Introduction
Adenoid cystic carcinoma (ACC) is rare form of
cancer that is not often observed in clinical practice, accounting
for ~1% of head and neck malignant tumors. The tumors are most
commonly observed in the salivary gland, nasal cavity and sinus, as
well as the lacrimal gland (1).
Adenoid cystic carcinoma of the lacrimal gland (LGACC) is one of
the most common types of malignant epithelial tumor in the lacrimal
gland, with an incidence of 29%. In spite of the low clinical
incidence, ACC is difficult to be cured and relapse is common
following surgery due to the high malignant degree and strong
invasion capacity in local tissue. Local lymph node metastasis is
rare; however, long distance metastasis is very common (2). In addition, the five-year survival rate
is relatively high; however, the survival rate between 10 and 20
years is considerably reduced (3,4). ACC
originating from the lacrimal gland had a higher tendency to invade
the cranial cavity, as compared with tumors from the salivary
gland, and the corresponding incidence rate is between 4 and 22%
(5).
However, the current early diagnosis and treatment
is far from achieving the considerable clinical requirement. The
majority of antitumor drug-mediated cell death occurs via the
suppression of cell proliferation and the induction of apoptosis.
As a typical anti-apoptosis protein, survivin is characterized by
inhibitory functions with regard to cell apoptosis and the
regulation of cell division (6).
Survivin has also been shown to affect the biological behavior of
tumors via inhibiting apoptosis, participating in cellular division
and promoting neovascularization, and the protein has been
recognized as one of the most powerful anti-apoptosis factors
(7,8). In addition, a previous study
demonstrated that normal tissue does not express survivin, while
the protein is predominantly and specifically expressed in tumor
tissues (9). Arsenic trioxide
(As2O3) has been demonstrated to induce the
apoptosis of ACC-2 cells (10).
Materials and methods
Patients and specimens
In total, 13 patients with LGACC (female, 4; male,
9) were recruited from Renmin Hospital of Wuhan University (Wuhan,
China) between 2007 and 2012. None of the patients had undergone
any chemotherapy, radiotherapy or hormonotherapy prior to surgery.
On the basis of the 7th edition of the American Joint Committee on
Cancer TNM Classification for Lacrimal Gland Tumors stages, the 13
patients were divided into two groups, namely the TNM1–2 and TNM3–4
groups. In total, eight samples comprised the TNM1–2 group, while
five samples were included in the TNM3–4 group. Primary cells were
obtained using tissue culture techniques. Mixed cells were removed
via a number of methods and cell morphological characteristics were
observed using a phase contrast microscope. In addition, normal
lacrimal gland tissues were collected from six patients who
underwent eye surgery as a result of a non-tumor-induced disease
during the same time period. All the patients provided informed
consent and the experiment was approved by the Ethics Committee of
Renmin Hospital of Wuhan University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of survivin were examined
using RT-qPCR, and the primers used are shown in Table I. β-actin was used as a control.
Total RNA was isolated with TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and treated with RNase-free DNase
I (Takara Bio, Inc., Shiga, Japan) to avoid genomic DNA
contamination. Yields and purities were estimated using the
A260/A280 ratio using a NanoDrop 2000c spectrophotometer (Thermo
Fisher Scientific, Waltham, MA, USA). Samples with an absorbance
ratio of 1.9–2.0 were reverse transcribed using a Revert Aid
First-Strand cDNA Synthesis kit (Thermo Fisher Scientific) with a
random hexamer primer (Shanghai Sangon Biotechnology Co., Ltd.,
Shanghai, China). PCR amplification of the cDNA template was
conducted using Thunderbird SYBR qPCR mix (Toyobo Co., Ltd., Osaka,
Japan) on an ABI PRISM 7300 sequence detection system (Applied
Biosystems Life Technologies, Carlsbad, CA, USA). The conditions
for PCR were as follows: Initial denaturation at 95°C for 5 min,
followed by 40 amplification cycles consisting of denaturation at
95°C for 5 sec, annealing at 58°C for 30 sec and elongation at 72°C
for 30 sec. PCR assays were run in triplicate, and the results were
averaged. All PCR products were visualized by electrophoresis and
ethidium bromide staining in 2% agarose gels. The relative
expression level of the genes was normalized against an internal
β-actin control, using the ddCt method.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| Survivin |
CCCAGCACAATGAAGATCAAGATCAT |
ATCTGCTGGAAGGTGGACAGCGA |
| β-actin |
CAGCCCTTTCTCAAGGACCAC |
GCAAACATCAGGCTCTTCCTC |
Western blot analysis
For western blot analysis, 50 µg protein was loaded
per well on a 12% sodium dodecyl sulfate-polyacrylamide
electrophoresis gel and resolved via standard electrophoresis.
