Introduction
A glioma is a tumor of the brain or spinal cord,
which occurs most commonly in the brain (1). Gliomas account for ~30% of all brain
and central nervous system tumors, and are the largest group of
primary brain tumors (2). According
a previous report (3), the annual
incidence of gliomas is ~4/100,000, which accounts for 1.9% of the
total tumor incidences worldwide (4). Gliomas are typically characterized by
rapid growth, high infiltration and difficulty in surgical
resection, and the majority of patients with gliomas are diagnosed
at stage IV (5). Currently, the
treatment of gliomas primarily consists of surgical resection and
postsurgical radiotherapy and chemotherapy. However, there are a
number of side effects associated with these treatments, resulting
in poor efficacy and final clinical outcomes (6,7). It has
been estimated that >50% of patients with a glioma succumb
within one year of diagnosis (5). In
addition, the pathogenesis underlying gliomas remains unclear
(8). Therefore, an improved
understanding of the molecular pathogenesis of gliomas may be
useful for the improvement of treatment efficacy and the
development of novel treatment schemes.
A microRNA (miRNA or miR) is a small non-coding RNA
molecule, which is produced by endogenous transcripts and contains
19–25 nucleotides. miRNAs have been implicated in the regulation of
gene expression, and are known to be key regulators of various
biological and metabolic processes in humans (9). Previous studies have indicated that
miRNAs are involved in a range of physiological processes in tumor
cells, including cell differentiation, apoptosis and metabolism,
which play a crucial role in tumor development and progression
(10–12). Furthermore, numerous studies have
indicated that miRNAs are involved in the pathogenesis of gliomas,
and may subsequently be useful in the diagnosis and treatment of
gliomas (13–18). However, to the best of our knowledge,
the role of miR-200b in the pathogenesis of gliomas remains
unknown.
Sex determining region Y-box 2 (SOX2) is a
transcription factor that is essential for maintaining the
self-renewal, or pluripotency, of undifferentiated embryonic stem
cells (19). As a member of the Sox
family of transcription factors, SOX2 serves key functions during
embryonic development and is involved in cancer stem cell
maintenance, in which the transcription factor impairs cell growth
and tumorigenicity (20,21). SOX2 is known to be involved in the
development and progression of multiple types of tumor, in which
aberrant SOX2 expression has been detected (22,23–26). In
addition, SOX2 has been demonstrated to be a glioma-specific marker
and a potential target for therapy (27–30).
In the current study, the expression levels of
miR-200b and SOX2 were determined in glioma tissues. Subsequently,
the associations between miR-200b and SOX2 expression with patient
gender and age, and the clinical staging and pathological staging
of the gliomas, were analyzed. In addition, the associations
between miR-200b and SOX2 expression with the prognosis of patients
with a glioma were assessed.
Subjects and methods
Study subjects
Medical records of 123 patients with a primary
glioma were collected from the Henan Provincial People's Hospital
(Zhengzhou, China). All subjects had a definite diagnosis of glioma
and complete medical records. The performance status of the
subjects was scored according to the Karnofsky Performance Scale
(KPS) scoring system, with a score range between 0 and 100. The
scoring system was defined as follows: 100, normal, no symptoms or
evidence of disease; 90, able to perform normal activity, minor
signs or symptoms of disease; 80, normal activity with effort,
certain signs or symptoms of disease; 70, able to care for self,
unable to perform normal activity or to do active work; 60,
requiring occasional assistance, but able to care for the majority
of personal needs; 50, requiring considerable assistance and
frequent medical care; 40, disabled, or requiring special care and
assistance; 30, severely disabled, hospital admission, although not
in a critical condition; 20, notably sick, hospital admission
necessary or active supportive treatment necessary; 10, moribund,
or fatal processes progressing rapidly; 0, mortality. Pathological
staging of the glioma was performed using the tumor, node,
metastasis staging system, while the glioma tissue was graded and
classified according to the tumor grading system outlined by the
World Health Organization (31).
Surgically dissected glioma specimens were fixed in 10% neutral
formalin and embedded in paraffin wax for the subsequent
experiments.
