Introduction
Ischemic heart disease is one of the leading causes
of morbidity and mortality worldwide (1). The survival of the ischemic myocardium
is dependent upon early reperfusion; however, reperfusion is known
as a ‘double-edged sword’ due to the spectrum of
reperfusion-associated pathologies, which are collectively referred
to as reperfusion injury (2).
Ventricular arrhythmia is a potentially lethal consequence of
myocardial ischemia/reperfusion (I/R) injury.
The administration of free radical scavengers and
antioxidants has become an important approach in the treatment of
myocardial ischemia or I/R injury and the suppression of
ventricular arrhythmia. The key antioxidant enzyme Cu/Zn superoxide
dismutase (SOD1) acts to diminish the effects of oxidative stress
by catalyzing the dismutation of the superoxide anion into hydrogen
peroxide plus oxygen, thereby protecting the cells from oxidative
damage; however, due to the considerable damage to the endogenous
antioxidant activity induced by I/R, the vulnerability of the
myocardium to oxygen free radicals is enhanced following I/R injury
(3). Furthermore, the selectivity
and limited permeability of the cell membrane prevents the delivery
of exogenous SOD1 into living cells, thus limiting the ability of
SOD1 to protect the cells/tissues from oxidative stress-induced
damage (4).
PEP-1 is a peptide carrier that possesses the
ability to deliver full-length, native peptides or proteins into
cells without the requirement for conformational changes (5). Through protein transduction technology
and the construction of full-length PEP-1 fusion proteins, numerous
proteins, including enhanced green fluorescent protein,
β-galactosidase, full-length specific antibodies, human copper
chaperone for SOD1, catalase and SOD, have been successfully
delivered into cultured cells and the nervous system (6–10). Our
previous studies have indicated that PEP-1-SOD1 fusion proteins can
be delivered into myocardial tissues to protect the myocardium from
I/R-induced injury in rats (11–13);
however, no studies, to the best of our knowledge, have considered
the possible use of PEP-1-SOD1 as a therapeutic agent to treat the
acute symptoms of myocardial ischemia or I/R injury-induced
arrhythmia. The aim of the present study, therefore, was to
evaluate the antiarrhythmic effects of PEP-1-SOD1 on myocardial I/R
injury in rats subjected to transient coronary artery occlusion and
reperfusion.
Materials and methods
Animals
Male Sprague Dawley rats (280–320 g) were obtained
from Beijing HFK Bioscience Co. Ltd. (Beijing, China). The rats
were kept in an animal house with a 12-h light/dark cycle. The
study was approved by the Animal Ethics Committee of Hubei
University of Medicine (Shiyan, China).
Generation of biologically active
PEP-1-SOD1 fusion protein
PEP-1-SOD1 fusion protein was isolated and purified
in accordance with the method described in one of our previous
studies (13,14). Briefly, two prokaryotic expression
plasmids for SOD1 and PEP-1-SOD1 (Novogen, Billerica, MA, USA) were
constructed using the TA-cloning method. Six histidine residues
(His-tag; Promega, Madison, WI, USA) were used to tag the two
recombinant at the amino terminus. The two proteins were expressed
and purified separately, as previously described (14).
Induction of I/R
The rats were systemically heparinized [1,000 U/kg,
intraperitoneal (i.p.)] and anesthetized with sodium pentobarbital
(60 mg/kg, i.p.; Shanghai No.1 Biochemical and Pharmaceutical Co.,
Ltd., Shanghai, China). The heart was rapidly excised and a
modified Langendorff apparatus was used to retrogradely perfuse the
heart via the aorta under constant pressure (80 mmHg) (95%
O2 and 5% CO2) with Krebs-Henseleit buffer
(118 mmol/l NaCl, 24.5 mmol/l NaHCO3, 4.7 mmol/l KCl,
1.2 mmol/l KH2PO4, 3.4 mmol/l
MgSO4·7H2O, 2.5 mmol/l CaCl2 and
5.5 mmol/l glucose; Shanhhai Zongze Biotechnology, Co., Ltd.,
Shanghai, China) at 37°C, as previously described (15). A Kreb's solution-filled latex balloon
was inserted into the left ventricle in order to monitor the left
ventricular hemodynamic parameters using a digital acquisition and
analysis system (Chengdu Taimeng Technology Co., Ltd., Chengdu,
China). The hemodynamic parameters included heart rate (HR), left
ventricular systolic pressure (LVSP) and the maximal rate of
pressure rise (+dp/dtmax). Coronary flow (CF) was monitored by
collecting pulmonary artery effluent. Following the recording of
baseline data, each heart was subjected to 30 min of global
ischemia, followed by 60 min of reperfusion. The elevation of the
S-T segment on the electrocardiogram was indicative of the
successful induction of ischemia.
