Introduction
Acute cerebral infarction is one of the most common
clinical cardiovascular and cerebrovascular diseases. Due to its
high incidence and fatality rate, it has become one of the most
serious diseases affecting human health (1,2). The
pathological basis of acute cerebral infarction is the disruption
of blood supply in the brain, with ischemia and hypoxia causing
ischemic necrosis and cerebral malacia in focal brain tissue
(3). In the acute stage of cerebral
infarction, vascular occlusion can lead to a reduction in blood
flow in the affected region and create a hypoxic state; cerebral
hypoxia and recanalization cause a stress response, leading to the
release of a large number of free radicals and the stimulation of
brain cells, causing neuronal cell apoptosis that ultimately
results in a loss of biological function (4,5).
Therefore, apoptosis plays an important role in the development
process of acute cerebral infarction.
A large number of studies have indicated that the
apoptosis of brain cells and neurons in rats and other animal
models with acute cerebral infarction is significantly increased
(6). Apoptosis is a cascade reaction
mediated by cysteine proteinases (caspases). These include
caspase-3, a protein that is affected by the cell surface death
receptor-mediated and mitochondrial apoptosis pathways and thus
plays an important role in the mediation of cell apoptosis
(7–9). Studies have shown that caspase-3
expression levels and protein activity are significantly increased
in the brain cells of patients with acute cerebral infarction, and
there is a certain correlation with the prognosis of the patient
(10). Therefore, the effective
delay and inhibition of brain cell or neuronal apoptosis in
patients with acute cerebral infarction reduces brain tissue damage
and necrosis, decreases the fatality and disability rates, and has
a protective effect on the brain. At present, acute cerebral
infarction is usually treated by anti-free radical, anti-oxidation
and thrombolytic therapies, among others, and there are very few
reports concerning the direct use of anti-apoptotic drugs for the
treatment of acute cerebral infarction (11). Therefore, the present study explored
the effect of the caspase-3 inhibitor z-DEVD-fmk on the apoptosis
of brain tissue in rats with acute cerebral infarction, with the
aim of identifying a new treatment option for the clinical
treatment of acute cerebral infarction.
Materials and methods
Establishment of the acute cerebral
infarction model
Sprague-Dawley rats (180–200 g) were purchased from
the Shanghai Laboratory Animal Center (SLAC, Shanghai, China). The
method of Longa et al was adopted to establish a rat model
of middle cerebral artery infarction (12). At 2 h after the surgery, rats showed
listlessness, homolateral Horner's syndrome, contralateral forelimb
prolapse, internal adduction and rotation, and spontaneously
homolateral circling, indicating the successful establishment of
model. If there were no such symptoms, then the establishment of
model was considered to have failed, and the rat was removed from
the study.
The rats were divided into three groups,
specifically the sham group (n=15), model group (n=15) and
treatment group (n=15). Models were established according to the
literature method (12) for the
model and treatment groups. In the sham group, the vasculature of
the rats was separated, but occlusion of the artery by suture was
not conducted. At 3 h after the successful establishment of the
model in the treatment group, an intravenous injection of the
caspase-3 inhibitor z-DEVD-fmk (2.5 µg/kg; BioVision, Milpitas, CA,
USA) was injected immediately via the tail vein. The same volume of
phosphate-buffered saline (PBS) solution was injected into the tail
veins of rats in the model and sham groups. After 48 h, rats were
sacrificed and analyses were conducted. This study was carried out
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (Eighth Edition, 2012; Bethesda, MD, USA). The animal use
protocol has been reviewed and approved by the Institutional Animal
Care and Use Committee (IACUC) of the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China).
