Introduction
Skeletal development and metabolic homeostasis
depend primarily on the activity of osteoblast cells, which are
derived from mesenchymal stem cells (1). Three osteoblast-specific transcription
factors, activating transcription factor 4, Sp7 (also known as
Osterix) and runt-related transcription factor 2 (RUNX2),
coregulate the differentiation of osteochondral progenitor cells
into fully differentiated osteoblasts (2,3).
Furthermore, transcription factors, such as cAMP-response element
binding protein, forkhead box protein O1 and members of the
activator protein 1 family, also contribute to osteoblast
differentiation and function (4,5).
Increasing evidence has indicated that osteogenic differentiation
is regulated by post-transcriptional mechanisms, most significantly
by temporally expressed microRNAs (miRNAs) (6,7).
miRNAs are short (17–25 nucleotides), non-coding
RNAs that regulate gene expression at the post-transcriptional
level (8). The biological function
of the majority of miRNAs are not known; however, they are
hypothesized to play critical roles in the regulation of almost all
physiological and pathological processes, including cell
proliferation, apoptosis, skeletal muscle development and
tumorigenesis (9–11). Previous studies have indicated that
certain miRNAs are involved in the regulation of osteoblast
differentiation (12). For example,
miRNA-204/211 targets RUNX2 in bone marrow-derived mesenchymal stem
cells and inhibits osteoblastic differentiation (13). An additional study observed that
miR-27a was able to regulate osteoblast differentiation by
specifically targeting the pro-osteoblastic transcription factor,
stabilin 2 (14). The aim of the
present study was to characterize the expression of miR-375 and
investigate its effects on osteoblast differentiation.
Materials and methods
Reagents, antibodies and plasmids
Recombinant bone morphogenetic protein 2 (BMP2) was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Anti-RUNX2 (sc-390351) and anti-GAPDH (sc-365062) mouse monoclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). miR-375 mimic/inhibitor, non-specific control
and RUNX2-pcDNA3.1 were obtained from Santa Cruz Biotechnology,
Inc.
Cell culture, differentiation and
transfection
A C2C12 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
Dulbeccos modified Eagles medium (DMEM; Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100
µg/ml streptomycin and 100 U/ml penicillin (Sigma-Aldrich, St.
Louis, MO, USA). Cultured cells were incubated in a humidity
chamber (Thermo Fisher Scientific, Waltham, MA, USA) containing 5%
CO2 at 37°C. To induce osteogenic differentiation, cells
were treated with 2 nM BMP2 (Invitrogen Life Technologies) for 24
h. For transfection, Lipofectamine 2000 (Invitrogen Life
Technologies) was mixed with the aforementioned small interfering
(si)RNA or DNA, according to the manufacturers instructions. The
solutions were subsequently combined with the C2C12 cells in
24-well culture plates at a density of 3.0×104
cells/well.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies), and cDNA was synthesized from 1 µg
total RNA using a Reverse Transcription kit (Invitrogen Life
Technologies), according to the manufacturers instructions. qPCR
was performed using an ABI 7500 Real-Time PCR System (Applied
Biosystems Life Technologies, Foster City, CA, USA), using the
following protocol: 95°C for 3 min, 40 cycles of 95°C for 15 sec,
60°C for 15 sec and 72°C for 30 sec. The primers used were as
follows: Osteocalcin (OC) forward, 5-TGC TTG TGA CGA GCT ATC AG-3
and reverse, 5-GAG GAC AGG GAG GAT CAA GT-3; collagen, type I, α 1
(COL1A1) forward, 5-GAG AGC ATG ACC GAT GGA TT-3 and reverse, 5-ATG
TAG GCC ACG CTG TTC TT-3; alkaline phosphatase (ALP) forward, 5-GAC
AAG AAG CCC TTC ACT GC-3 and reverse, 5-AGA CTG CGC CTG GTA GTT
GT-3; GAPDH forward, 5-ACC ACA GTC CAT GCC ATC AC-3 and reverse,
5-TCC ACC CTG TTG CTG TA-3. For the quantification of miRNA
expression, specific primers for miR-375 and U6 (Applied Biosystems
Life Technologies) were used.