Subsequently, the gels were electrophoretically transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membranes were blocked with 5% bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline
(PBS) containing 0.1% Tween-20 (PBS-T) for 1 h at room temperature.
Next, the membranes were probed overnight at 4°C with polyclonal
antibodies targeting survivin (1:800; 2803; Cell Signaling
Technology, Inc., Danvers, MA, USA) and β-actin (1:600; 4967; Cell
Signaling Technology, Inc.). Protein molecular weight markers were
included on each blot. To verify that equivalent amounts of the
protein samples were loaded, the membranes were probed overnight at
4°C. Subsequently, the membranes were extensively washed in PBS-T
and incubated with a peroxidase-conjugated anti-rabbit IgG (1:3,000
w/v; #8888; Cell Signaling Technology, Inc., Danvers, MA, USA) to
reveal the presence of the survivin and β-actin primary antibodies.
The bands were visualized using an enhanced chemiluminescence kit
(PerkinElmer, Inc., Waltham, MA, USA), and their optical densities
were measured using the Densitometry Function in the Scion Image
software for Windows, beta version 4.02 (NIH Image programme).
Cell culture
Primary human ACC cells and normal cells of the
lacrimal gland were prepared and cultured, as described previously
(10), with certain minor
modifications. The lacrimal gland was decapsulated to remove the
majority of other tissues, and the remaining tissue was minced and
digested with 1.0 g/l collagenase I (#C0130) and 5.0 mg/l DNase I
(#D5025; Sigma-Aldrich) in Dulbecco's modified Eagle's medium
(#SH30023.01B, HyClone, Logan, UT, USA): Nutrient mixture F-12
(DMEM/F12) at 37°C for 15 min. The supernatants were diluted with
modified DMEM/F12 medium, which contained 10% fetal bovine serum
(#SH30084.03; HyClone). Subsequently, the mixtures were centrifuged
at 60 × g for 8 min at 4°C, and the resulting cell pellets were
resuspended in medium and repelleted, as aforementioned. The cells
were plated at a density of ~1×106 cells/ml in six-well
plates and incubated in a humidified atmosphere of 5%
CO2 at 37°C until the cells had adhered. Six replicates
were used. After 24 h, the medium was replaced (2 ml/well).
RNA interference
Primer sequences for survivin were as follows:
Forward, 5′-CCC GTT GGC AGA GGT GGC-3′ and reverse, 5′-TGG GAC CAG
GCA GCT CCG-3′. Specific short interfering RNA (siRNA) fragments of
survivin were obtained using a BLOCK-iT™ Complete Dicer RNAi kit
(Invitrogen Life Technologies). Subsequently, the ACC cells of the
lacrimal gland were transfected with 25 ng siRNA using 5 ml
Lipofectamine 2000 (Invitrogen Life Technologies). At 48 h after
transfection, the cells were collected for the subsequent RT-qPCR
and MTT assays.
As2O3
treatment
As2O3 treatment was applied
following the adoption and modification of a previous method
(10). Cells in an exponential
growth phase were cultured in serum-free medium for 24 h, after
which the cells were seeded into three independent six-well plates
and treated with 0, 2, 4 and 6 µM As2O3 (200
µl per well) for 24, 48 and 72 h.
MTT assay
Following As2O3 treatment for
24 and 48 h, cell survival was determined using an MTT assay. The
cells were cultured in 96 well-plates at a density of
1×104 cells per well. A 20-µl volume of MTT solution (5
mg/ml) was added to each well and subsequently incubated for 4 h at
37°C. Following removal of the medium, the formazan salts were
dissolved in 100 µl dimethyl sulfoxide and the optical absorbance
was determined using a microplate reader at a wavelength of 490 nm
(BioTek Instruments, Inc., Winooski, VT, USA). Cell viability was
expressed as a percentage of the corresponding control.