Analysis of neurological function
The Bederson scale was adopted to analyze the
neurological impairment of rats in the sham, model and treatment
groups at 12, 24 and 48 h, respectively, after surgery (13). The scores were as follows: 0, no
symptoms of neurological deficit; 1, unable to fully extend the
right front paw; 2, circling to the left; 3, toppling over to one
side; 4, loss of spontaneous walking and loss of consciousness.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from brain tissues according
to instructions of the RNA extraction kit (Invitrogen Life
Technologies, Carlsbad, CA, USA). Following the extraction and
purification of the RNA, a spectrophotometer was used to measure
the concentration of RNA. The RNA was transcribed into cDNA with
the use of reverse transcription reagent (Takara Biotechnology Co.,
Ltd., Dalian, China). PCR primers for rat caspase-3 were designed,
with sequences as follows: forward, 5′-GGT ATT GAG ACA GAC AGT
GG-3′ and reverse, 5′-CAT GGG ATC TGT TTC TTT GC-3′. Then, the qPCR
reaction mixture was prepared as follows: 10 µl 2X SYBR Green
Universal qPCR Master Mix (Roche, Basel, Switzerland),
upstream/downstream primers (10 µmol/l; 1 µl of each) and 1 µl
cDNA, with double-distilled water to a final volume of 20 µl. The
volume prepared was adjusted according to the required number of
test samples, and 20 µl was the volume used in each hole of the PCR
plate. The reaction mixture was centrifuged for 10 min at 2,000 ×
g. PCR was carried out according to the following reaction
conditions: Pre-denaturation at 95°C for 30 sec; denaturation at
95°C for 3 sec; and annealing extension at 60°C for 30 sec; with
the generation of dissociation curves. Finally, the data were
directly provided by the real-time fluorescent quantitative PCR
instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot assay
In this assay, 1 g rat brain tissue was placed in a
mortar filled with liquid nitrogen, and was ground to a powder. To
the powdered tissue was added 300 µl cell lysis buffer (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) and 3 µl protease
inhibitor, and the mortar was placed on ice for 30 min. The mixture
was then centrifuged at 15,000 × g for 15 min. The supernatant was
taken in order to measure the protein concentration. A 4X sample
buffer solution was added to the sample, which was then boiled for
30 min followed by centrifugation. SDS-PAGE was conducted to
transfer the protein to polyvinylidene difluoride membranes.
Following blocking with 5% skimmed milk, the membrane was incubated
with primary polyclonal goat anti-caspase-3 antibody (1:100;
sc-189; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 37°C. The membrane was then washed with PBS with Tween
20 (PBST) three times, and then incubated with horseradish
peroxide-coupled sheep anti-mouse secondary antibody (Beijing
Kangwei Technology Group Co., Ltd., Beijing, China) at room
temperature for 1 h. After washing with PBST three times,
chemiluminescent reagent (GS009; Beyotime Institute of
Biotechnology, Shanghai, China) was added to the blots and images
were captured. β-actin was also measured as an internal control,
and gray-scale computing software (Tocan, Inc., Shanghai, China)
was used to analyze the protein band intensities and calculate the
relative expression of the target protein.
Caspase-3 activity assay
This was carried in accordance with the instructions
provided with the caspase-3 protein activity detection reagent
(Beyotime Institute of Biotechnology, Beijing, China).
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick end labeling
(TUNEL) staining
Antigen retrieval was conducted according to the
equipment manufacturer's instructions (Roche) and Following
dewaxing, 20-µm rat brain sections from each group were washed with
PBST three times. The TUNEL staining procedure was executed in
strict accordance with the instructions provided with the TUNEL kit
(Roche). The specimens were observed at a magnification of x400,
and 10 visual fields of the infarct zone and peripheral area were
randomly selected. In each field, the proportion of apoptotic cells
among all cells was calculated as the apoptotic index of the brain
cells.
2,3,5-Triphenyltetrazolium chloride
(TTC) analysis
Following sacrifice, brain tissues were extracted
from the mice, the olfactory bulb, cerebellum and brain stem were
removed, and the remaining tissue was cut into coronal slices. The
slices were put into 2% TTC phosphate buffer solution
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C for staining for 20
min. Then, paraformaldehyde was used to fix the tissue slices. The
normal tissue was stained red and the infarcted tissue was white.
The infarcted tissues were separated from the normal tissues under
a microscope, and an analytical balance was used to weigh the wet
normal tissues and infarcted tissues. The infarction scope was
calculated as the weight of the infarcted tissues as a percentage
of the total weight of infarcted tissues plus normal brain
tissues.