ALP staining and measurement
C2C12 cells transfected with the indicated siRNA
were fixed with 10% formalin for 20 min, followed by fixation in an
ice-cold solution of ethanol and acetone. Subsequent to washing
with phosphate-buffered saline (PBS), the cells were stained with
an ALP staining solution (Sigma-Aldrich) at 37°C for 20 min. For
the measurement of ALP activity, a p-Nitrophenyl Phosphate
Liquid Substrate System (Sigma-Aldrich) was added and the
absorbance was measured at 405 nm using a microplate reader (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Cells were washed twice with PBS and lysed in a
buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM EDTA, 10%
glycerol and 0.5% NP-40 and protease inhibitor cocktail
(Sigma-Aldrich). Protein samples were separated using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto nitrocellulose membranes. The membranes were incubated with
the primary antibodies at 4°C overnight, followed by incubation
with a horseradish peroxide-conjugated mouse IgG secondary antibody
(sc-2025; Santa Cruz Biotechnology, Inc.). Proteins were visualized
using an enhanced chemiluminescence method kit (GE Healthcare Life
Sciences, Piscataway, NJ, USA), and the protein band intensity was
quantified via densitometric analysis using Quantity One software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Luciferase Reporter Assay
miRanda (http://www.microrna.org/microrna/getDownloads.do),
TargetScan (http://www.targetscan.org) and PicTar
database (http://pictar.mdc-berlin.de) software
was used in the study to identify miR-375 targets. Then, cells were
seeded in 24-well plates at a density of 5×104 cells per
well, one day prior to transfection. miRNA-375 mimic or inhibitor
(50 pmol), 500 ng luciferase reporter and 40 ng pRL-TK were added
to each well. Cells were collected at 48 h after transfection and
analyzed using a Dual-Luciferase Reporter Assay System (Promega
Corporation, Madison, WI, USA).
Statistical analysis
Each experiment was performed in triplicate and
repeated a minimum of three times, with all the data presented as
the mean ± standard deviation. Statistical analyses were performed
using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
Comparisons between groups were conducted using the Students t-test
or analysis of variance, where P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-375 is downregulated during