Immunohistochemical detection of SOX2
in glioma specimens
Glioma specimens were surgically dissected, fixed in
Bouin's solution (Sigma-Aldrich, St Louis, MO, USA) for 1 h, rinsed
with phosphate-buffered saline and dehydrated in a series of 70,
80, 95 and 100% ethanol. Subsequently, the specimens were cleared
with xylene, embedded in paraffin wax and cut into sections. Normal
brain tissues, adjacent to the glioma tissues were also excised and
served as controls. The sections were treated with citrate antigen
retrieval solution for 60 min in a humidity chamber (Henan Yuantong
Chemical Co., Ltd., Anyany, China) in order to unmask the epitopes.
Mouse anti-SOX2 polyclonal antibody (cat. no. sc-17320; 1:100;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added to
the sections and incubated overnight at 4°C. Detection was
performed with biotinylated goat anti-mouse IgG secondary antibody
(cat. no. 115-065-003; 1:100; Santa Cruz Biotechnology, Inc.),
using the avidin-biotin-peroxidase technique with
3,3-diaminobenzidine (Dako Denmark A/S, Glostrup, Denmark) as a
chromogen. The tissue samples were counterstained with
Lillie-Mayer's hematoxylin. The proportion of SOX2-positive cells
was determined by counting all the cell nuclei, in addition to the
nuclei positively stained for SOX2, in three randomly selected
high-power fields using an Eclipse 80i microscope (Nikon
Corporation, Tokyo, Japan; magnification, x400) in the tumor core
of each sample. Proportions of SOX2-positive cells at 0–10%,
10–30%, 30–70% and >70% were scored 0, 1, 2 and 3, respectively.
Score 0 indicated SOX2-negative expression and scores 1, 2 and 3
indicated SOX2-positive expression.
miR-200b amplification and
quantification using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR)
Total RNA was extracted from the glioma tissues
using standard procedures (TriPure Reagent; Roche Diagnostics GmbH,
Mannheim, Germany). miR-200b was amplified using the following
forward primer, CGT AAC ACT GTC TGG TAA CGA TGT; and U6 was used a
control with the following former primer, CTC GCT TCG GCA GCA CA.
The reverse primers are defined in the EXPRESS SYBR GreenER miRNA
RT-qPCR kit (Life Technologies, Grand Island, NY, USA). All primers
were synthesized by Shanghai Invitrogen Biotechnology Co., Ltd.
(Shanghai, China). PCR amplification of miR-200b was performed in a
20-µl system containing 10 µl SYBR Premix DimerEraser (Takara
Biotechnology Co., Ltd., Dalian, China), 1.5 µl forward primer (20
µmol/l), 1.5 µl reverse primer (20 µmol/l), 0.4 µl ROX Reference
Dye II (Takara Biotechnology Co., Ltd.), 1 µl cDNA template and 5.6
µl ddH2O. The RT was conducted using the following
protocol: Initial denaturation for 30 sec at 95°C, followed by 45
cycles of denaturation for 5 sec at 95°C, annealing for 30 sec at
60°C and elongation for 30 sec at 72°C. miR-200b expression levels
were detected using qPCR in a 10-µl system that contained 5.0 µl
qPCR SuperMix Universal (Takara Biotechnology Co., Ltd.), 0.2 µl
miRNA-specific forward primer (10 µmol/l), 0.2 µl Universal qPCR
Primer (10 µmol/l), 1.0 µl cDNA template and 3.6 µl RNase-free
ddH2O. The reaction was conducted using the following
protocol: Initial denaturation for 30 sec at 95°C, followed by 45
cycles of denaturation for 10 sec at 95°C, annealing for 15 sec at
55–60°C, and elongation for 15–20 sec at 72°C. The expression of
miR-200b was normalized against that of U6. miR-200b relative
expression levels <0.5 indicated low expression and >1
indicated high expression.
Ethical approval
This study was approved by the Ethics Review
Committee of Henan Provincial People's Hospital (ERC-000117).