Statistical analysis
All data were analyzed using SPSS software, version
17.0 (SPSS, Inc., Chicago, IL, USA). Measurement data are expressed
as mean ± standard deviation, measurement data of multiple groups
were compared by variance analysis, and date between two groups
were compared by the least significant difference method. P<0.05
was considered statistically significant.
Results
Comparison of caspase-3 mRNA and
protein levels among the three groups
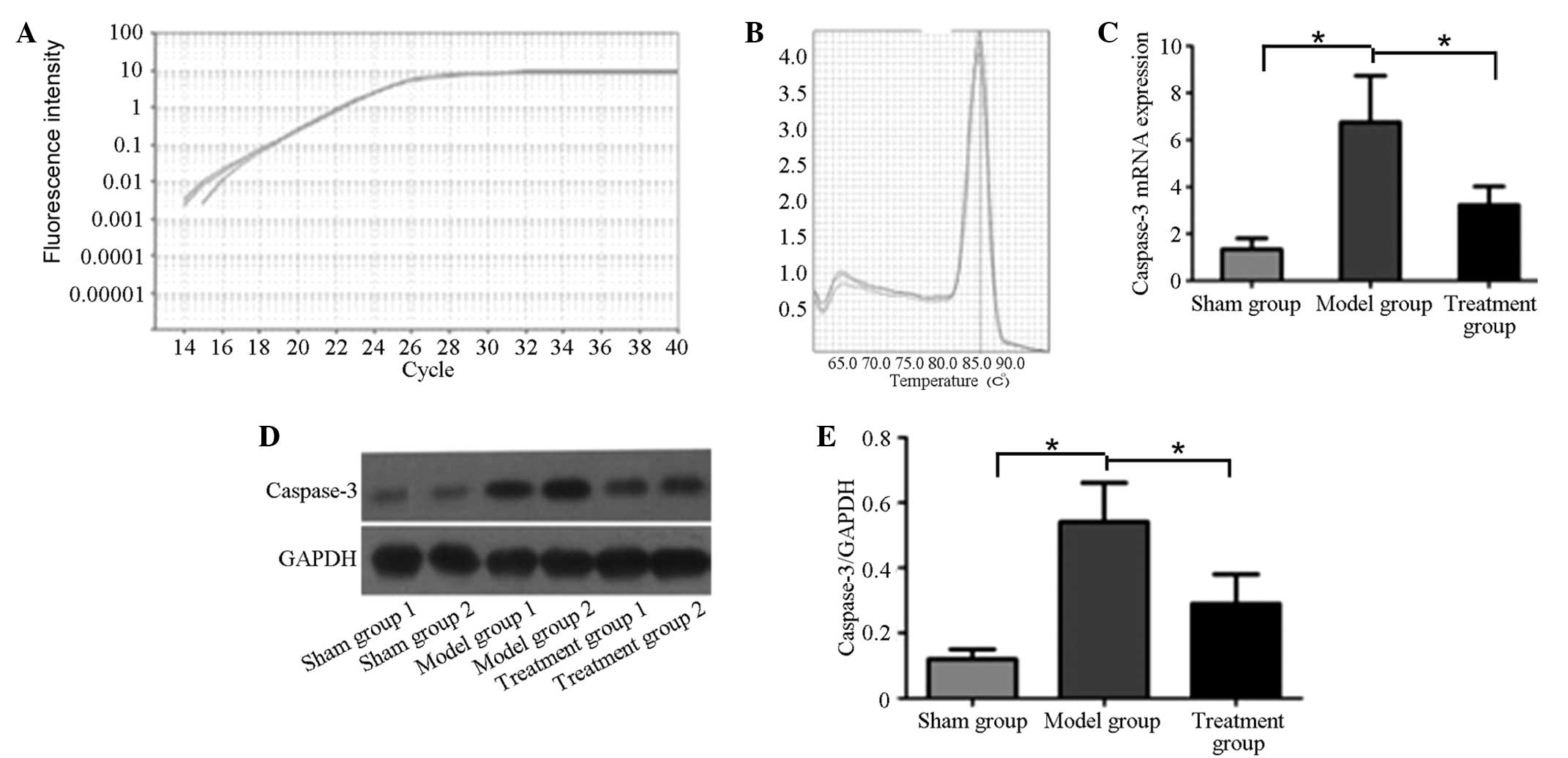
Fluorescence qPCR was used to analyze the caspase-3
mRNA levels in the sham, model and treatment groups. The
amplification curve is shown in Fig.