Experimental groups
The rats were assigned to one of six groups
(n=10/group), as follows: Sham (no global ischemia), control
(subjected to global ischemia), PEP-1-SOD1-treated (25, 50 and 100
µmol/l) and SOD1-treated (100 µmol/l). In the drug-treated groups,
PEP-1-SOD1 and SOD1 were administered as components of the
perfusion medium 15 min prior to ischemia. The hearts of the rats
in the untreated group were perfused for an additional 15 min with
perfusion medium only.
Measurement of cardiac marker
enzymes
Coronary effluent was collected 60 min after
reperfusion in order to measure the activity of lactate
dehydrogenase (LDH) and serum creatine kinase-MB (CK-MB) using
assay kits obtained from the Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). Enzyme activity was determined
spectrophotometrically.
Infarct size assessment
Myocardial infarct size was assessed with Evans Blue
dye and 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich,
St. Louis, MO, USA). Following the excision of the heart, the left
ventricle was cut into 2-mm transverse slices, from apex to base.
Following incubation in 1% TTC (pH 7.4) at 37°C for 15 min, the
slices were placed in 4% formaldehyde for 1 day. The infarcted
myocardium was not stained by the TTC and thus appeared white,
while the non-ischemic myocardium was stained brick-red by the TTC.
Infarct area analyses were performed using ImageJ software
(National Institutes of Health, Bethesda, MD, USA), and the results
are expressed as the percentage of left ventricular volume for each
heart.
Reperfusion-induced arrhythmia
evaluation
Arrhythmias were evaluated based on the guidelines
of the Lambeth Conventions (16).
Ventricular tachycardia (VT) was defined as the occurrence of ≥4
consecutive premature ventricular contractions, while ventricular
fibrillation (VF) was defined as an inability to distinguish
individual QRS deflections. The prevalence and total duration of VT
and VF during the reperfusion period were measured.
Intracellular reactive oxygen species
(ROS) measurement
ROS generation was measured with the sensitive
fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Cardiomyocytes were dispersed from the hearts using digestion
buffer, washed with KB solution and then incubated with 5 µmol
DCFH-DA for 20 min, according to the instructions of commercial
kits. The fluorescence intensity was measured using a
fluorospectrophotometer with 488-nm excitation and 525-nm emission
filters.
Statistical analysis
All experiments were performed in triplicate, and
the data are presented as the mean ± standard deviation.
Statistical significance was determined using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects on hemodynamics
The LVSP, +dp/dtmax, CF and HR in the control group
were observed to have decreased markedly after 60 min of
reperfusion (P<0.01) (Table I).
Compared with the control group, a significant recovery of LVSP,
+dp/dtmax and CF was apparent in the PEP-1-SOD1-treated groups, and
this effect was concentration-dependent (P<0.05). The rats in
the SOD1 pretreated group had less obvious improvements in LVSP,
+dp/dtmax and CF. No significant difference in HR was found between
the control and pretreated groups.
 | Table I.Effect of PEP-1-SOD1 fusion protein on
hemodynamics (n=10/group). |
Table I.
Effect of PEP-1-SOD1 fusion protein on
hemodynamics (n=10/group).