Written informed consent was obtained from all the participants
prior to the study.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Differences were assessed for statistical significance using the
χ2 or Fisher's exact test. In addition, correlation
analysis of ranked data was performed using the Spearman rank
correlation test, while survival analyses were performed using the
Kaplan-Meier survival curve approach and the Cox regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
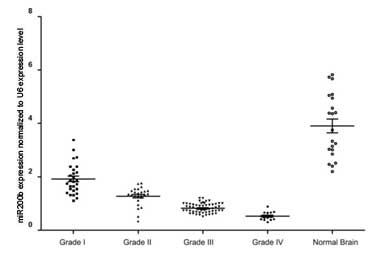
miR-200b expression levels are
increased in glioma tissues compared with normal brain tissues
qPCR analysis detected increased miR-200b expression
levels in the glioma tissues when compared with the normal brain
tissues. Furthermore, a reduction in miR-200b expression was
observed to correlate with the increasing pathological grade of the
gliomas, with the lowest expression detected in grade IV gliomas
(Fig. 1). Thus, the results
demonstrated that miR-200b expression levels may correlate with the
malignancy of the gliomas, with reduced miR-200b expression
detected in gliomas with an increased malignancy.
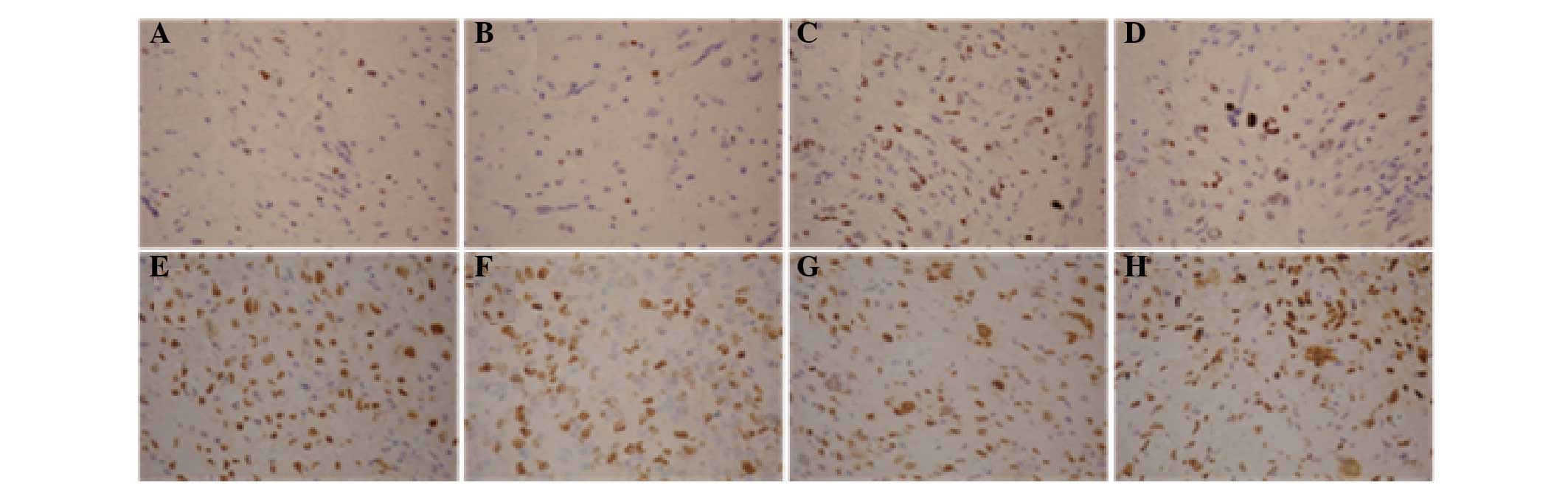
SOX2 expression levels in the glioma
specimens
Immunohistochemical analysis revealed that SOX2
expression was predominantly positive in the nuclei, while SOX2 was
rarely expressed in the cytoplasm. The gross expression rate of
SOX2 in the glioma tissues was 53.7% (66/123; Fig. 2).
Association between SOX2 and miR-200b
expression with clinicopathological characteristics of patients
with gliomas
Of the 123 patients with gliomas, 55 were male and
68 were female, with a median age of 41 years (range, 31–80 years).
Histological classification indicated an astrocytoma in 38 cases
(30.89%), glioblastoma in 53 cases (43.08%) and ependymoma in 32
cases (26.01%). Furthermore, histological grading revealed grade I
in 25 cases, grade II in 27 cases, grade III in 57 cases and grade
IV in 14 cases (Table I). SOX2 and
miR-200b expression levels were observed to correlate with the
histological grading of the gliomas (P=0.002 and 0.004,
respectively). However, no associations were identified with the
patient gender or age, or the pathological classification and
clinical staging of the gliomas (P>0.05).
 | Table I.Associations between miR-200b and
SOX2 expression with the clinicopathological characteristics of
glioma patients. |
Table I.