1A. The caspase-3 primers were well designed, and the melting
curve had no impurity peak (Fig.
1B). According to the quantitative analysis results presented
in Fig. 1C, the caspase-3 mRNA
levels in the model and treatment groups were significantly higher
than that in the sham group (P<0.05). However, following
treatment with the caspase-3 inhibitor z-DEVD-fmk, the caspase-3
mRNA level in the treatment group was significantly lower than that
in the model group (P<0.05).
Western blot analysis was used to analyze the
caspase-3 protein expression levels in the brain tissues of the
three groups. As shown in Fig. 1C and
D, the caspase-3 protein expression level in the model group
was significantly higher than that in the sham and treatment
groups. Following treating with the caspase-3 inhibitor, the
caspase-3 protein expression level in the treatment group was
significantly decreased compared with that in the model group
(P<0.05), but remained significantly higher than that in the
sham group (P<0.05).
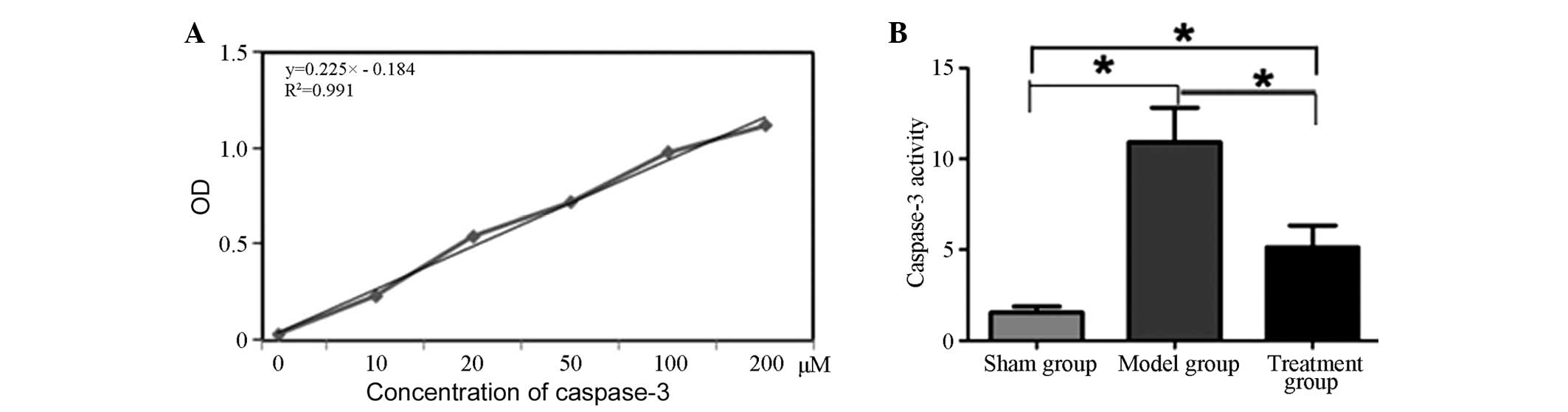
Comparison of caspase-3 activity in
rat brain tissues among the three groups
Firstly, a standard curve for caspase-3 activity was
established (Fig. 2A). The standard
curve had good linearity (R2=0.999). Analysis of the
caspase-3 activity in the rat brain tissues of the three groups
indicated that the caspase-3 activity in the brain tissues of the
rats in the model group was significantly higher than that of rats
in the sham and treatment groups (P<0.05). Following treatment
with caspase-3 inhibitor, the caspase-3 activity in the brain
tissues of rats in the treatment group was significantly lower than
that of the model group (P<0.05), but remained higher than that
of the sham group (P<0.05; Fig.
2).