osteogenic differentiation
Expression levels of miR-375 during osteogenic
differentiation were analyzed using qPCR. C2C12 cells were cultured
in DMEM without serum and treated with 2 nM BMP2 for 24 h to induce
osteogenic differentiation. A number of osteogenic factors,
including OC, ALP and COL1A1, were used as phenotypic markers of
osteogenic differentiation. The results from the qPCR revealed that
the mRNA expression levels of OC, ALP and COL1A1 were markedly
increased in the cells treated with BMP2, indicating that
osteogenic differentiation had been successfully induced (Fig. 1A–C). In addition, a significant
reduction in miR-375 expression was observed in the BMP2-treated
cells, as compared with the non-BMP2-induced cells during
osteogenic differentiation (Fig.
1D). Collectively, these results demonstrated that miR-375 was
involved in the progression of osteogenic differentiation.
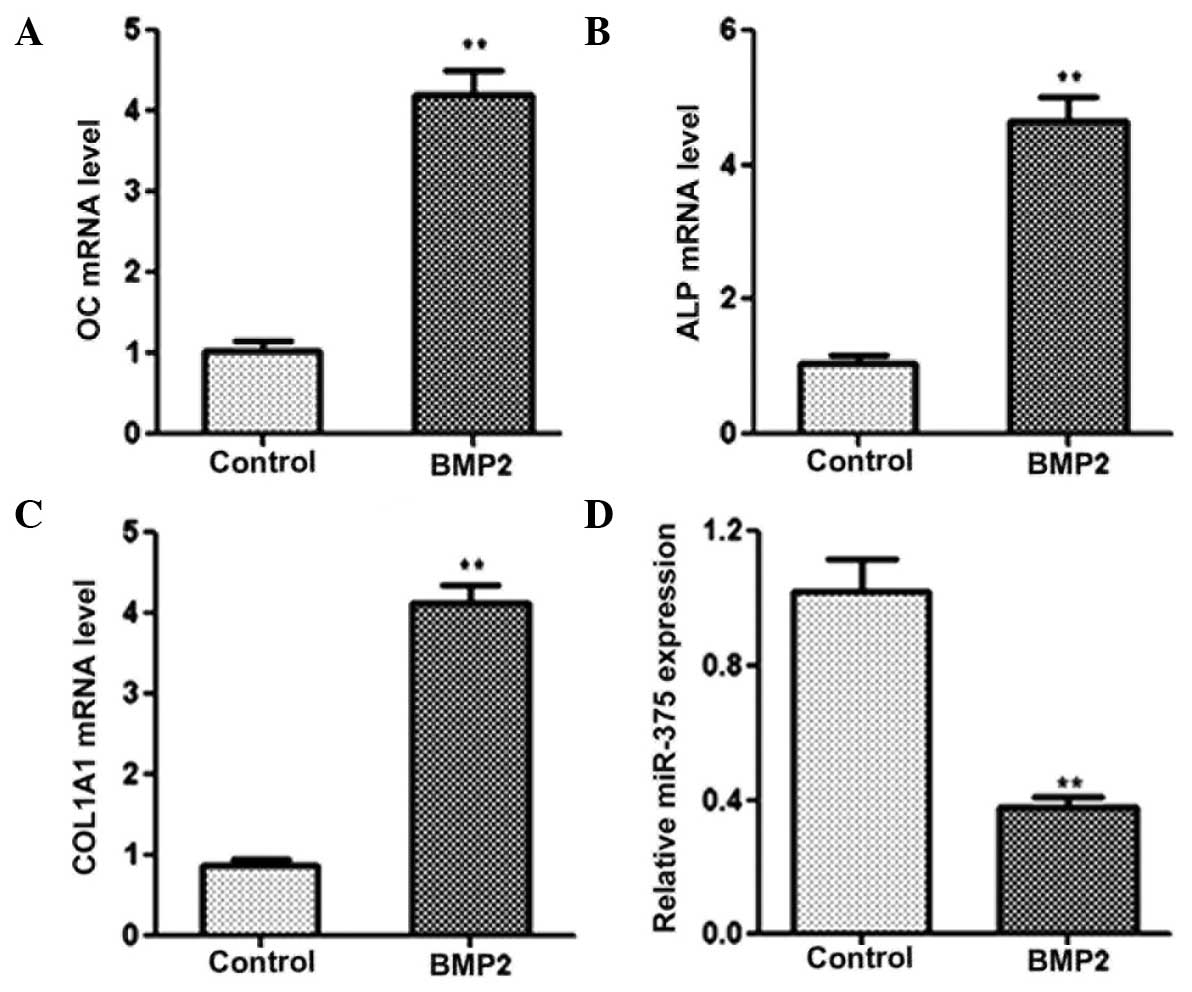 | Figure 1.miR-375 expression is downregulated
during osteogenic differentiation. Quantitative polymerase chain
reaction was performed to determine the expression levels of (A)
OC, (B) ALP, (C) COL1A1 and (D) miR-375 in control and BMP2-induced
C2C12 cells. GAPDH was used as a control for the mRNA expression
levels, while U6 was used as an internal control for miRNA
expression. **P<0.01, vs. control group. BMP2, bone
morphogenetic protein 2; OC, osteocalcin; ALP, alkaline
phosphatase; COL1A1, collagen, type I, α 1; miRNA, microRNA. |
miR-375 inhibits osteogenic
differentiation
C2C12 cells were transfected with miR-375 mimic or
inhibitor in order to study the physiological role of miR-375 on
osteogenic differentiation. The qPCR results revealed the mRNA
expression levels of OC, ALP and COL1A1 to be markedly reduced in
the cells transfected with the miR-375 mimic for 48 h (Fig. 2A). By contrast, C2C12 cells
transfected with the miR-375 inhibitor exhibited increased mRNA
expression levels of OC, ALP and COL1A1 (Fig. 2B). Furthermore, cells that
overexpressed miR-375 exhibited a significant reduction in ALP
activity, while the inhibition of miR-375 expression resulted in a
notable increase in ALP activity (Fig.
2C). qPCR was also performed to determine the efficiency of
transfection with miR-375 mimic/inhibitor (Fig. 2D). Collectively, the results
indicated that miR-375 was able to inhibit osteogenic
differentiation.