Associations between miR-200b and
SOX2 expression with the clinicopathological characteristics of
glioma patients.
|
|
| miR-200b
expression, n (%) |
| SOX2 expression, n
(%) |
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Cases (n) | Low | High | P-value | – | + | P-value |
|---|
| Gender |
|
|
| 0.431 |
|
| 0.498 |
|
Male | 68 | 31 (45.6) | 37 (54.4) |
| 40 (58.8) | 28 (41.2) |
|
|
Female | 55 | 29 (52.7) | 26 (47.3) |
| 29 (52.7) | 26 (47.3) |
|
| Age (years) |
|
|
| 0.666 |
|
| 0.059 |
|
<41 | 57 | 29 (50.9) | 28 (49.1) |
| 26 (45.6) | 31 (54.4) |
|
|
≥41 | 66 | 31 (47.0) | 35 (53.0) |
| 43 (65.2) | 23 (34.8) |
|
| Grade |
|
|
| 0.004 |
|
| 0.002 |
| I | 25 | 15 (60.0) | 10 (40.0) |
| 21 (84.0) | 4 (16.0) |
|
| II | 27 | 20 (74.1) | 10 (25.9) |
| 17 (63.0) | 10 37.0) |
|
|
III | 57 | 20 (35.1) | 37 (64.9) |
| 27 (47.4) | 30 (52.6) |
|
| IV | 14 | 5 (35.7) | 9 (64.3) |
| 4 (28.6) | 10 (71.4) |
|
| Pathological
classification |
|
|
| 0.601 |
|
| 0.560 |
|
Astrocytoma | 38 | 16 (42.1) | 22 (57.9) |
| 18 (47.4) | 20 (52.6) |
|
|
Glioblastoma | 53 | 27 (50.9) | 26 (49.1) |
| 31 (58.5) | 22 (41.5) |
|
|
Ependymoma | 32 | 17 (53.1) | 15 (46.9) |
| 20 (62.5) | 12 (37.5) |
|
| Clinical
staging |
|
|
| 0.927 |
|
| 0.269 |
|
I–II | 45 | 25 (55.6) | 20 (44.4) |
| 19 (42.2) | 26 (57.8) |
|
|
III–IV | 78 | 44 (56.4) | 34 (43.6) |
| 41 (52.6) | 37 (47.4) |
|
Correlation between miR-200b and SOX2
expression levels in glioma tissues
No statistically significant association was
observed between miR-200b and SOX2 expression levels in grade I and
II gliomas; however, a significant correlation was detected between
miR-200b and SOX2 expression levels in grade III and IV glioma
tissues (Table II).
 | Table II.Associations between miR-200b and
SOX2 expression and the grade of glioma. |
Table II.
Associations between miR-200b and
SOX2 expression and the grade of glioma.
|
|
| miR-200b
expression, n (%) |
|
|---|
|
|
|
|
|
|---|
| SOX2
expression | Cases (n) | Low | High | P-value |
|---|
| Grade I glioma |
|
|
|
|
| – | 21 | 12 (57.1) | 9 (42.9) | 0.504 |
| + | 4 | 3 (75.0) | 1 (25.0) |
|
| Grade II
glioma |
|
|
|
|
| – | 17 | 14 (82.4) | 3 (17.6) | 0.201 |
| + | 10 | 6 (60.0) | 4 (40.0) |
|
| Grade III
glioma |
|
|
|
|
| – | 27 | 7 (25.9) | 20 (74.1) | 0.019 |
| + | 30 | 17 (56.7) | 13 (43.3) |
|
| Grade IV
glioma |
|
|
|
|
| – | 4 | 3 (75.0) | 1 (25.0) | 0.045 |
| + | 10 | 2 (20.0) | 8 (80.0) |
|
| Total |
|
|
|
|
| – | 69 | 36 (52.2) | 33 (47.8) | 0.395 |
| + | 54 | 24 (44.4) | 30 (55.6) |
|
Association between miR-200b and SOX2
expression with the prognosis of patients with a glioma
A total of 123 patients with a glioma were
followed-up until December 29, 2009, with a median follow-up period
of 52 months (range, 7–69.5 months). The gross 1-, 3- and 5-year
survival rates were 82.1, 50.0 and 30.7%, respectively. Univariate
analysis indicated no association between the patient survival rate
and the patient age, patient gender, KPS score, histological
grading and clinical staging of the glioma (P>0.05). However,
univariate and multivariate analyses indicated that miR-200b and
SOX2 expression levels were independent prognostic factors for
gliomas (Table III).
 | Table III.Univariate and multivariate analyses
of prognostic factors in patients with a glioma. |
Table III.