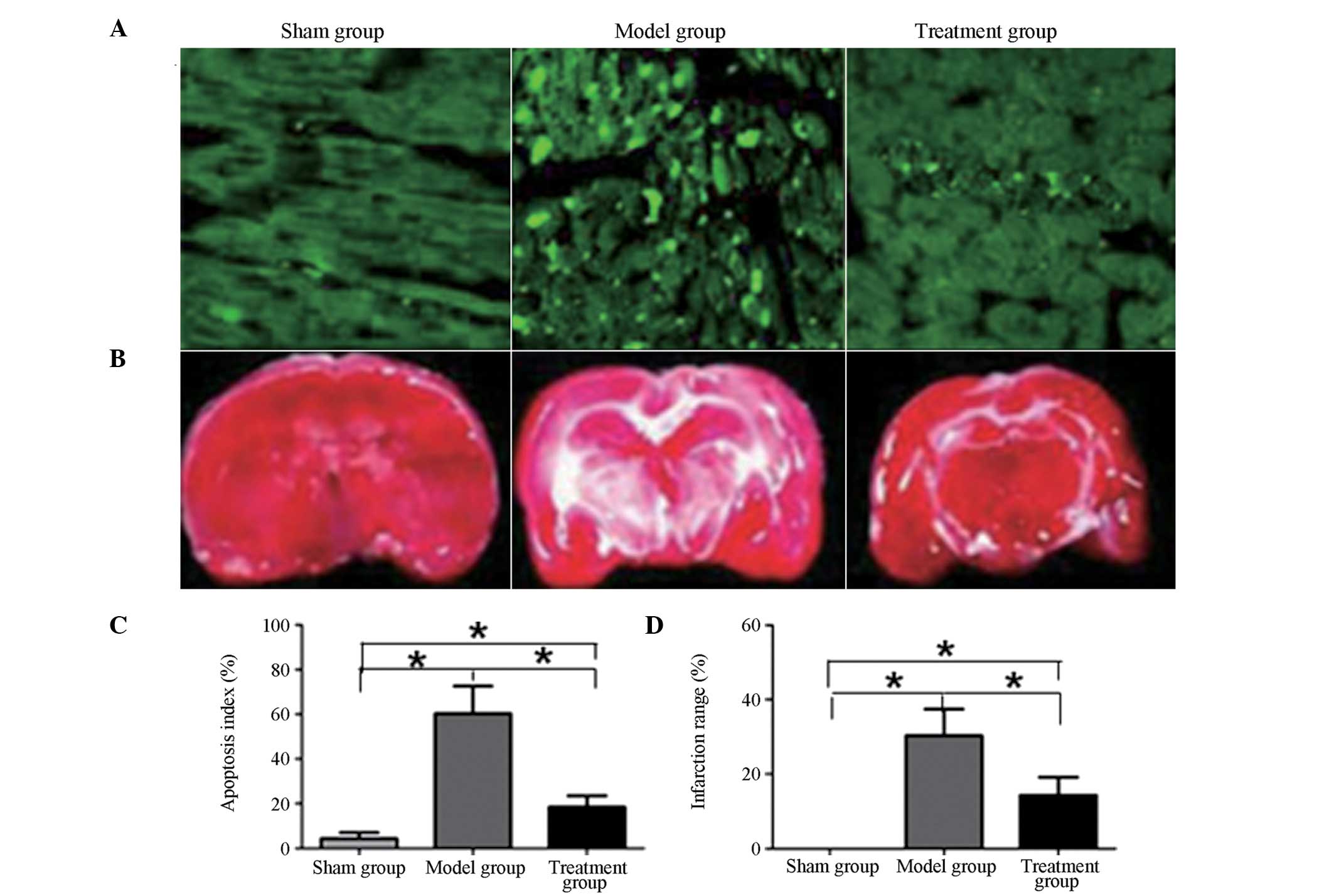
Comparison of apoptotic index and
infarction scope in rat brain tissues among the three groups
As shown in Fig. 3,
the apoptotic index and infarction scope of the rat brain tissues
in the model and treatment groups were significantly increased
compared with those in the sham group (P<0.05). Following
treatment with caspase-3 inhibitor, the apoptotic index and
infarction scope in the treatment group were significantly
decreased compared with those in the model group (P<0.05).
However, the apoptotic index and infarction scope of rat brain
tissues in the treatment group remained significantly higher than
those in the sham group (P<0.05).