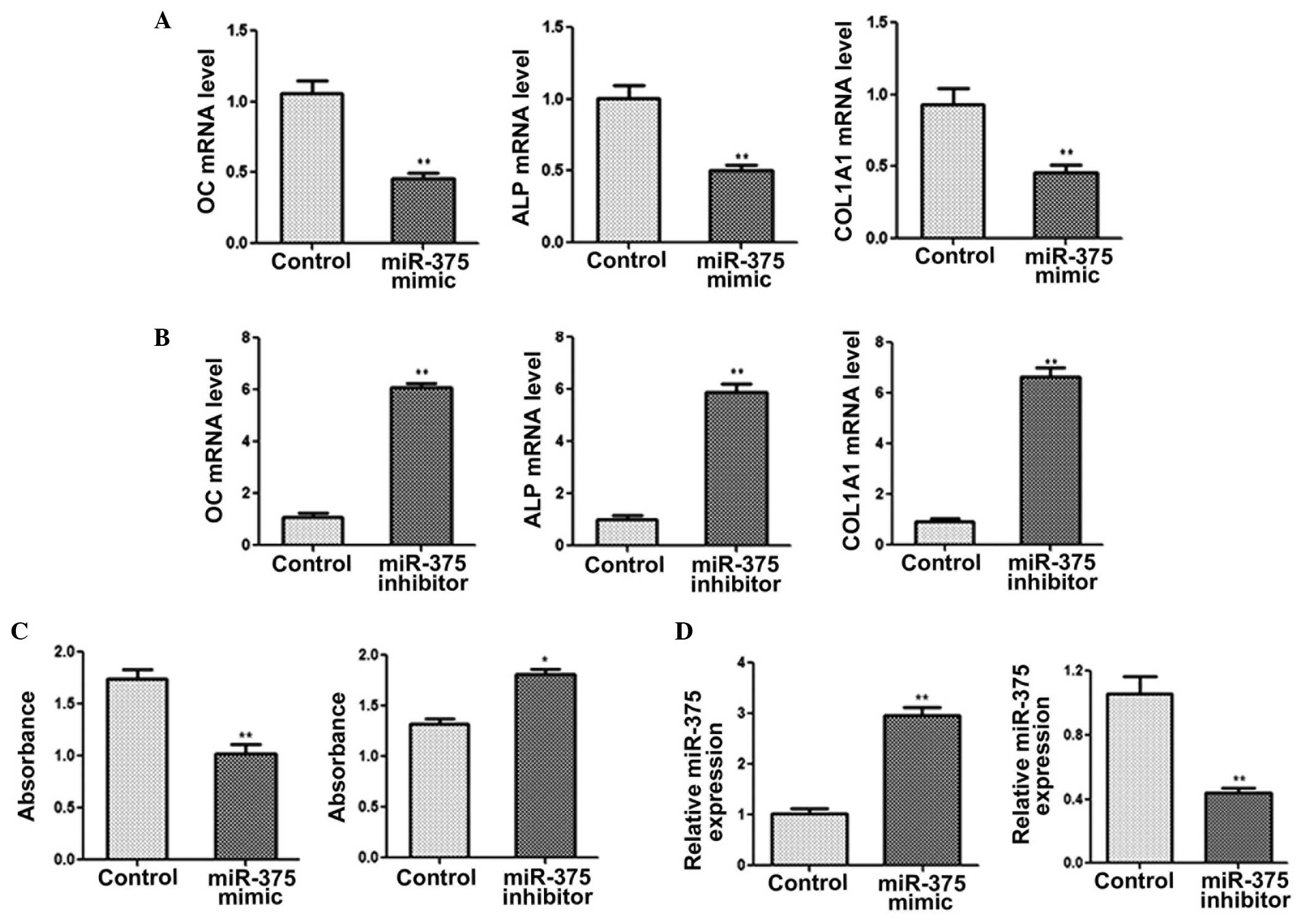 | Figure 2.miR-375 inhibits osteogenic
differentiation. Quantitative polymerase chain reaction (qPCR) was
performed to determine the mRNA expression levels of OC, ALP and
COL1A1 in C2C12 cells transfected with (A) miR-375 mimic or (B)
miR-375 inhibitor. (C) ALP activity was measured following
transfection with miR-375 mimic or inhibitor. (D) Expression levels
of miR-375 were determined using qPCR in C2C12 cells transfected
with miR-145 mimic, miR-145 inhibitor or with no treatment
(control) for 48 h. GAPDH was used as a control for the mRNA
expression levels, while U6 was used as an internal control for
miRNA expression. *P<0.05 and **P<0.01, vs. control group.
