognosis of patients with coronary heart
diseases, particularly those with acute coronary syndrome (1). However, in-stent restenosis (ISR) is a
major concern that can compromise the long-term outcome of PCI
(2). The mechanisms of restenosis
secondary to PCI injury are very complex, and include local
reendothelialization and vascular remodeling mediated by a variety
of inflammatory cells, cytokines and growth factors. Poor
reendothelialization, and excessive migration and proliferation of
vascular smooth muscle cells in the tunica media, can result in
obstructive neointimal hyperplasia, and are considered to be the
major mechanisms involved in restenosis following PCI (3).
Vascular endothelial growth factor (VEGF) is a
homodimer glycoprotein (molecular weight, 45 kDa) composed of two
identical peptide chains connected by disulfide bonds. There are
five isoforms of the VEGF gene resulting from alternate splicing:
VEGF121, VEGF145, VEGF165, VEGF189 and VEGF206; among these,
VEGF165 is the biologically active form (4). The human VEGF gene is ~14 kb in length
and consists of eight exons. VEGF is primarily secreted by
endothelial cells, macrophages and fibroblasts. VEGF can stimulate
mitosis and angiogenesis by binding to VEGF receptors on the
surface of vascular endothelial cells (5).
The majority of animal studies indicate that local
delivery of the VEGF gene is able to promote vascular
reendothelialization and prevent restenosis (6–8),
although this remains controversial. Asahara et al
demonstrated that VEGF protein stimulated vascular
reendothelialization after local delivery into rat carotid arteries
following balloon injury (9). The
authors concluded that VEGF reduced the intimal thickening
resulting from the proliferation of smooth muscle cells. In
addition, the authors succeeded in treating balloon injuries in
rabbit iliac arteries using a locally delivered plasmid DNA
construct, phVEGF165, which supported the concept of using VEGF
gene therapy against restenosis (7).
However, Dulak et al (7)
demonstrated that a different plasmid DNA construct (pSG5VEGF165)
was unable to inhibit intimal hyperplasia in a rabbit model of
hypercholesterolemia. Normocholesterolemic rabbits were found to
benefit from VEGF following an arterial injury; however, since
hypercholesterolemia per se appeared to increase plasma VEGF
levels in the model, hypercholesterolemic rabbits did not receive
any benefit from exogenous VEGF, which may be due to the already
increased levels of VEGF and the decreased availability of nitric
oxide (7,10). This is consistent with the
observations of a previous study that performed adenoviral transfer
of VEGF in rabbits, and demonstrated that the therapeutic effect of
VEGF was nitric oxide-dependent (11). Two studies using a pig model revealed
that liposome-mediated VEGF gene transfer prevented the regression
of microvessels, enhanced the accumulation of elastin in the
adventitia, reduced the amount of myofibroblasts in the adventitia
and induced a healing inflammatory response. These mechanisms
indicated a potential role for VEGF transfer in the prevention of
restenosis (12,13). Furthermore, a mouse model of
adenovirus-mediated VEGF transfer showed that VEGF accelerated
endothelial repair and inhibited neointima formation following an
arterial injury (14). An additional
study investigating adenoviral transfer of VEGF in rabbits revealed
that VEGF accelerated the restoration of endothelium integrity and
decreased intimal hyperplasia following an arterial injury
(15). In addition, rabbits
implanted with VEGF-eluting stents were found to undergo
accelerated reendothelialization in the injured artery (16). However, results from randomized
controlled studies indicate that local delivery of the VEGF gene
into an injured coronary artery, using an adenovirus or liposome as
a vector, is not effective at preventing restenosis and improving
the long-term outcomes of patients (17,18).
This may be due to inadequate VEGF concentrations or the short
period of time that effective concentrations of VEGF are available
for action on local blood vessels.
Nanoparticles are an emerging vector for delivering
gene therapy, with excellent tissue penetration ability, good
absorption and sustained release (19). In addition, the use of nanoparticles
bypasses the requirement for conventional vectors to carry the
gene. Viral vectors are known to be associated with certain
limitations, including the induction of a host immune response,
random insertional mutagenesis, the eventual presence of a
wild-type vector in the administered preparation and unsuitable
tissue tropism (20,21). Similarly, a low efficiency and
transient gene expression have been reported with the use of
liposomal vectors (22). By
contrast, polylactic-polyglycolic acid (PLGA) is safe and has an
excellent biocompatibility, and is extensively used in medicine
with US Food and Drug Administration approval (23,24).
Nanoparticles can further enhance local drug concentrations and
thereby yield the desired therapeutic effects with excellent tissue
penetration and high cellular absorption rates. A previous study
demonstrated that nano- and microparticles can maintain measurable
drug concentrations for days after the injection (25). Nanoparticles are becoming valued as a
potential method for the treatment of restenosis (26). Guzman et al investigated PLGA
nanoparticles incorporated with dexamethasone for local delivery in
a rat carotid model of restenosis. The authors used
immunofluorescence to show that nanoparticles were present in each
of the arteries' three layers at 3 h and 24 h post-treatment, and
were present in the adventitial layer from days three to seven
(27). Furthermore, the arterial
vasa vasorum provided a path for nanoparticles to reach the
adventitial layer (8). The
adventitial layer has been hypothesized to function as a storage
pool for nanoparticles, facilitating their release. A previous
study in mice revealed that VEGF delivery using PLGA nanoparticles
enhanced vascular growth and connectivity (23). An additional study in dogs
demonstrated that stents coated with VEGF nanoparticles enhanced
the reendothelialization of injured arteries (28). Furthermore, a pig model that coeluted
VEGF and paclitaxel from a nanoparticle-coated stent was shown to
have similarly favorable results (29).