Statistical analysis
All data were expressed as the mean ± standard error
of the means. Comparisons between two groups were performed using
the unpaired Students t-test. Differences among groups were
determined using one-way analysis of variance followed by
Student-Newman-Keuls tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
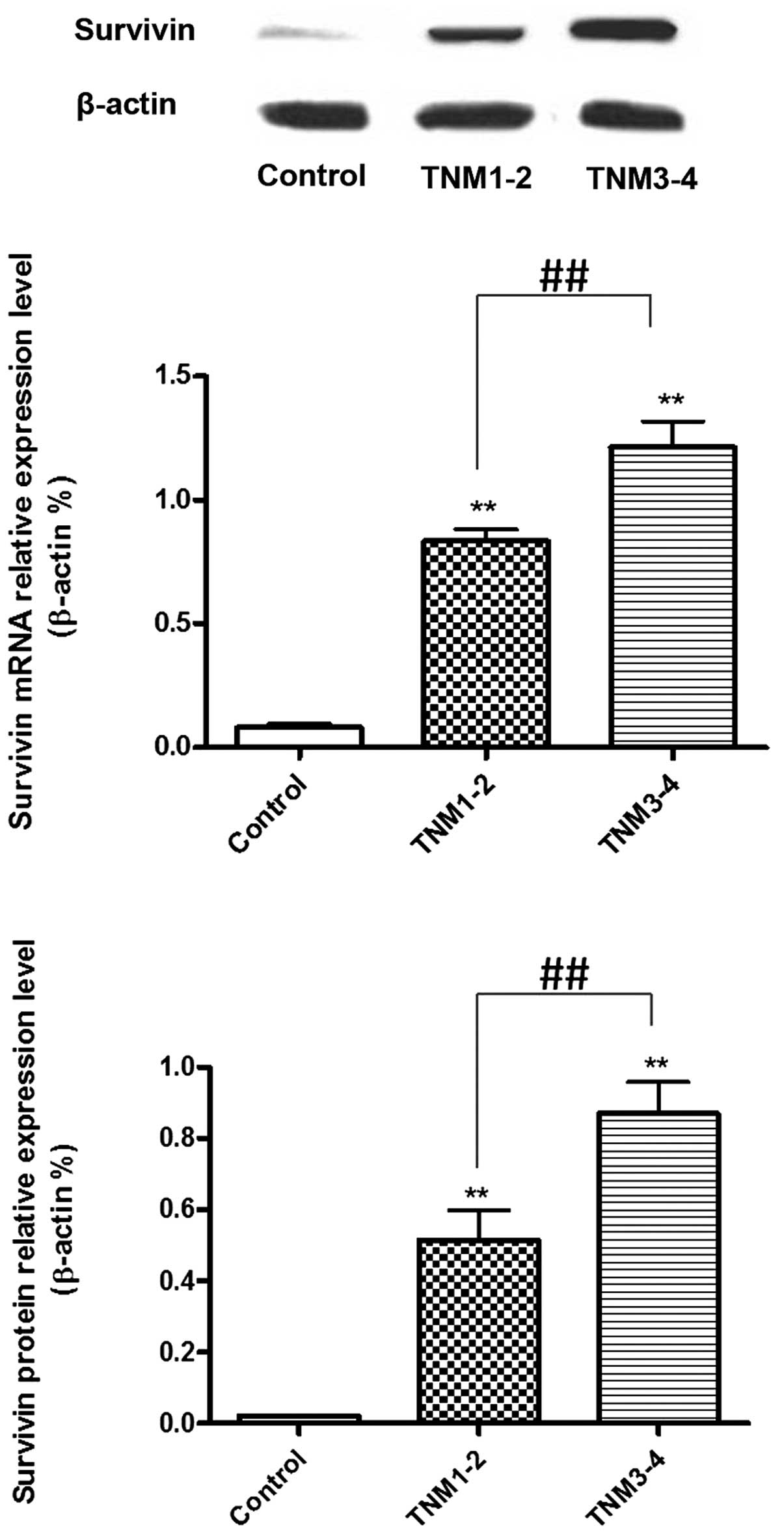
mRNA and protein expression levels of
survivin in tumor tissues collected from LGACC patients with a
different pathological classification
The mRNA and protein expression levels of survivin
in the TNM1–2 and TNM3–4 groups were higher when compared with the
control samples (P<0.01). In addition, when comparing the TNM
groups, the TNM3–4 patients exhibited higher mRNA and protein
expression levels of survivin when compared with the TNM1–2
patients (P<0.01; Fig. 1).