OC, osteocalcin; ALP, alkaline phosphatase; COL1A1, collagen, type
I, α 1; miRNA, microRNA. |
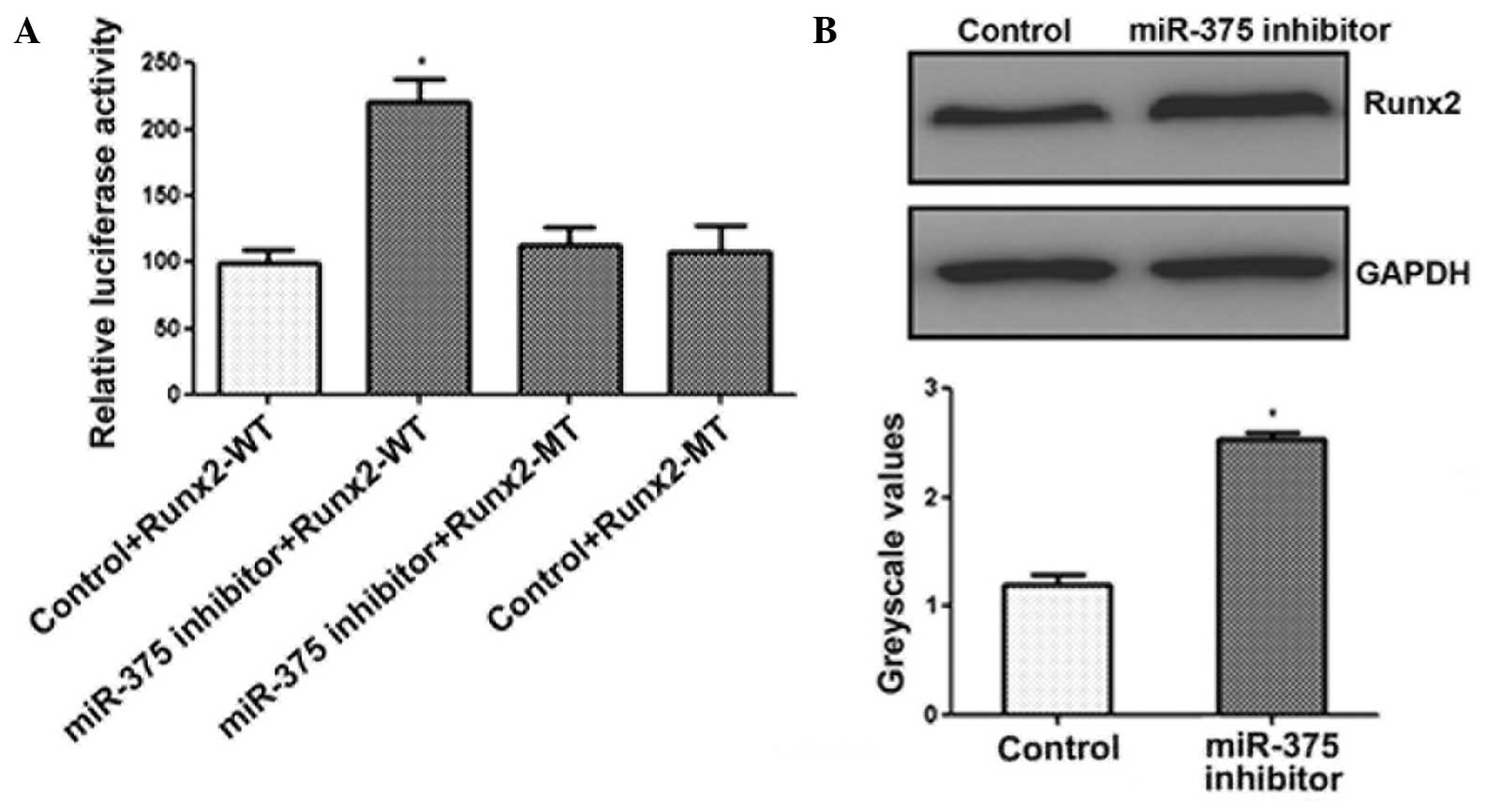
miR-375 reduces the expression levels
of RUNX2
To elucidate the molecular mechanism by which
miR-375 suppressed osteogenic differentiation, miRanda, TargetScan
and PicTar databases were used to search for putative miR-375
targets. The search identified a 3′ untranslated region of RUNX2
that contained the conserved putative miR-375 binding site. A
dual-luciferase activity assay was performed to confirm that RUNX2
was a target of miR-375. As shown in Fig. 3A, luciferase activity was observed to
increase significantly in the C2C12 cells following transfection
with the miR-375 inhibitor, whereas no increase was observed in the
activity of the mutant luciferase (Fig.