|
| LVSP (mmHg) | +dp/dtmax
(mmHg/sec) | CF (ml) | HR (bpm) |
|---|
|
|
|
|
|
|
|---|
| Group | Baseline | RP 60 min | Baseline | RP 60 min | Baseline | RP 60 min | Baseline | RP 60 min |
|---|
| Sham |
102±2 |
97±4 |
2,207±135 |
2,145±155 |
10.8±0.9 |
10.8±0.8 |
263±20 |
265±18 |
| Control |
101±6 |
58±4a |
2,189±179 |
1,312±82a |
10.6±1.8 |
4.9±1.1a |
265±18 |
170±19a |
| SOD1 |
98±5 |
59±5 |
2,175±165 |
1,280±73 |
11.0±1.9 |
4.8±0.8 |
270±19 |
162±15 |
| PEP-1-SOD1 (25
µmol/l) |
100±5 |
66±3b |
2,203±144 |
1,394±32c |
10.2±1.7 |
5.8±0.8c |
262±12 |
155±18 |
| PEP-1-SOD1 (50
µmol/l) |
98±6 |
70±3b |
2,098±129 |
1,503±42b |
11.0±1.7 |
6.9±0.7b |
257±16 |
158±14 |
| PEP-1-SOD1 (100
µmol/l) |
100±6 |
74±2b |
2,122±150 |
1,583±99b |
10.7±1.4 |
7.8±1.1b |
269±18 |
154±18 |
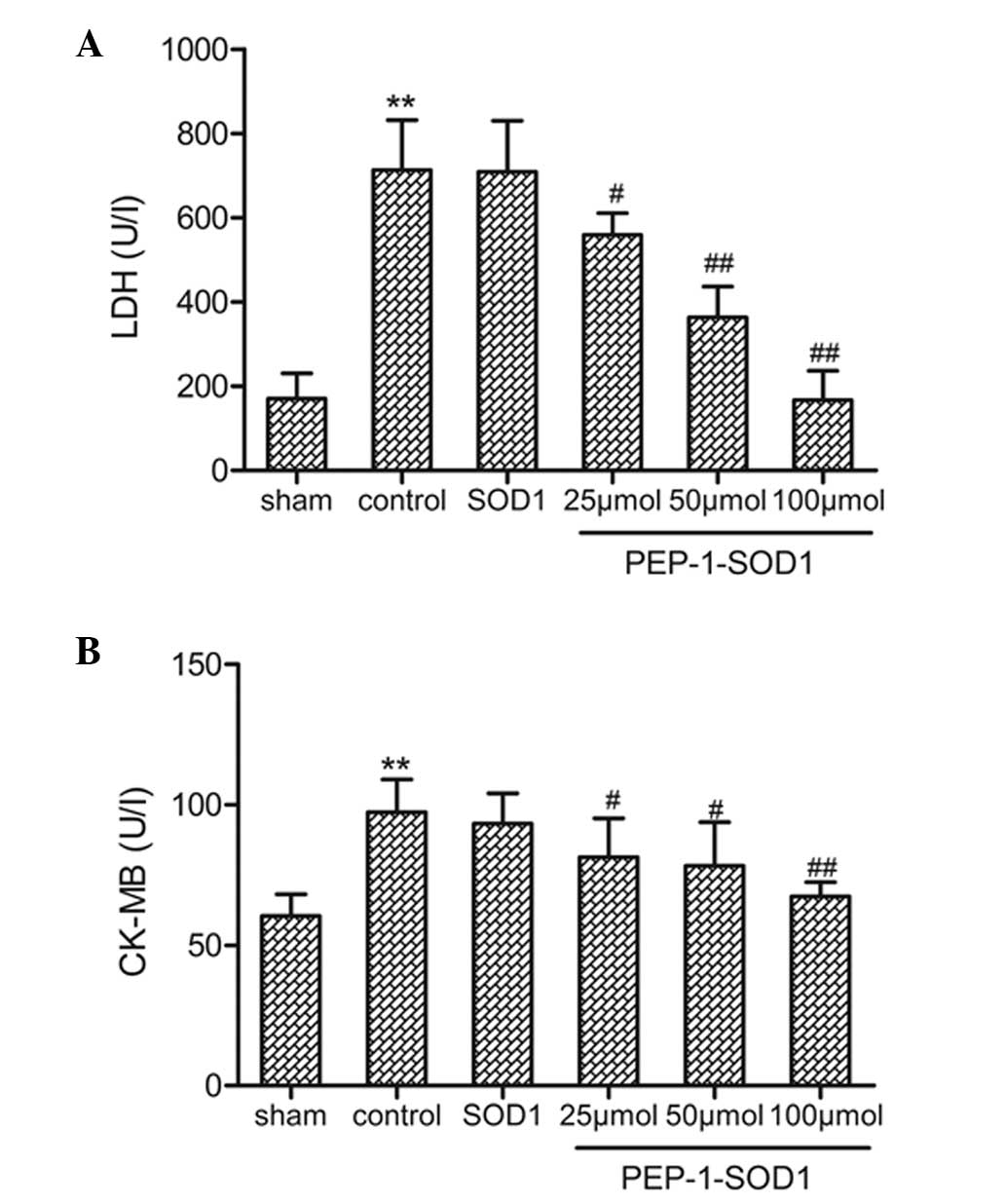
Effects on LDH and CK-MB activity
The activity of the cardiac biomarkers in the
coronary effluent was measured as an index of myocardial cellular
injury following I/R and found to be elevated during reperfusion.