Since PLGA particles exhibit a promising efficacy,
VEGF nanoparticles were hypothesized to effectively induce the
expression of VEGF in a rabbit model of vascular restenosis induced
by aorta balloon injury. In the present study, a rabbit model of
vascular restenosis was established by abdominal aorta balloon
injury. The VEGF gene nanoparticles were prepared using nanoscale
particle technology and were locally delivered to determine their
beneficial effects on the restenosis of injured arteries. The
results of the present study may lead to novel therapeutic options
to limit restenosis following percutaneous coronary interventions
in patients with myocardial infarction.
Materials and methods
Ethical statement
Experimental protocols of the study were approved by
the Ethics Committee of Peking Union Medical College Hospital
(Beijing, China; approval ID, XJYYLL-2012107). Animal testing was
performed in accordance with the international guiding principles
for biomedical research (30), and
the animals used in the experiments were cared for according to
these guidelines.
Preparation and characterization of
the VEGF gene nanoparticles
A phacoemulsification method was used to prepare the
nanoparticles (31). Briefly, 200 mg
PLGA (Birmingham Polymers, Inc., Pelham, AL, USA) was dissolved in
a solution of indichloromethane containing the VEGF165 cDNA (4 ml;
obtained from the Department of Cardiology of the Peking Union
Medical College Hospital). Next, a 0.5% polyvinyl alcohol solution
(Sigma-Aldrich, St. Louis, MO, USA) was added and the samples were
placed in sonicating, ice-bath conditions. The solution was
centrifuged at 64,000 × g for 30 min until complete volatilization
was achieved. The VEGF gene nanoparticles were subsequently
freeze-dried into pellets, and stored in a dry environment at low
temperatures. Prior to use, the VEGF gene nanoparticles (6.6 mg/ml)
were dissolved in 0.9% NaCl to create a final VEGF gene
concentration of ~0.4 mg/ml. The nanoparticles' range of diameters
and ζ-potential were detected using a laser particle sizer
(Brookhaven Instruments Corporation, Holtsville, NY, USA). Particle
morphology was observed using scanning electron microscopy
(Hitachi, Ltd., Tokyo Japan), and the microstructure was observed
using transmission electron microscopy (Hitachi, Ltd.). VEGF gene
encapsulation rates were calculated using the following formula:
Encapsulation rate (%) = (total amount of loaded gene - amount of
gene in the supernatant)/total amount of loaded gene × 100%.
VEGF nanoparticle bioactivity
assay
A bioactivity assay was used to demonstrate that the
VEGF nanoparticles were biologically active; thus, if a VEGF gene
product was detectable in the cells. Briefly, the media layer of a
rabbit coronary artery was treated with 1.0 mg/ml collagenase I
(Gibco®, Invitrogen Life Technologies, Hong Kong, China) and 10
U/ml elastase (Gibco®, Invitrogen Life Technologies) at 37°C for 10
h. The resulting mixture was centrifuged at 120 × g for 7 min and
the supernatant was discarded. Primary cells were prepared for
culture using the collagen gel embedded method (32), with a collagen gel matrix and Media
199 supplemented with 10% fetal bovine serum and antibiotics (all
from Gibco®, Invitrogen Life Technologies). Primary cells were
subcultured in a culture dish, and maintained in culture medium at
37°C with 5% carbon dioxide. Subsequently, 2 µg VEGF nanoparticle
solution (20 µg/ml) or 0.9% NaCl was added to the cells. VEGF
expression levels in the medium were measured using a commercial
ELISA kit (CytImmune Sciences, Rockville, MD, USA). Experiments
were performed in triplicate.
Establishment of an animal model of
restenosis and drug delivery through a perfusion balloon
catheter
A total of 18 New Zealand male rabbits (aged, 4
months; purchased from The institute of Laboratory Animal Science,
Chinese Academy of Medical Sciences and Peking Union Medical
College, Beijing) were randomly divided into the control (n=6;
receiving normal saline), empty nanoparticles (n=6; receiving empty
nanoparticles) and VEGF nanoparticles (n=6; receiving VEGF gene
nanoparticles) groups. A further two rabbits did not receive any
treatment and were not allocated into a group. A rabbit model of
vascular restenosis was established in all rabbits by abdominal
aorta balloon injury (33). Briefly,
each rabbit received aspirin (12.5 mg/day) intragastrically from
the day prior to surgery until euthanasia. Rabbits were
anesthetized via an ear vein injection of sodium pentobarbital (30
mg/kg). A 30-mm incision was created over the right femoral artery
and the femoral artery was dissected. The distal end of the femoral
artery was ligated, and the proximal end was occluded. The femoral
artery was subsequently removed using ophthalmic scissors, and a
sheath guide wire was implanted into the artery, followed by the
insertion of a 5-French sheath (Cordis Corporation, Fremont, CA,
USA). A 40-mm incision was made along the ventral (right) side of
the midline and the abdominal aorta was dissected. The aortas
obtained from the two untreated rabbits were termed ‘normal
aortas’; the aortas in control group were from the restenosis
rabbits receiving normal saline. A site 10 mm below the renal
artery was selected as the nanoparticle delivery site, and a suture
was placed at the proximal end. The size of the balloon catheter
(Cordis Corporation) was selected according to the outer diameter
of the abdominal aorta. The balloon catheter was located at the
labeled site (~250 mm) in the abdominal aorta along the guide wire.