3A). In addition, the results of the western blot analysis
demonstrated that inhibition of miR-375 expression in C2C12 cells
significantly enhanced the protein expression levels of RUNX2
(Fig. 3B). These results
demonstrated that miR-375 downregulated the expression of
RUNX2.
miR-375 inhibits osteogenic
differentiation by targeting RUNX2
Finally, whether overexpression of RUNX2 attenuated
the inhibitory effect of miR-375 on osteogenic differentiation was
investigated. The results of the qPCR (Fig. 4A) and western blot analysis (Fig. 4B and C) indicated that RUNX2
expression was upregulated. Furthermore, cotransfection of miR-375
mimic with a RUNX2 overexpression plasmid significantly increased
the mRNA expression levels of OC, ALP and COL1A1 (Fig. 4D–F). Thus, overexpression of RUNX2
appeared to attenuate the miR-375-mediated suppression of
osteogenic differentiation. These results indicated that miR-375
inhibited osteogenic differentiation, in part via the
downregulation of RUNX2 expression.
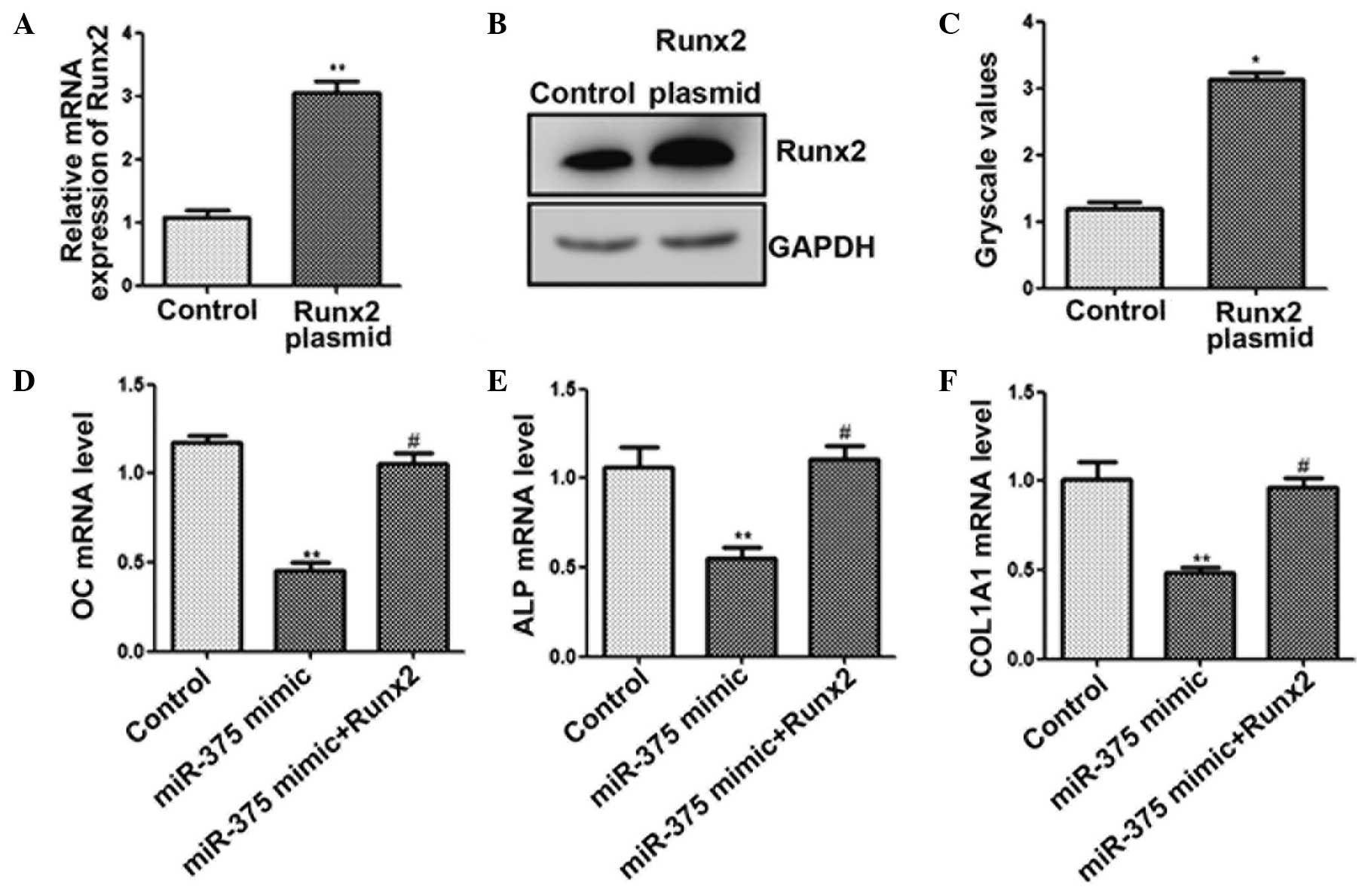 | Figure 4.miR-375 inhibits osteogenic
differentiation by targeting RUNX2. C2C12 cells were transfected
with a RUNX2 plasmid for 48 h. (A) Quantitative polymerase chain
reaction (qPCR) and (B and C) western blot analysis was used to
determine the expression levels of RUNX2. C2C12 cells were
cotransfected with miR-375 mimic and RUNX2-pcDNA3.1 for 48 h. The
mRNA expression levels of (D) OC, (E) ALP and (F) COL1A1 (F) were
determined using qPCR. *P<0.05 and **P<0.01, vs. control
group; #P<0.05, vs. miR-375 mimic transfection group.