Univariate and multivariate analyses
of prognostic factors in patients with a glioma.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender, male vs.
female | 1.537 | 1.227–1.860 | 0.527 |
|
|
|
| Age, <41 vs. ≥41
years | 0.567 | 0.344–0.711 | 0.334 |
|
|
|
| KPS, <70 vs. ≥70
score | 1.301 | 0.917–1.785 | 0.278 |
|
|
|
| Grade, I–II vs.
III–IV | 1.607 | 1.308–1.957 | 0.674 |
|
|
|
| Pathology, stage N
vs. stage M | 0.801 | 0.608–1.056 | 0.097 |
|
|
|
| miR-200b
expression, low vs. high | 0.857 | 0.365–1.467 | 0.014 | 0.687 | 0.288–1.175 | 0.004 |
| SOX2 expression,
positive vs. negative | 2.059 | 1.388–2.634 | 0.001 | 2.221 | 1.674–2.812 | 0.001 |
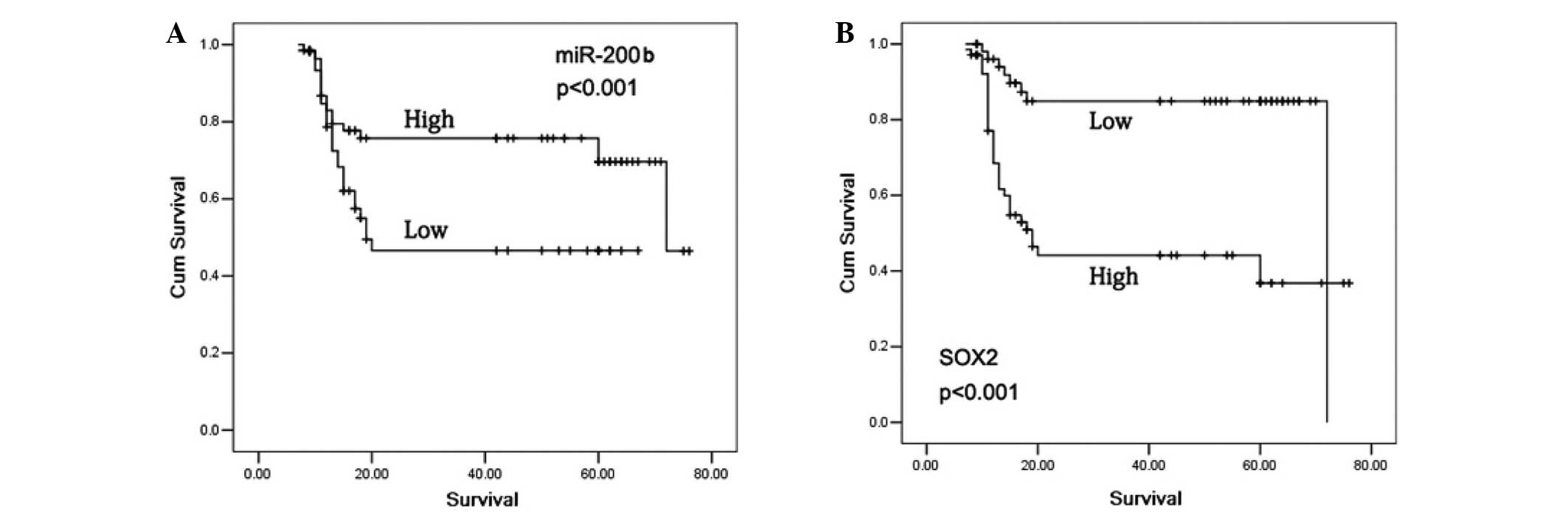
The 5-year survival rate in the glioma patients who
exhibited positive SOX2 expression was significantly reduced
compared with patients with negative SOX2 expression (P<0.001).