The balloon injury model was created by inflating the balloon to a
pressure of 10 atm, and moving the balloon retrograde by 100 mm
three times for 15 sec each. The balloon catheter was then
removed.
The perfusion balloon, GENIE Catheter™ (Acrostak
Corporation, Geneva, Switzerland), is a new local drug delivery
catheter designed to deliver various liquid therapeutic agents into
arteries. A GENIE Catheter™ with an outer diameter matched to the
aorta's lumen size was located at the injury site using a guide
wire via a 5-French sheath. VEGF gene nanoparticles, empty
nanoparticles or normal saline (0.9%) were delivered to the injury
site using the GENIE Catheter™, with a perfusion pressure of 2–3
atm over 5 min. The GENIE Catheter™, guide wire and 5-French sheath
were subsequently removed. The proximal end of the artery was
ligated and each wound layer was individually sutured. Penicillin
(80 MU) was intramuscularly administrated daily for three days
after surgery.
Intimal hyperplasia of the injured
artery
All rabbits were euthanized through air embolization
at day 28 after surgery. The injured abdominal aorta was removed
from the site where the drug had been delivered. The removed aorta
was washed with 10% phosphate-buffered saline, fixed in
formaldehyde, and embedded in paraffin for hematoxylin and eosin
staining. The neointima area (NIA), media area (MA) and
proliferation index (PI) of the aorta were calculated following