RUNX2, runt-related transcription factor 2; OC, osteocalcin; ALP,
alkaline phosphatase; COL1A1, collagen, type I, α 1; miR,
microRNA. |
Discussion
miRNAs have been reported to function as regulators
in skeletal muscle development, myeloblast differentiation and
osteoblastogenesis (15). The
results of the present study indicated that miR-375 functions as an
inhibitor of osteogenic differentiation by modulating RUNX2
expression.
The C2C12 cell line is a typical pluripotent
mesenchymal precursor cell line that possesses the potential to
differentiate into myoblasts, chondroblasts and osteoblasts, which
are commonly used in cellular models of osteogenic differentiation
(16,17). The results of the present study
indicated that the expression of miR-375 was downregulated in the
C2C12 cells during osteogenic differentiation.
Furthermore, OC, ALP and COL1A1 are typical
osteoblast differentiation markers (18). In the present study, C2C12 cells were
used as a model of osteoblast differentiation and the successful
establishment of the model was indicated by a significant elevation
in the expression levels of OC, ALP and COL1A1. Overexpression of
miR-375 was shown to suppress osteogenic differentiation, as
indicated by reductions in the mRNA expression levels of OC, ALP
and COL1A1. By contrast, the inhibition of miR-375 expression
resulted in an increase in the expression levels of these markers.
In addition, ALP activity was reduced in miR-375 mimic-transfected
cells; however, this effect was reversed by the inhibition of
miR-375 expression. Collectively, these results suggested that
miR-375 was able to suppress osteogenic differentiation.
Previous studies have reported aberrant expression
of miR-375 in a number of tumor types, in addition to the
inhibition of cell proliferation and invasion by miR-375 through
targeting numerous key genes (19,20).
Furthermore, a number of additional studies have indicated that
miR-375 is involved in the regulation of insulin secretion, the
maintenance of blood homeostasis and the inhibition of neurite
differentiation (21,22). The results of the present study
revealed that miR-375 was capable of suppressing the osteogenic
differentiation of C2C12 cells. Further investigation into the
mechanism underlying this suppressive effect, by searching for
target genes using bioinformatic analysis, identified RUNX2 as a
potential target of miR-375.
RUNX2 is a member of the RUNX family of
transcription factors and has a runt DNA-binding domain. RUNX2 has
been identified as a key transcription factor in the regulation of
osteogenesis and chondrogenesis (23). miRNAs, as small molecular regulators
of gene expression, have critical roles in osteoblast function by
regulating vital proteins involved in differentiation (24). For example, miR-204 and miR-211 are
known to function as crucial endogenous negative regulators of
RUNX2, promoting adipogenesis and inhibiting the osteogenesis of
mesenchymal progenitor cells (13).
Furthermore, the results of a previous study indicated that
miR-3960 and miR-2861 affected osteoblast differentiation via a
novel RUNX2/miR-3960/miR-2861 regulatory feedback loop (25). In the present study, miR-375
overexpression was demonstrated to result in the suppression of
RUNX2 protein expression, indicating that RUNX2 is regulated by
miR-375. A dual-luciferase reporter assay further identified RUNX2
as a direct target of miR-375. In addition, cotransfection of
miR-375 mimic with a RUNX2 overexpression plasmid was demonstrated
to attenuate the miR-375-mediated inhibition of osteogenic
differentiation. Therefore, these results indicated that miR-375
negatively targets RUNX2 and subsequently inhibits osteogenic
differentiation.
In conclusion, the results of the present study
indicated that miR-375 inhibits osteoblast differentiation via the
regulation of RUNX2 expression. These observations further the
understanding into the biological roles played by miRNAs in
osteoblast differentiation. These observations further
understanding of the biological functions miRNAs in osteoblast
differentiation, and may provide a novel therapeutic target for
treatment of skeletal dysfunction.