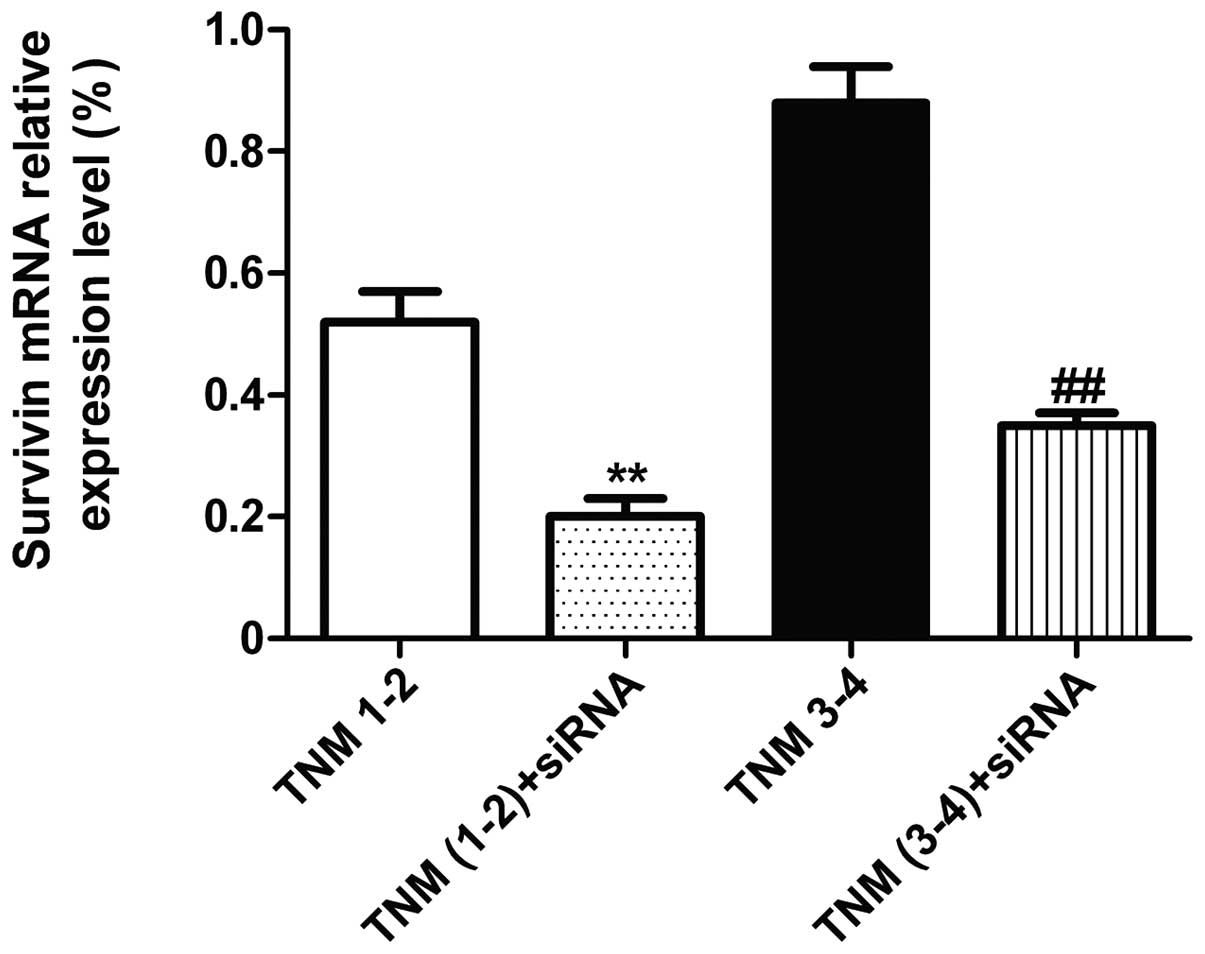
Effects of siRNA interference on the
mRNA expression of survivin and the cell proliferation of primary
cultured LGACC cells
At 48 h after siRNA interference, the mRNA
expression levels of survivin in the TNM1–2 and TNM3–4 groups were
significantly decreased when compared with the non-siRNA
interference group (P<0.01; Fig.