As shown in Fig. 1, the LDH
(Fig. 1A) and CK-MB (Fig. 1B) activities were significantly
increased in the control group compared with those in the sham
group. Pretreatment with PEP-1-SOD1 markedly decreased the LDH
activity after 60 min of reperfusion compared with the control
(P<0.05). Similar to the LDH activity, the CK-MB activity was
also significantly reduced by PEP-1-SOD1 pretreatment
(P<0.05).
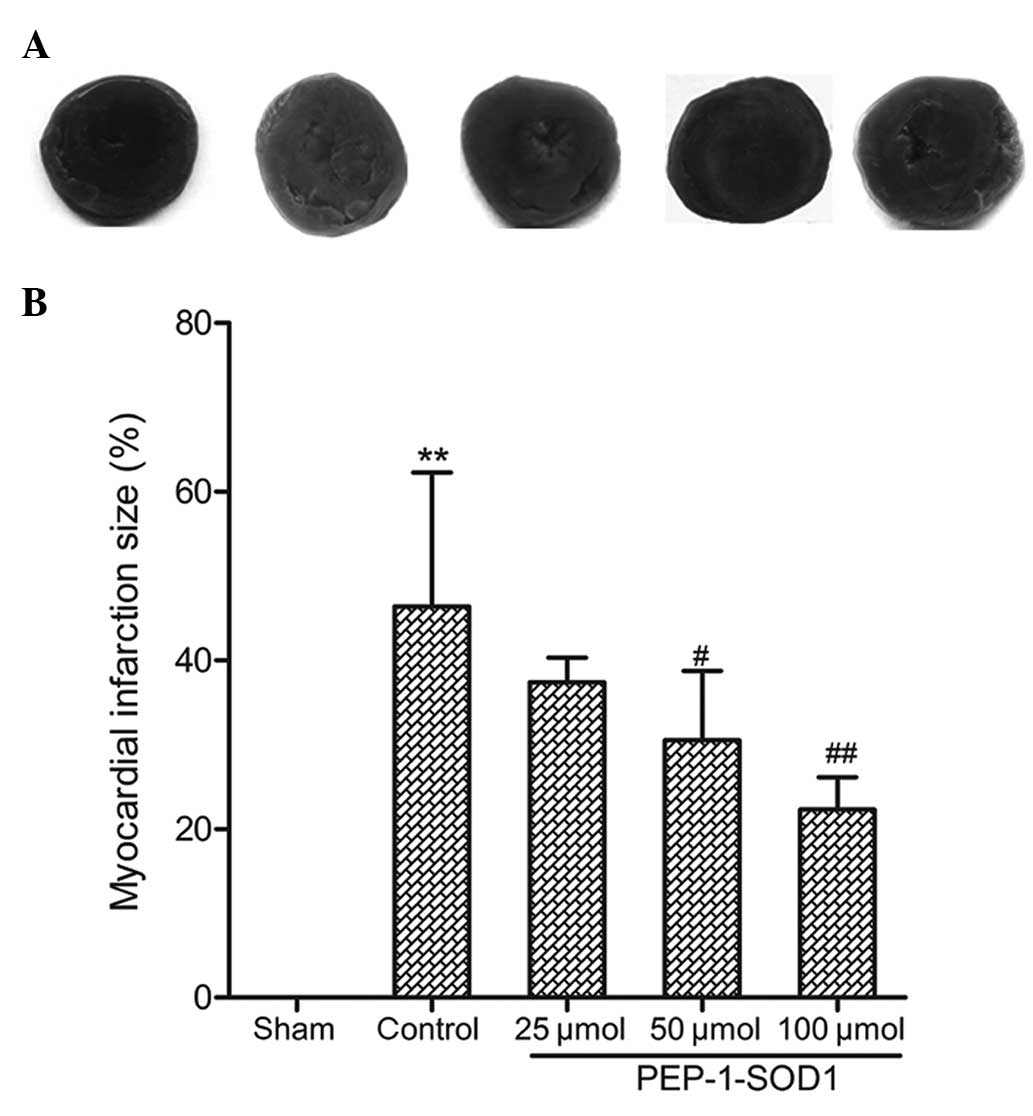
Effect on infarct size
As shown in Fig. 2A and
B, infarct size was significantly decreased in the
PEP-1-SOD1-pretreated groups compared with that in the control
group. The effect of PEP-1-SOD1 was concentration-dependent
(P<0.05).