References
|
1
|
Sabbieti MG, Agas D, Marchetti L, et al:
BMP-2 differentially modulates FGF-2 isoform effects in osteoblasts
from newborn transgenic mice. Endocrinology. 154:2723–2733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu S, Zhu K, Lai Y, et al: atf4 promotes
β-catenin expression and osteoblastic differentiation of bone
marrow mesenchymal stem cells. Int J Biol Sci. 9:256–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeong HM, Choi YH, Jeong HG, et al:
Bromopropane compounds inhibit osteogenesis by ERK-dependent Runx2
inhibition in C2C12 cells. Arch Pharm Res. 37:276–283. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang R, Edwards JR, Ko SY, et al:
Transcriptional regulation of BMP2 expression by the PTH-CREB
signaling pathway in osteoblasts. PLoS One. 6:e207802011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long F: Building strong bones: molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Hassan MQ, Volinia S, et al: A
microRNA signature for a BMP2-induced osteoblast lineage commitment
program. Proc Natl Acad Sci USA. 105:13906–13911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Hassan MQ, Jafferji M, et al:
Biological functions of miR-29b contribute to positive regulation
of osteoblast differentiation. J Biol Chem. 284:15676–15684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
9
|
Roy S, Banerjee J, Gnyawali SC, et al:
Suppression of induced microRNA-15b prevents rapid loss of cardiac
function in a Dicer depleted model of cardiac dysfunction. PLoS
One. 8:e667892013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rissland OS, Hong SJ and Bartel DP:
MicroRNA destabilization enables dynamic regulation of the miR-16
family in response to cell-cycle changes. Mol Cell. 43:993–1004.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rasheed SA, Teo CR, Beillard EJ, et al:
MicroRNA-182 and microRNA-200a control G-protein subunit α-13
(GNA13) expression and cell invasion synergistically in prostate
cancer cells. J Biol Chem. 288:7986–7995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei J, Shi Y, Zheng L, et al: miR-34s
inhibit osteoblast proliferation and differentiation in the mouse
by targeting SATB2. J Cell Biol. 197:509–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364.
2010.PubMed/NCBI
|
|
14
|
Guo D, Li Q, Lv Q, et al: MiR-27a targets
sFRP1 in hFOB cells to regulate proliferation, apoptosis and
differentiation. PLoS One. 9:e913542014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamouille S, Subramanyam D, Blelloch R and
Derynck R: Regulation of epithelial-mesenchymal and
mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell
Biol. 25:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katagiri T, Yamaguchi A, Komaki M, et al:
Bone morphogenetic protein-2 converts the differentiation pathway
of C2C12 myoblasts into the osteoblast lineage. J Cell Biol.
127:1755–1766. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin CS, Lecanda F, Sheikh S, et al:
Relative abundance of different cadherins defines differentiation
of mesenchymal precursors into osteogenic, myogenic, or adipogenic
pathways. J Cell Biochem. 78:566–577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gámez B, Rodríguez-Carballo E, Bartrons R,
et al: MicroRNA-322 (miR-322) and its target protein Tob2 modulate
Osterix (Osx) mRNA stability. J Biol Chem. 288:14264–14275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding L, Xu Y, Zhang W, et al: MiR-375
frequently downregulated in gastric cancer inhibits cell
proliferation by targeting JAK2. Cell Res. 20:784–793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Li Y, Zhou J, et al: miR-375 is
down-regulated in squamous cervical cancer and inhibits cell
migration and invasion via targeting transcription factor SP1. Am J
Pathol. 179:2580–2588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelmohsen K, Hutchison ER, Lee EK, et
al: miR-375 inhibits differentiation of neurites by lowering HuD
levels. Mol Cell Biol. 30:4197–4210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krützfeldt J and Stoffel M: MicroRNAs: a
new class of regulatory genes affecting metabolism. Cell Metab.
4:9–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takarada T, Hinoi E, Nakazato R, et al: An
analysis of skeletal development in osteoblast-specific and
chondrocyte-specific runt-related transcription factor-2 (Runx2)
knockout mice. J Bone Miner Res. 28:2064–2069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inose H, Ochi H, Kimura A, et al: A
microRNA regulatory mechanism of osteoblast differentiation. Proc
Natl Acad Sci USA. 106:20794–20799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu R, Liu W, Li H, et al: A
Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse
osteoblast differentiation. J Biol Chem. 286:12328–12339. 2011.
View Article : Google Scholar : PubMed/NCBI
|