Weigert's staining. The PI was calculated using the following
formula: PI = NIA/MA. Picro-sirius red staining was used to detect
collagen expression, while immunohistochemistry was used to
determine the expression of α-actin, proliferating cell nuclear
antigen (PCNA), matrix metalloproteinase-2 (MMP-2), tissue
inhibitor of MMP-2 (TIMP-2), VEGF and C-reactive protein (CRP). The
numbers of positively stained cells were counted in five randomly
selected fields from each section. The positive expression index
(PEI) was calculated using the following formula: PEI (%) = number
of positively-stained cells/total number of cells in five fields ×
100%.
Statistical analysis
Statistical analyses were performed using SPSS 10.0
software (SPSS, Inc., Chicago, USA). The results are expressed as
the mean ± standard deviation for continuous data. The
Kolmogorov-Smirnov test was used for normalized tests. Differences
among three groups were assessed by analysis of variance, while
intergroup differences were further evaluated using the Bonferroni
method. The χ2 and Fisher's exact tests were used to
analyze categorical data, where P<0.05 was considered to
indicate a statistically significant difference.
Results
Nanoparticle characterization and
biological activity
The average diameter of the nanoparticles was 78.82
nm (range, 58.28–105.7 nm). The average ζ-potential was −12.2. The
VEGF gene encapsulation efficiency was 98% and the amount of loaded