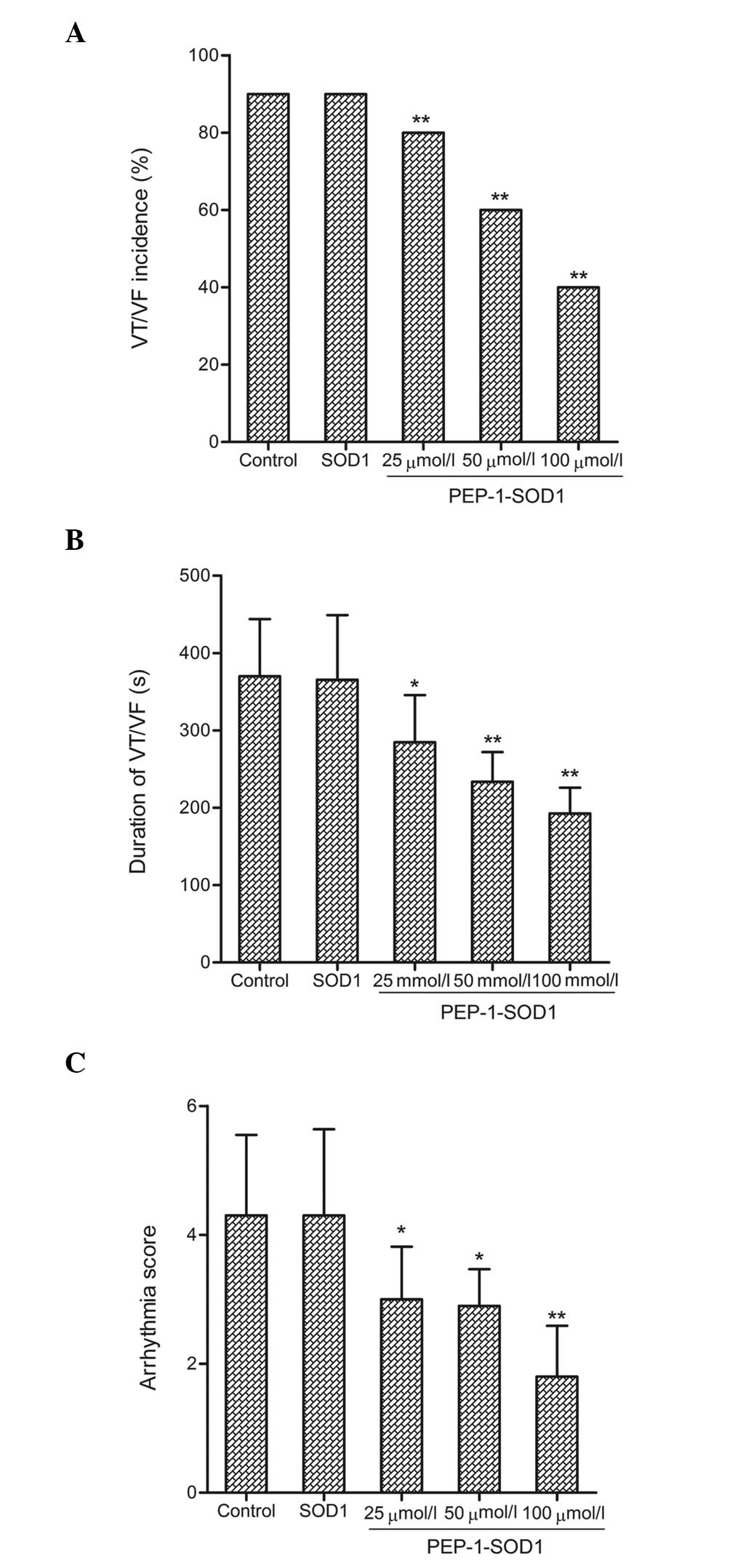
Effect on arrhythmias
To assess the I/R-induced arrhythmia, all
ventricular arrhythmias that occurred during the 60-min reperfusion
period were recorded. In the sham group, no obvious ventricular
arrhythmias were noted. Compared with the control group, PEP-1-SOD1
significantly reduced both the duration and incidence of VT/VF
(Fig. 3A and B). I/R-induced
arrhythmias were additionally evaluated using a scoring system. The
I/R-induced arrhythmia score was 4.3 in the control group; however,
PEP-1-SOD1 administration was shown to significantly decrease this
score (Fig. 3C, P<0.05).