Comparison of the neurological
impairment of the three groups
The neurological impairment of the three groups of
rats was analyzed 12, 24 and 48 h after the establishment of the
model. As shown in Table I, the
neurological impairment scores of the model and treatment groups
were significantly increased compared with that of the sham group
prior to treatment, (P<0.05). As the time after treatment
increased, the neurological impairment score of the treatment group
gradually decreased, and were significantly lower than those in the
model group (P<0.05). However, the neurological impairment
scores of the treatment group at the different time-points remained
significantly higher than those in the sham group (P<0.05).
 | Table I.Comparison of neurological impairment
scores in the three groups. |
Table I.
Comparison of neurological impairment
scores in the three groups.
|
|
|
| Post-treatment
score |
|---|
|
|
|
|
|
|---|
| Groups | No. of cases | Pre. treatment
score | 12 h | 24 h | 48 h |
|---|
| Sham | 15 |
0.28±0.22 |
0.35±0.41 |
0.20±0.20 |
0.09±0.03 |
| Model | 15 |
3.18±0.91a |
2.87±0.74a |
2.31±0.63a |
1.98±0.51a |
| Treatment | 15 |
3.13±1.02a,b |
2.23±0.59a,b |
1.57±0.31a,b |
0.51±0.15a,b |
Discussion
Cell apoptosis, also known as programmed cell death,
is an independent death process regulated by genes under
physiological or pathological conditions. In the process of acute
cerebral infarction, due to cerebral hypoxia and ischemia, brain
tissues may undergo necrosis and apoptosis (14,15).
Currently, treatments for acute cerebral infarction are focused on
the early recanalization of blood flow and saving the reversible
ischemic penumbra. Apoptosis is the main form of cell death in the
ischemic penumbra; therefore, inhibition of the apoptosis of cells
in the ischemic penumbra is the primary method for saving the
reversible ischemic penumbra (16).
Caspase-3 is a member of the cysteine protease family, and is the
major protease that participates in cell apoptosis. It activates
the apoptotic pathway by degrading corresponding intracellular
substrates, and causes apoptosis; it is therefore known as a
molecular switch (17,18). At present, a number of experimental
studies have shown that caspase-3 plays an important role in the
pathological injury process of myocardial ischemia and cerebral
ischemia, and caspase-3 has received great attention due to its
role in the final common pathway in apoptosis (19–21).
Since the caspase-3 protein is affected by the cell
surface death receptor-mediated and mitochondrial apoptosis
pathways, it plays an important role in mediating the apoptosis of
cells (7). On the basis of this, the
present study explored the role of caspase-3 inhibition in
modulating caspase-3 expression, protecting against brain cell
apoptosis and maintaining the neurological functions of rats
following acute cerebral infarction.
The results showed that the caspase-3 mRNA and
protein levels and enzyme activity increased significantly in the
rat brain tissues of an established acute cerebral infarction
model, indicating that the apoptosis of brain and nerve cells
significantly increases following acute cerebral infarction. This
was verified by TUNEL staining, in which quantitative results
indicated that the apoptotic index of brain tissues in the model
group was significantly increased compared with that in the control
group. With the increase of the level of apoptosis, the necrotic
scope of the brain tissues in the model group was significantly
increased compared with that in the control group, accompanied by a
decline of neurological function. When the rats with acute cerebral
infarction received caspase-3 inhibitor treatment, it was observed
that the caspase-3 mRNA and protein expression levels and enzyme
activity in the brain tissue decreased significantly, and the
apoptosis index of the brain tissue decreased significantly. Due to
a significant reduction in the level of apoptosis, the infarction
scope of the brain tissue also significantly declined; the
neurological impairment of the rats also significantly declined.
This result suggests that the caspase-3 inhibitor z-DEVD-fmk can
significantly inhibit the apoptosis of brain tissue cells in rats
with acute cerebral infarction, and has a protective effect on
brain tissue.