gene was 4.67%.
The bioactivity assay results revealed that the
cells treated with VEGF nanoparticles expressed VEGF at 243.5±111.5
ng/l, while the saline-treated cells did not express VEGF.
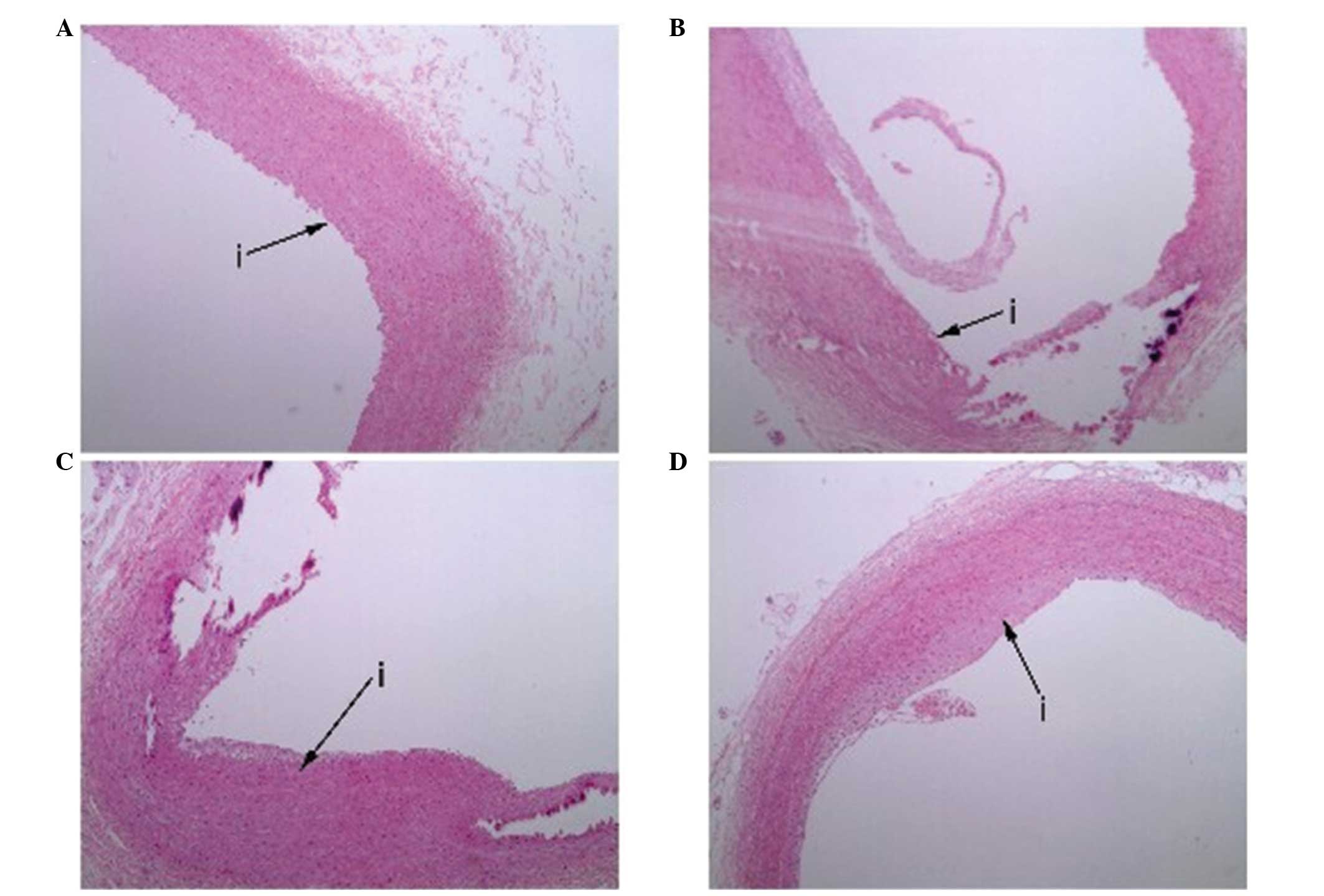
Histological examination of the intima
following injury and treatment
All the rabbits survived following the aorta balloon
injury and the local delivery of VEGF nanoparticles. In the control
and empty nanoparticle groups, histological examination revealed
partially denuded endothelial cells with intimal thickening, and
hyperplasia of foam cells, smooth muscle cells and fibrous tissue,
as well as the rupture of the internal elastic lamina (Fig. 1). By contrast, these pathological
changes were rarely observed in the VEGF nanoparticles group at day
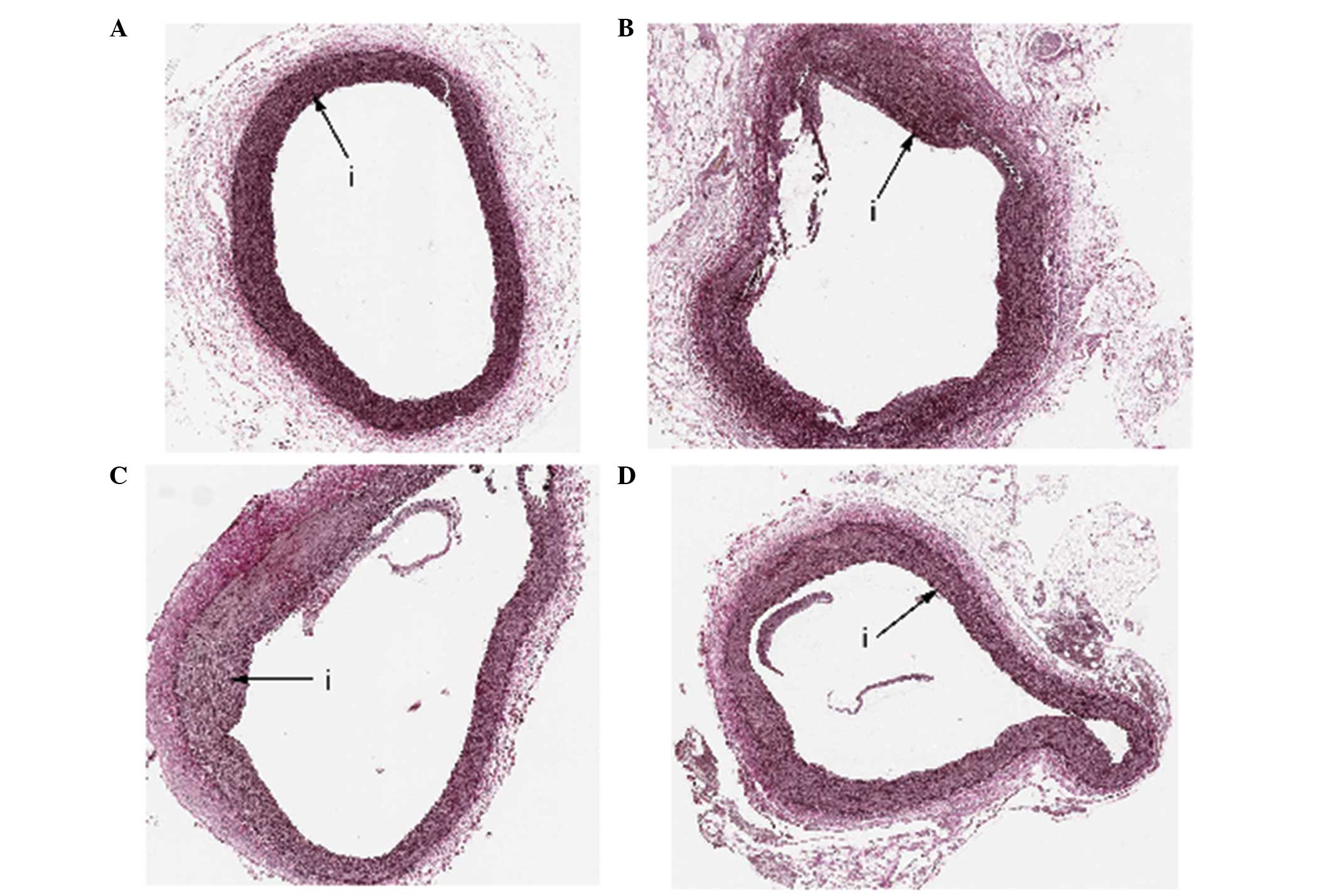
28 after balloon injury. Based on Weigert's staining (Fig. 2), the VEGF nanoparticles group
exhibited a decreased neointima area (VEGF nanoparticles, 0.19±0.11
mm2 vs. empty nanoparticles, 0.48±0.08 mm2
and controls, 0.49±0.09 mm2; P<0.001) and a decreased
proliferation index (VEGF nanoparticles, 0.13±0.06 vs. empty
nanoparticles, 0.32±0.05 and controls, 0.32±0.03; P<0.001) when
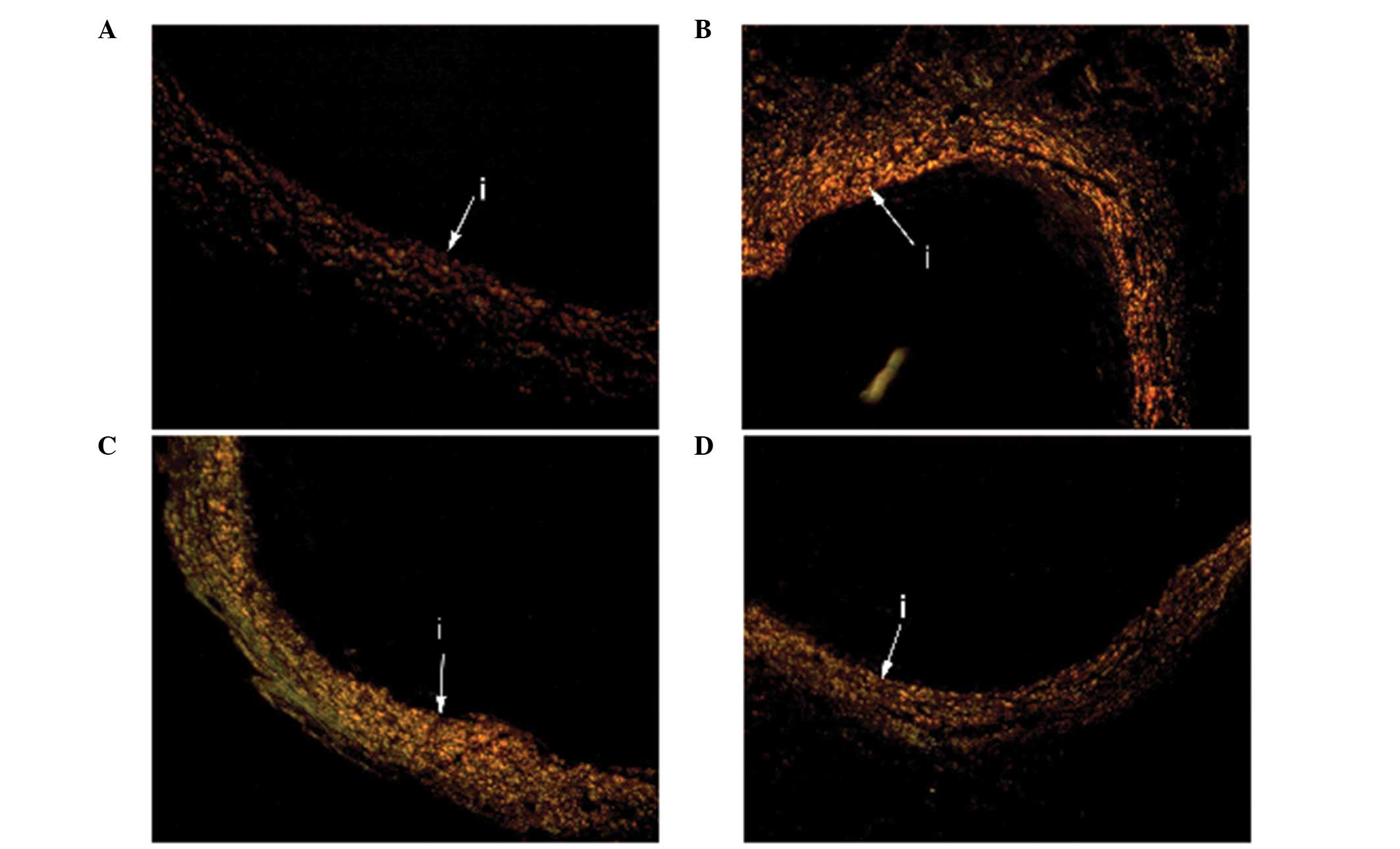
compared with the two other groups (Table I). Small amounts of type III and type
II collagen were observed in the media and adventitia of the vessel
walls from the three groups (Fig.