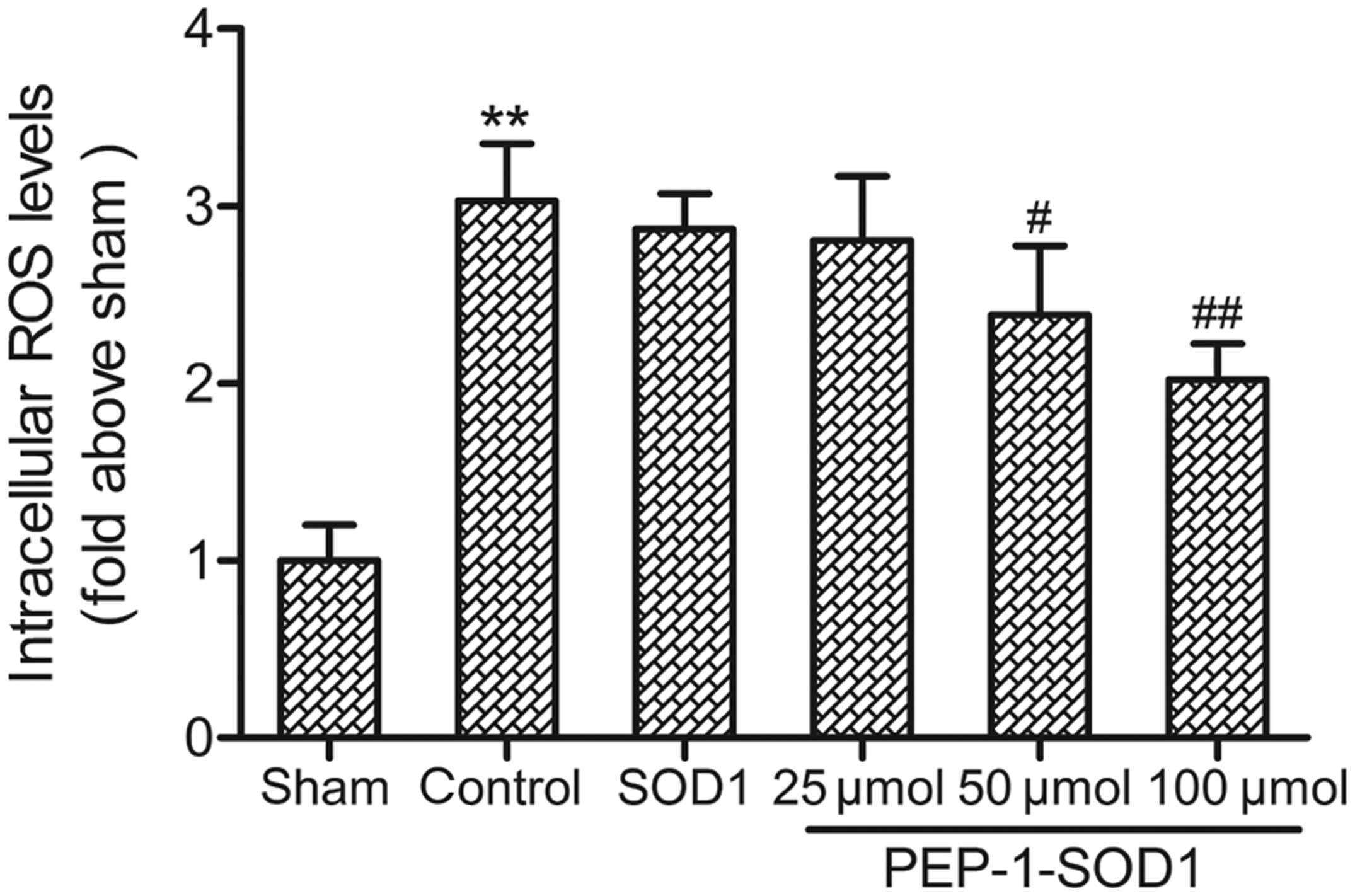
Effect on intracellular ROS
levels
It is generally accepted that ROS are one of the
major contributors to I/R-induced myocardial damage; therefore, the
effect of PEP-1-SOD1 on I/R-induced ROS generation was measured. As
shown in Fig. 4, the intracellular
ROS level in the control group increased to 303% of the level of
the sham group. Significant reductions in ROS levels were observed
in the PEP-1-SOD1-treated groups compared with the control group
(P<0.05).