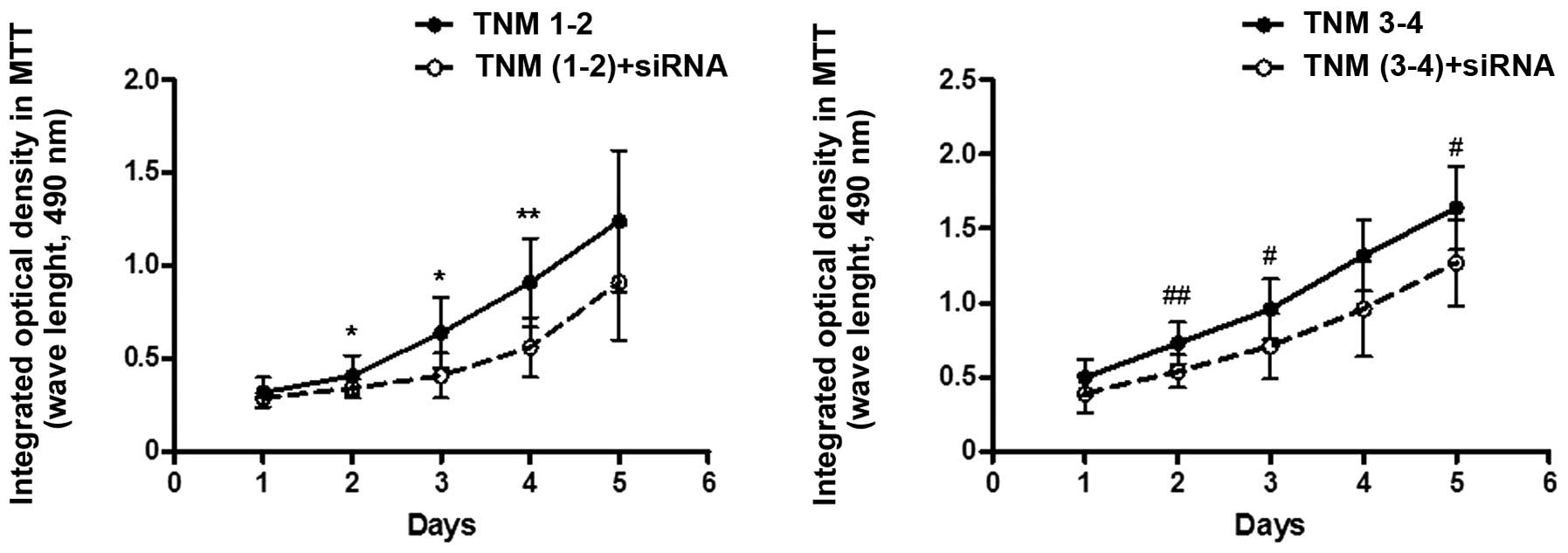
2). Furthermore, the rate of cell proliferation in the TNM1–2
group was shown to decrease following siRNA treatment, and the cell
viability percentages at days 2, 3 and 4 were all lower when
compared with the non-siRNA interference control (days 2 and 3,
P<0.05; day 4, P<0.01 vs. TNM1–2 + siRNA). The rate of cell
proliferation in the TNM3–4 group was also significant decreased
following siRNA treatment, and the cell viability percentages at
days 2, 3 and 5 were all lower when compared with the non-siRNA
interference control (days 3 and 5, P<0.05; day 2, P<0.01 vs.
TNM3–4 + siRNA; Fig. 3).