3).
 | Table I.Indices of intimal proliferation at
day 28 after balloon injury and treatment. |
Table I.
Indices of intimal proliferation at
day 28 after balloon injury and treatment.
| Parameters | Control group
(n=6) | Empty nanoparticles
group (n=6) | VEGF nanoparticles
group (n=6) | P-value |
|---|
| Neointima area,
mm2 | 0.49±0.09 |
0.48±0.08 |
0.19±0.11a | <0.001 |
| Media area,
mm2 |
1.53±0.26 |
1.55±0.39 |
1.75±1.43 | 0.889 |
| Proliferation
index |
0.32±0.03 |
0.32±0.05 |
0.13±0.06a | <0.001 |
Immunohistochemical examination of the
intima following injury and treatment
α-actin was used to identify smooth muscle cells,
while PCNA was used to determine the extent of cell proliferation.
MMP-2 plays an important role in extracellular matrix degradation
and cell migration, while TIMP-2 is the inhibitor of MMP-2. Thus,
the VEGF nanoparticles group showed decreases in the PEI of α-actin
(VEGF nanoparticles, 34.7±9.6% vs. empty nanoparticles, 65.7±16.2%
and controls, 65.0±21.3%; P=0.001) and PCNA (VEGF nanoparticles,
21.0±8.6% vs. empty nanoparticles, 69.5±13.7% and controls,
63.0±17.3%; P<0.001), and an increase in the PEI of VEGF (VEGF
nanoparticles, 45.8±10.5% vs. empty nanoparticles, 27.5±12.5% and
controls, 25.7±10.2%; P=0.01). The PEIs of MMP-2, TIMP-2 and CRP
were similar between the three groups (Table II).
 | Table II.Positive expression indexes (%) at
day 28 after balloon injury and treatment. |
Table II.
Positive expression indexes (%) at
day 28 after balloon injury and treatment.
|
| Control | Empty
nanoparticles | VEGF
nanoparticles |
|
|---|
| Parameters | group (n=6) | group (n=6) | group (n=6) | P-value |
|---|
| α-actin |
65.0±21.3 |
65.7±16.2 |
34.7±9.6a | 0.001 |
| PCNA |
63.0±17.3 |
69.5±13.7 |
21.0±8.6b | <0.001 |
| MMP-2 |
61.7±14.4 |
56.8±8.7 |
57.2±11.6 | 0.735 |
| TIMP-2 |
56.8±8.7 |
61.5±15.0 |
49.8±9.0 | 0.229 |
| VEGF |
25.7±10.2 |
27.5±12.5 |
45.8±10.5c | 0.012 |
| CRP |
61.7±11.5 |
60.5±10.3 |
57.8±12.1 | 0.836 |
Discussion
The aim of the present study was to investigate
whether VEGF nanoparticles can effectively induce the expression of
VEGF in a rabbit model of vascular restenosis. Immunohistochemical
analyses of the injured abdominal aortas from the experimental
rabbits demonstrated that treatment with VEGF nanoparticles
significantly increased the number of cells that were positive for
VEGF expression when compared with the control cells or those that
had been treated with empty nanoparticles, indicating that the VEGF
nanoparticles were able to induce VEGF expression.
The present study also investigated whether local
delivery of VEGF was able to effectively improve intimal
hyperplasia in the rabbit restenosis model. This issue is important
since previous studies investigating the use of VEGF for the
treatment of restenosis have produced conflicting results (6–14). In
general, the results indicate that when high cholesterol is
involved in model establishment, the beneficial effects of VEGF are
mitigated (7,12); however, a number of studies using
liposome- and virus-mediated VEGF transfer to injured arteries in a
variety of animal models have demonstrated similar beneficial
results (13,15). The present study showed that
characteristics observed in the rabbit model of vascular
restenosis, including intimal thickening, proliferation of foam
cells and smooth muscle cells and an increase in fibrous tissues,
were all decreased following VEGF nanoparticle treatment. The lower
rate of proliferation in the muscle cells was confirmed by the
decreased numbers of α-actin-positive cells, while the lower
overall rate of cell proliferation was demonstrated by the lower
number of PCNA-positive cells following VEGF nanoparticle
treatment. Therefore, the results from the present study concur
with the observations from previous studies, despite using a
completely different delivery method.
The two major differences between the present study
and previous studies were the use of a perfusion balloon catheter
as a delivery system and nanoparticles as gene vectors. The GENIE
Catheter™ has been approved for clinical use in the local delivery
of medication for ISR, branch lesions and small vessel diseases. A
key feature of the GENIE Catheter™ is that it can maintain drug
concentrations and perfusion pressure at the site of injury with
small doses and without any damage to the vessel walls. Herdeg
et al demonstrated the safety and efficacy of locally
administering paclitaxel through the GENIE Catheter™ as a PCI
strategy for treating patients with coronary heart disease
(34).