Discussion
Myocardial I/R injury can induce ventricular
arrhythmia, leading to circulatory collapse and ultimately sudden
mortality. The proposed underlying pathophysiological mechanisms
include a burst generation of reactive oxygen intermediates,
intracellular Ca2+ handling and ion channels. It is
therefore believed that the effective inhibition of oxygen free
radical production or the elimination of oxygen free radicals could
reduce the ventricular arrhythmia caused by I/R injury (17). In the present study, the results
showed that pretreatment with PEP-1-SOD1 led to a recovery in
cardiac function (Table I), a
reduction in myocardial-specific biomarker release (Fig. 1) and decreased infarct size (Fig. 2) in isolated rat hearts exposed to
I/R injury. These findings were similar to those of previous
studies (11,13). In addition, it was found that
PEP-1-SOD1 could attenuate reperfusion-induced arrhythmia, as shown
by reductions in the VT/VF duration, VT/VF incidence and arrhythmia
score (Fig. 3).
At present, the precise mechanism underlying the
antiarrhythmic effects elicited by PEP-1-SOD1 remains elusive. In
this study, PEP-1-SOD1 was shown to reduce the myocardial I/R
injury-induced ROS generation; the generation of ROS plays an
important role in the regulation of reperfusion-induced arrhythmia.
A recent study (18) reported that
ion channels, such as the ATP-sensitive potassium channel, L-type
calcium channel, Na+-Ca2+ exchanger and
Na+-H+ exchanger, also have critical
functions in the pathophysiology and electrophysiology of I/R
injury. It has yet to be determined whether these ion channels are
involved in the modulation of reperfusion-induced arrhythmia by
PEP-1-SOD1. These findings, in combination with those of our
previous studies, indicate that the cardioprotective effects
elicited by PEP-1-SOD1 are associated with the regulation of lipid
peroxidation and ROS generation.
The present study was subject to several
limitations. It should be noted that the study evaluated
reperfusion-induced arrhythmia in the Langendorff model, in which
the hearts were denervated and were not affected by extrinsic
factors; however, reperfusion-induced arrhythmia is associated not
only with the dysfunction of the intact hearts, but also with
neurohumoral factors. The antiarrhythmic effects of PEP-1-SOD1
therefore still require verification in in vivo models.
In conclusion, this study has provided evidence that
PEP-1-SOD1 can effectively improve the hemodynamic parameters,
decrease the activity of myocardial-specific biomarkers, reduce the
infarct size and downregulate ventricular arrhythmias in isolated
rat hearts subjected to I/R insult. PEP-1-SOD1 reduces both the
duration and incidence of VT/VF, and this effect may be associated
with the regulation of lipid peroxidation and ROS generation.
Acknowledgements
This study was supported by the Hubei Province
Outstanding Scientific Innovation Team Plans (grant no. T200811)
and the Shiyan City Significant Technological Project (grant no.
2006030Z6).
References
|
1
|
Forini F, Nicolini G and Iervasi G:
Mitochondria as key targets of cardioprotection in cardiac ischemic
disease: Role of thyroid hormone triiodothyronine. Int J Mol Sci.