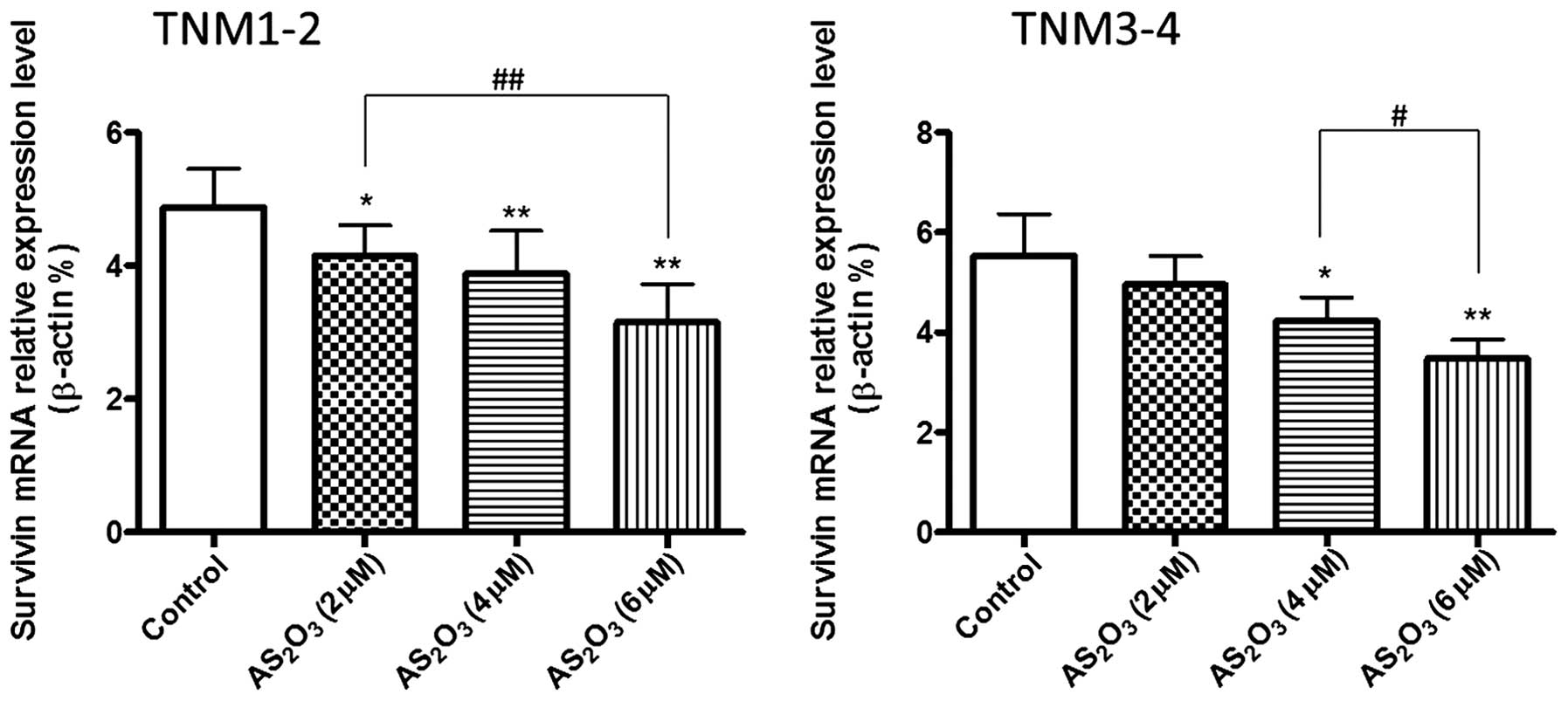
Effects of different doses of
As2O3 treatment on the mRNA expression levels
of survivin
At 48 h after treatment with various doses of
As2O3 (2, 4 and 6 µΜ), the mRNA expression
levels of survivin in the TNM1–2 group were significantly decreased
when compared with the control (P<0.05, P<0.01). Furthermore,
As2O3 treatment was shown to dose-dependently
decrease the mRNA expression levels of survivin in the TNM3–4
group, particularly at a concentration of 4 and 6 µM
As2O3 (Fig. 4;
P<0.05, P<0.01).
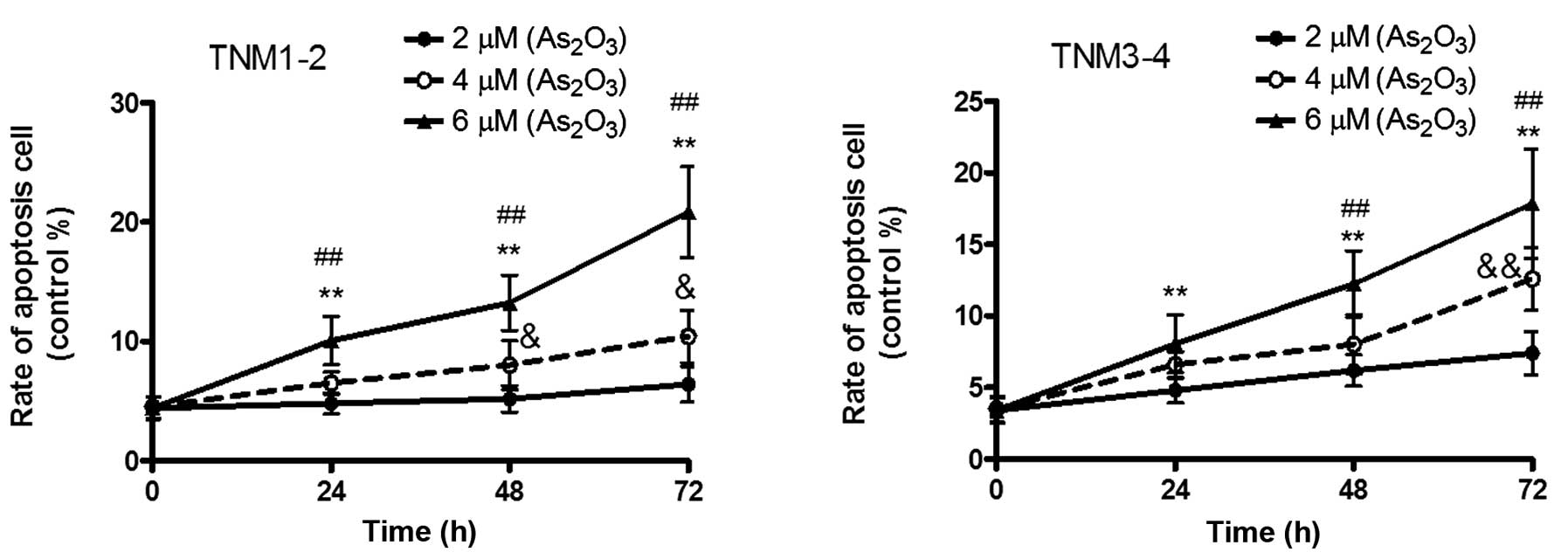
Effects of different doses of
As2O3 treatment on cell apoptosis
After 72 h of treatment, As2O3
was demonstrated to dose-dependently decrease the cell viability in
the TNM1–2 and TNM3–4 groups (P<0.05, P<0.01), indicating
that As2O3 was able to significantly promote
cell apoptosis. In the TNM1–2 group, the apoptotic rate was shown
to increase from 4.80 to 6.51%, and finally to 10.06%, according to
the concentration of As2O3 (2, 4 and 6 µM) in
24 h. Furthermore, the apoptotic rates at 48 h were 5.20, 8.02 and
13.24% for the various concentrations, respectively, and 6.40,
10.41 and 20.84% at 72 h, respectively. In the TNM3–4 group, the
apoptotic rates following treatment with 2, 4 and 6 µM
As2O3 were 4.82, 6.59 and 8.06% at 24 h,
respectively, 6.20, 8.02 and 12.19% at 48 h, respectively, and
7.40, 12.59 and 17.84% at 72 h, respectively (Fig. 5).
Discussion
Survivin is one of eight members of the inhibitor of
apoptosis protein family, which was initially identified in 1997
(11). Although survivin is
predominantly expressed in fetal tissues, little or no expression