An additional potential benefit of the method used
in the present study is that unlike certain methods, such as
adenovirus delivery, nanoparticle delivery is unlikely to trigger
an immune response. The results of the current study indicated that
nanoparticles themselves do not trigger an immune response, since
the results observed with empty nanoparticles were similar to those
obtained in the control group. The uptake of nanoparticles by cells
has been shown to be dependent on particle size (35). The average and range of particle
sizes used in the present study were in accordance with those that
have been shown to have the most success at transferring into
arterial walls (36). Results from
the present study and from previous studies performed in different
animal models strongly suggest that the use of nanoparticles for
the sustained delivery of VEGF is an appropriate and efficient
method of promoting reendothelialization following an arterial
injury (23,28,29).
A small number of studies have assessed the effects
of VEGF transfer to arterial injuries in humans. Three studies used
adenoviruses and liposomes to transfer VEGF in patients undergoing
PCI for a coronary event (17,18,37).
These three studies demonstrated that the short- and long-term
safety was adequate. In addition, the results showed an increase in
vascularity, but without any effect on the clinical restenosis
rate. As previously discussed, the efficacy of adenovirus- and
liposome-mediated VEGF transfer is lower compared with the efficacy
achieved using VEGF-containing PLGA nanoparticles. Therefore,
future clinical trials using VEGF nanoparticles in humans may
result in a higher efficacy (8,23,24).
The present study has several limitations. Firstly,
the efficacy of VEGF gene nanoparticles was demonstrated without
comparing with other VEGF gene vectors. However, the previously
used vectors, viruses and liposomes, have been shown to have a low
efficacy and a number of issues associated with safety (17,38,39).
Nevertheless, a future study should compare all three modalities.
Secondly, the positive expression rate was calculated using an
immunohistochemistry assay, rather than a more direct
semi-quantitative polymerase chain reaction (PCR) or real-time PCR
method, to assess the expression of the proteins in the vessel
wall. Thirdly, only one concentration of VEGF was applied in the
nanoparticles; thus, future studies should investigate the effects
of different concentrations. Finally, the biodistribution,
bioavailability and biodegradation of the nanoparticles were not
analyzed in the model used in the present study. Assessment of
these parameters is planned for a future study.
In conclusion, the present study demonstrated the
efficacy of VEGF gene nanoparticles for the treatment of restenosis
following vascular injury, using an animal model. The results
provide a new direction for the clinical application of VEGF gene
therapy. However, this conclusion requires further confirmation by
future studies.
Acknowledgements
The study was supported by the PUMCH Young
Investigator Grant (no. 81271706) and the National Natural Science
Foundation of China (no. 30800225).
References
|
1
|
Singh M, Rihal CS, Berger PB, et al:
Improving outcome over time of percutaneous coronary interventions
in unstable angina. J Am Coll Cardiol. 36:674–678. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim MS and Dean LS: In-stent restenosis.
Cardiovasc Ther. 29:190–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jukema JW, Verschuren JJ, Ahmed TA and
Quax PH: Restenosis after PCI. Part 1: pathophysiology and risk
factors. Nat Rev Cardiol. 9:53–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu Y, Hoareau-Aveilla C, Oltean S, Harper
SJ and Bates DO: The anti-angiogenic isoforms of VEGF in health and
disease. Biochem Soc Trans. 37:1207–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrara N: The role of VEGF in the
regulation of physiological and pathological angiogenesis. EXS.
94:209–231. 2005.PubMed/NCBI
|
|
6
|
Hiltunen MO, Laitinen M, Turunen MP, et
al: Intravascular adenovirus-mediated VEGF-C gene transfer reduces
neointima formation in balloon-denuded rabbit aorta. Circulation.
102:2262–2268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dulak J, Schwarzacher SP, Zwick RH, et al:
Effects of local gene transfer of VEGF on neointima formation after
balloon injury in hypercholesterolemic rabbits. Vasc Med.
10:285–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simón-Yarza T, Formiga FR, Tamayo E, et
al: Vascular endothelial growth factor-delivery systems for cardiac
repair: an overview. Theranostics. 2:541–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asahara T, Bauters C, Pastore C, et al:
Local delivery of vascular endothelial growth factor accelerates
reendothelialization and attenuates intimal hyperplasia in
balloon-injured rat carotid artery. Circulation. 91:2793–2801.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaffney MM, Hynes SO, Barry F and O'Brien
T: Cardiovascular gene therapy: current status and therapeutic
potential. Br J Pharmacol. 152:175–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rutanen J, Turunen AM, et al: Gene
transfer using the mature form of VEGF-D reduces neointimal
thickening through nitric oxide-dependent mechanism. Gene Ther.
12:980–987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deiner C, Schwimmbeck PL, Koehler IS, et
al: Adventitial VEGF165 gene transfer prevents lumen loss through
induction of positive arterial remodeling after PTCA in porcine
coronary arteries. Atherosclerosis. 189:123–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pels K, Deiner C, Coupland SE, et al:
Effect of adventitial VEGF(165) gene transfer on vascular
thickening after coronary artery balloon injury. Cardiovasc Res.
60:664–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hutter R, Carrick FE, Valdiviezo C, et al:
Vascular endothelial growth factor regulates reendothelialization
and neointima formation in a mouse model of arterial injury.