In summary, the caspase-3 inhibitor z-DEVD-fmk
significantly reduced the expression of caspase-3 at the mRNA and
protein levels and caspase-3 activity in the brain tissues of rats
with cerebral infarction. In addition, it reduced apoptosis and
tissue infarction in the brain tissues and decreased the degree of
neurological impairment. Thus, the caspase-3 inhibitor played a
protective role for nervous tissues, and the findings of the
present study may act as a reference for the clinical treatment of
acute cerebral infarction.
References
|
1
|
Arboix A and Alio J: Acute cardioembolic
cerebral infarction: answers to clinical questions. Curr Cardiol
Rev. 8:54–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mokin M, Snyder KV, Siddiqui AH, Hopkins
LN and Levy EI: Endovascular management and treatment of acute
ischemic stroke. Neurosurg Clin N Am. 25:583–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsieh FI and Chiou HY: Stroke: morbidity,
risk factors and care in Taiwan. J Stroke. 16:59–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Candelario-Jalil E: Injury and repair
mechanisms in ischemic stroke: considerations for the development
of novel neurotherapeutics. Curr Opin Investig Drugs. 10:644–654.
2009.PubMed/NCBI
|
|
5
|
Sergeeva SP, Litvickiy PF, Gultyaev MM,
Savin AA and Breslavich ID: To the Fas-induced neurons apoptosis
mechanisms in stroke pathogenesis. Patol Fiziol Eksp Ter. 3:15–18.
2013.(In Russian). PubMed/NCBI
|
|
6
|
Wang JP, Yang ZT, Liu C, He YH and Zhao
SS: L-carnosine inhibits neuronal cell apoptosis through signal
transducer and activator of transcription 3 signaling pathway after
acute focal cerebral ischemia. Brain Res. 1507:125–133. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan W, Dai Y, Xu H, et al: Caspase-3
modulates regenerative response after stroke. Stem Cells.
32:473–486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang B, Ye D and Wang Y: Caspase-3 as a
therapeutic target for heart failure. Expert Opin Ther Targets.
17:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang ZN, Li JY, Zhao Y, Wang JQ, Huang C
and Fan GQ: Effects of Tongnao Huoluo acupuncture therapy on
Caspase-3 and Bcl-2 of rats with acute cerebral infarction.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 33:646–650. 2013.(In Chinese).
PubMed/NCBI
|
|
10
|
Rosell A, Cuadrado E, Alvarez-Sabín J, et
al: Caspase-3 is related to infarct growth after human ischemic
stroke. Neurosci Lett. 430:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kikuchi K, Tanaka E, Murai Y and
Tancharoen S: Clinical trials in acute ischemic stroke. CNS Drugs.
28:929–938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Belayev L, Alonso OF, Busto R, Zhao W and
Ginsberg MD: Middle cerebral artery occlusion in the rat by
intraluminal suture. Neurological and pathological evaluation of an
improved model. Stroke. 27:1616–1622. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oostveen JA, Dunn E, Carter DB and Hall
ED: Neuroprotective efficacy and mechanisms of novol
pyrrolopyrmidine lipid preoxidation inhibitors in the gerbil
forebrain ischemia model. J Gereb BIood FIow Metab. 18:539–547.
1998. View Article : Google Scholar
|
|
17
|
Charriaut-Marlangue C: Apoptosis: A target
for neuroprotection. Therapie. 59:185–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka K: Pathophysiology of brain injury
and targets of treatment in acute ischemic stroke. Rinsho
Shinkeigaku. 53:1159–1162. 2013.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang B, Ye D and Wang Y: Caspase-3 as a
therapeutic target for heart failure. Expert Opin Ther Targets.
17:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu SZ, Yan L, Wang Q, An TL and Guan XQ:
Effects of caspase-3 inhibitor on the neuronal apoptosis in rat
cerebral cortex after ischemia-reperfusion injury. Zhonghua Bing Li
Xue Za Zhi. 35:165–170. 2006.(In Chinese). PubMed/NCBI
|
|
21
|
Ferrer I, Friguls B, Dalfó E, Justicia C
and Planas AM: Caspase-dependent and caspase-independent signalling
of apoptosis in the penumbra following middle cerebral artery
occlusion in the adult rat. Neuropathol Appl Neurobiol. 29:472–481.
2003. View Article : Google Scholar : PubMed/NCBI
|