is observed in completely differentiated human tissues. However,
multiple malignant tumor tissues, such as human ACC, have been
shown to exhibit hyperexpression of survivin (12). In the present study, survivin was
demonstrated to be highly expressed in ACC cells of the lacrimal
gland, and the corresponding expression levels were shown to
correlate with the TNM classification. The expression of survivin
in the TNM3–4 group was significantly higher compared with the
TNM1–2 group and control.
Survivin plays a vital role in the entire cell cycle
since the protein exerts dual functions in inhibiting cell
apoptosis and promoting cell proliferation (12). Survivin has been recognized as a
putative biomarker and drug target for various types of tumor
(9). However, the underlying
mechanism remains unknown. A previous study hypothesized that
survivin may block apoptosis via inhibiting caspase-3 and −7
(13). In addition, survivin has
been proposed to interact with interleukin-3, Fas, Bax and p53
(14,15); however, the underlying mechanisms
require further investigation. During mitosis, survivin exists as a
multi-protein complex, known as the chromosomal passenger complex.
By participating in this complex, survivin may promote cell
proliferation by facilitating accurate sister chromatid segregation
and the stabilization of microtubules in late mitosis (16). In the present study, silencing
survivin expression using siRNA in primary cultured ACC cells of
the lacrimal gland was shown to significantly decelerate the rate
of cell proliferation, which may be attributed to the ability of
survivin to inhibit apoptosis and promote proliferation.