Circulation. 110:2430–2435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Lu Z, Yue Y, et al: Experimental
study of adenovirus vector mediated-hVEGF165 gene on prevention of
restenosis after angioplasty. J Huazhong Univ Sci Technolog Med
Sci. 24:132–133, 137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walter DH, Cejna M, Diaz-Sandoval L, et
al: Local gene transfer of phVEGF-2 plasmid by gene-eluting stents:
an alternative strategy for inhibition of restenosis. Circulation.
110:36–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hedman M, Hartikainen J, Syvänne M, et al:
Safety and feasibility of catheter-based local intracoronary
vascular endothelial growth factor gene transfer in the prevention
of postangioplasty and in-stent restenosis and in the treatment of
chronic myocardial ischemia: phase II results of the Kuopio
Angiogenesis Trial (KAT). Circulation. 107:2677–2683. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hedman M, Muona K, Hedman A, et al:
Eight-year safety follow-up of coronary artery disease patients
after local intracoronary VEGF gene transfer. Gene Ther.
16:629–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin S and Ye K: Nanoparticle-mediated drug
delivery and gene therapy. Biotechnol Prog. 23:32–41. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Chirmule N, Gao GP, et al: Acute
cytokine response to systemic adenoviral vectors in mice is
mediated by dendritic cells and macrophages. Mol Ther. 3:697–707.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunetti-Pierri N, Palmer DJ, Beaudet AL,
et al: Acute toxicity after high-dose systemic injection of
helper-dependent adenoviral vectors into nonhuman primates. Hum
Gene Ther. 15:35–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kircheis R, Wightman L and Wagner E:
Design and gene delivery activity of modified polyethylenimines.
Adv Drug Deliv Rev. 53:341–358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Golub JS, Kim YT, Duvall CL, et al:
Sustained VEGF delivery via PLGA nanoparticles promotes vascular
growth. Am J Physiol Heart Circ Physiol. 298:H1959–H1965. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jain RA: The manufacturing techniques of
various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA)
devices. Biomaterials. 21:2475–2490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panyam J and Labhasetwar V: Biodegradable
nanoparticles for drug and gene delivery to cells and tissue. Adv
Drug Deliv Rev. 55:329–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brito L and Amiji M: Nanoparticulate
carriers for the treatment of coronary restenosis. Int J
Nanomedicine. 2:143–161. 2007.PubMed/NCBI
|
|
27
|
Guzman LA, Labhasetwar V, Song C, et al:
Local intraluminal infusion of biodegradable polymeric
nanoparticles. A novel approach for prolonged drug delivery after
balloon angioplasty. Circulation. 94:1441–1448. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paul A, Shao W, Shum-Tim D and Prakash S:
The attenuation of restenosis following arterial gene transfer
using carbon nanotube coated stent incorporating TAT/DNA
(Ang1+Vegf) nanoparticles. Biomaterials. 33:7655–7664. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Zeng Y, Zhang C, et al: The
prevention of restenosis in vivo with a VEGF gene and paclitaxel
co-eluting stent. Biomaterials. 34:1635–1643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
World Medical Association: Guiding
principles for research involving animals and human beings. Am J
Physiol Heart Circ Physiol. 281:3 following. H27612001.
|
|
31
|
Xu YY, Li YJ, Guan H, et al: The effect of
vascular endothelia growth factor encapsulated in nanoparticles on
chronic limb ischemia. Zhonghua Wai Ke Za Zhi. 42:58–61.
2004.PubMed/NCBI
|
|
32
|
Sasaki M, Sawada N, Minase T, Satoh M and
Mori M: Collagen-gel-embedded three-dimensional culture of human
thyroid epithelial cells: comparison between the floating sandwich
method and the dispersed embedding method. Cell Struct Funct.
16:209–215. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagae T, Aizawa K, Uchimura N, et al:
Endovascular photodynamic therapy using mono-L-aspartyl-chlorin e6
to inhibit Intimal hyperplasia in balloon-injured rabbit arteries.
Lasers Surg Med. 28:381–388. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herdeg C, Göhring-Frischholz K, Haase KK,
et al: Catheter-based delivery of fluid paclitaxel for prevention
of restenosis in native coronary artery lesions after stent
implantation. Circ Cardiovasc Interv. 2:294–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kettler K, Veltman K, van de Meent D, van
Wezel A and Hendriks AJ: Cellular uptake of nanoparticles as
determined by particle properties, experimental conditions, and
cell type. Environ Toxicol Chem. 33:481–492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Westedt U, Barbu-Tudoran L, Schaper AK, et
al: Deposition of nanoparticles in the arterial vessel by porous
balloon catheters: localization by confocal laser scanning
microscopy and transmission electron microscopy. AAPS Pharm Sci.
4:E412002. View
Article : Google Scholar
|
|
37
|
Mäkinen K, Manninen H, et al: Increased
vascularity detected by digital subtraction angiography after VEGF
gene transfer to human lower limb artery: a randomized,
placebo-controlled, double-blinded phase II study. Mol Ther.
6:127–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stewart DJ, Kutryk MJ, Fitchett D, Freeman
M, et al: VEGF gene therapy fails to improve perfusion of ischemic
myocardium in patients with advanced coronarydisease: results of
the NORTHERN trial. Mol Ther. 17:1109–1115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wirth T, Hedman M, et al: Safety profile
of plasmid/liposomes and virus vectors in clinical gene therapy.
Curr Drug Saf. 1:253–257. 2006. View Article : Google Scholar : PubMed/NCBI
|