16:6312–6336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalogeris T, Bao Y and Korthuis RJ:
Mitochondrial reactive oxygen species: A double edged sword in
ischemia/reperfusion vs. preconditioning. Redox Biol. 2:702–714.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vijayasarathy K, Shanthi Naidu K and
Sastry BK: Melatonin metabolite 6-Sulfatoxymelatonin, Cu/Zn
superoxide dismutase, oxidized LDL and malondialdehyde in unstable
angina. Int J Cardiol. 144:315–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang YE, Fu SZ, Li XQ, et al: PEP-1-SOD1
protects brain from ischemic insult following asphyxial cardiac
arrest in rats. Resuscitation. 82:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim MJ, Jeong HJ, Kim DW, et al:
PEP-1-PON1 protein regulates inflammatory response in raw 264.7
macrophages and ameliorates inflammation in a TPA-induced animal
model. PLoS One. 9:e860342014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He XH, Wang Y, Yan XT, et al: Transduction
of PEP-1-heme oxygenase-1 fusion protein reduces myocardial
ischemia/reperfusion injury in rats. J Cardiovasc Pharmacol.
62:436–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong X, Wang JN, Huang YZ, Guo LY and Kong
X: Cell-penetrating peptide PEP-1-mediated transduction of enhanced
green fluorescent protein into human colorectal cancer SW480 cells.
Ai Zheng. 26:216–219. 2007.PubMed/NCBI
|
|
8
|
Henriques ST, Costa J and Castanho MA:
Translocation of beta-galactosidase mediated by the
cell-penetrating peptide pep-1 into lipid vesicles and human HeLa
cells is driven by membrane electrostatic potential. Biochemistry.
44:10189–10198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim W, Kim DW, Yoo DY, et al:
Neuroprotective effects of PEP-1-Cu,Zn-SOD against ischemic
neuronal damage in the rabbit spinal cord. Neurochem Res.
37:307–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Dong XW, Wang JN, et al:
PEP-1-CAT-transduced mesenchymal stem cells acquire an enhanced
viability and promote ischemia-induced angiogenesis. PLoS One.
7:e525372012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YE, Wang JN, Tang JM, et al: In vivo
protein transduction: Delivery of PEP-1-SOD1 fusion protein into
myocardium efficiently protects against ischemic insult. Mol Cells.
27:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke ZP, Wang JN, Tang JM, et al: The role
of PEP-1-SOD1 fusion protein on ischemia-reperfusion injury in
isolated perfused rat hearts. Zhonghua Xin Xue Guan Bing Za Zhi.
37:268–274. 2009.(In Chinese). PubMed/NCBI
|
|
13
|
Huang GQ, Wang JN, Tang JM, et al: The
combined transduction of copper, zinc-superoxide dismutase and
catalase mediated by cell-penetrating peptide, PEP-1, to protect
myocardium from ischemia-reperfusion injury. J Transl Med.
9:732011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JN, Ding P, Huang YZ, Luo LN, Guo LY,
Kong X and Shao F: The protective effect of PEP-1-SOD1
preconditioning on hypoxia/reoxygenation injury in cultured human
umbilical vein endothelial cells. Zhonghua Xin Xue Guan Bing Za
Zhi. 35:750–756. 2007.(In Chinese). PubMed/NCBI
|
|
15
|
Bhandary BI, Piao CS, Kim DS, Lee GH, Chae
SW, Kim HR and Chae HJ: The protective effect of rutin against
ischemia/reperfusion-associated hemodynamic alteration through
antioxidant activity. Arch Pharm Res. 35:1091–1097. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curtis MJ and Walker MJ: Quantification of
arrhythmias using scoring systems: An examination of seven scores
in an in vivo model of regional myocardial ischaemia. Cardiovasc
Res. 22:656–665. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong JS, Yao YT, Fang NX and Li LH:
Sevoflurane postconditioning attenuates reperfusion-induced
ventricular arrhythmias in isolated rat hearts exposed to
ischemia/reperfusion injury. Mol Biol Rep. 39:6417–6425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JI, Wook Nha K, Suh JS, Choo SK, Park
JH and Park JW: Remote postconditioning attenuates
ischemia/reperfusion injury in rat skeletal muscle through
mitochondrial ATP-sensitive K+ channel-dependent
mechanism. J Reconstr Microsurg. 29:571–578. 2013. View Article : Google Scholar : PubMed/NCBI
|