As2O3 is a broad-spectrum
anti-cancer drug that has been widely administered over a number of
years. The compound can significantly suppress the growth of solid
tumors, such as stomach and pulmonary carcinomas. The underlying
mechanism is hypothesized to involve As2O3
initiating the mitochondria-mediated apoptosis pathway, which
increases the production of reactive oxygen species, subsequently
activating cytochrome c pathway-induced apoptosis and
thereby facilitating the mutation of mitochondrial DNA in the
mitochondria (17). A previous study
demonstrated that As2O3 was able to induce
the apoptosis of ACC-2 cells, indicating a potential
anti-adenocarcinoma effect of As2O3 (10). In the present study,
As2O3 was demonstrated to dose-dependently
inhibit the mRNA expression of survivin in primary cultured cells
isolated from the tumor tissues of the TNM1–2 and TNM3–4 groups.
Furthermore, As2O3 was shown to enhance the
cell apoptosis rate in the TNM1–2 and TNM3–4 groups in a dose- and
time-dependent manner. Therefore, the results of the present study
indicate that As2O3 may exert an anti-LGACC
effect.
In conclusion, to the best of our knowledge the
present study is the first to demonstrate that survivin is
expressed in the tumor tissues of patients with LGACC, and is
positively associated with tumor deterioration. Furthermore, it was
observed that As2O3 induces the apoptosis of
LGACC cells by inhibiting survivin expression. Therefore, the
results of the present study indicate an association between
survivin and LGACC, with the overexpression of survivin suggesting
a poor prognosis in patients with LGACC. Survivin may be useful as
a diagnostic marker and a potential therapeutic target for the
treatment of LGACC and As2O3 may be a
feasible future therapy for the treatment of LGACC.
References
|
1
|
Kokemueller H, Eckardt A, Brachvogel P and
Hausamen JE: Adenoid cystic carcinoma of the head and neck - a 20
years experience. Int J Oral Maxillofac Surg. 33:25–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Q, Chang H, Han YD, Gao Y and Liu HG:
High-grade transformation in adenoid cystic carcinoma: A
clinicopathologic study. Zhonghua Bing Li Xue Za Zhi. 42:106–110.
2013.(In Chinese). PubMed/NCBI
|
|
3
|
Zeng J, Shi JT, Li B, Sun XL, An YZ, Li
LQ, Gao F, Xu JP and Jonas JB: Epithelial tumors of the lacrimal
gland in the Chinese: a clinicopathologic study of 298 patients.
Graefes Arch Clin Exp Ophthalmol. 248:1345–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Civit T, Klein O and Baylac F: Lacrimal
gland epithelial tumors. Neurochirurgie. 56:152–157. 2010.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JI, Kim YZ, Lee EH and Kim KH: Skull
base invasion of adenoid cystic carcinoma of the lacrimal gland: a
case report. J Korean Neurosurg Soc. 44:273–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKenzie JA and Grossman D: Role of the
apoptotic and mitotic regulator survivin in melanoma. Anticancer
Res. 32:397–404. 2012.PubMed/NCBI
|
|
7
|
Kanwar JR, Kamalapuram SK and Kanwar RK:
Targeting survivin in cancer: Patent review. Expert Opin Ther Pat.
20:1723–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanwar JR, Kamalapuram SK and Kanwar RK:
Targeting survivin in cancer: The cell-signalling perspective. Drug
Discov Today. 16:485–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang B, Mu HB, Xu XG, Liu W and Hu NR:
Role of survivin gene on the apoptosis of adenoid cystic
carcinoma-2 cells induced by arsenic trioxide. Hua Xi Kou Qiang Yi
Xue Za Zhi. 28:246–249. 2010.(In Chinese). PubMed/NCBI
|
|
11
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
12
|
Ryan BM, O'Donovan N and Duffy MJ:
Survivin: a new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC,
Hwang JI, Chung CW, Jung YK and Oh BH: An anti-apoptotic protein
human survivin is a direct inhibitor of caspase-3 and −7.
Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
15
|
Mirza A, McGuirk M, Hockenberry TN, Wu Q,
Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen
LL, Pickett CB and Liu S: Human survivin is negatively regulated by
wild-type p53 and participates in p53-dependent apoptotic pathway.
Oncogene. 21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang D, Welm A and Bishop JM: Cell
division and cell survival in the absence of survivin. Proc Natl
Acad Sci USA. 101:15100–15105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang CH, Kuo ML, Chen JC and Chen YC:
Arsenic trioxide sensitivity is associated with low level of
glutathione in cancer cells. Br J Cancer. 81:796–799. 1999.
View Article : Google Scholar : PubMed/NCBI
|