In addition, subjects with high miR-200b expression levels
exhibited a significantly increased 5-year survival rate compared
with patients with low miR-200b expression levels (P<0.001;
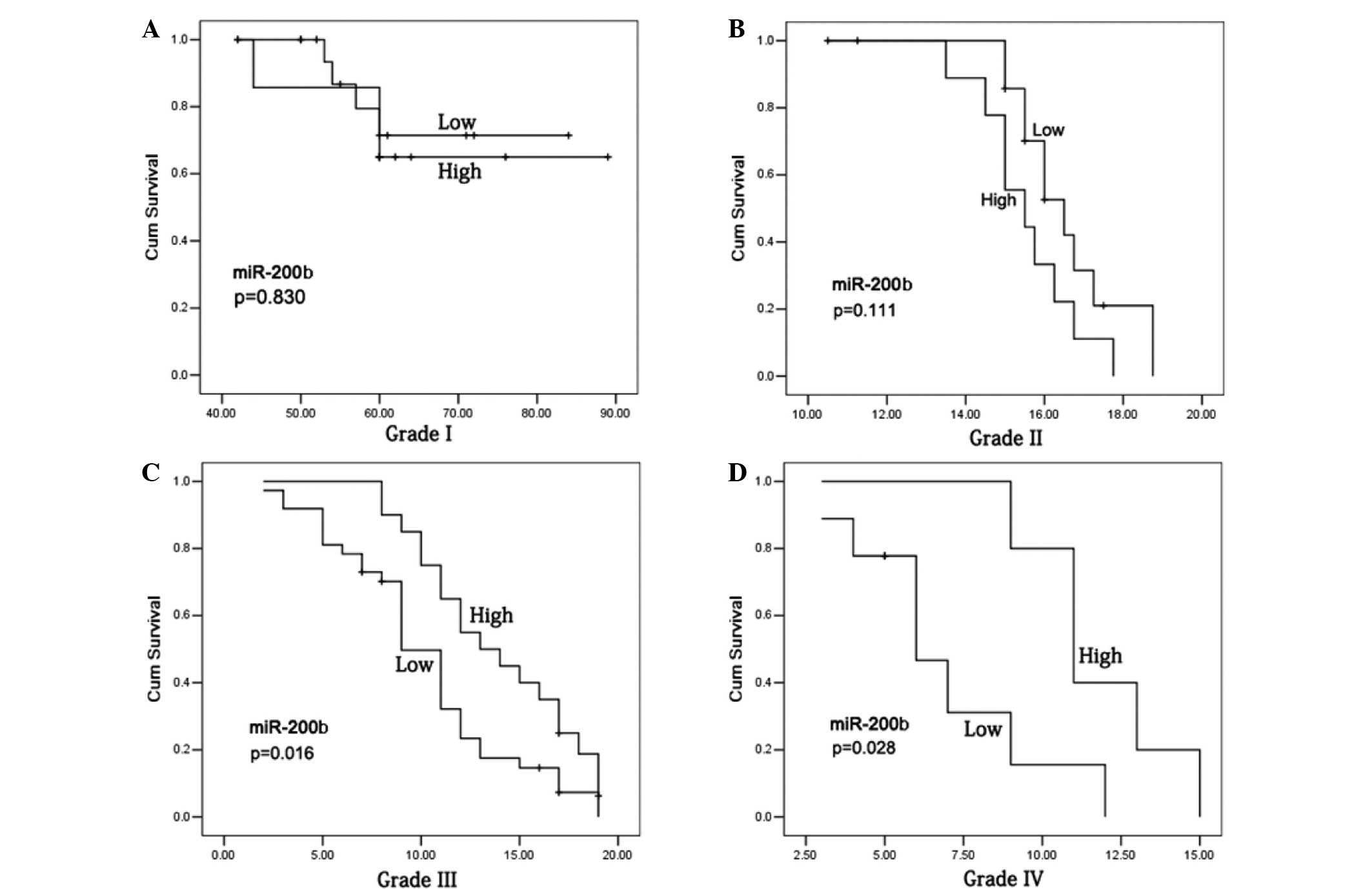
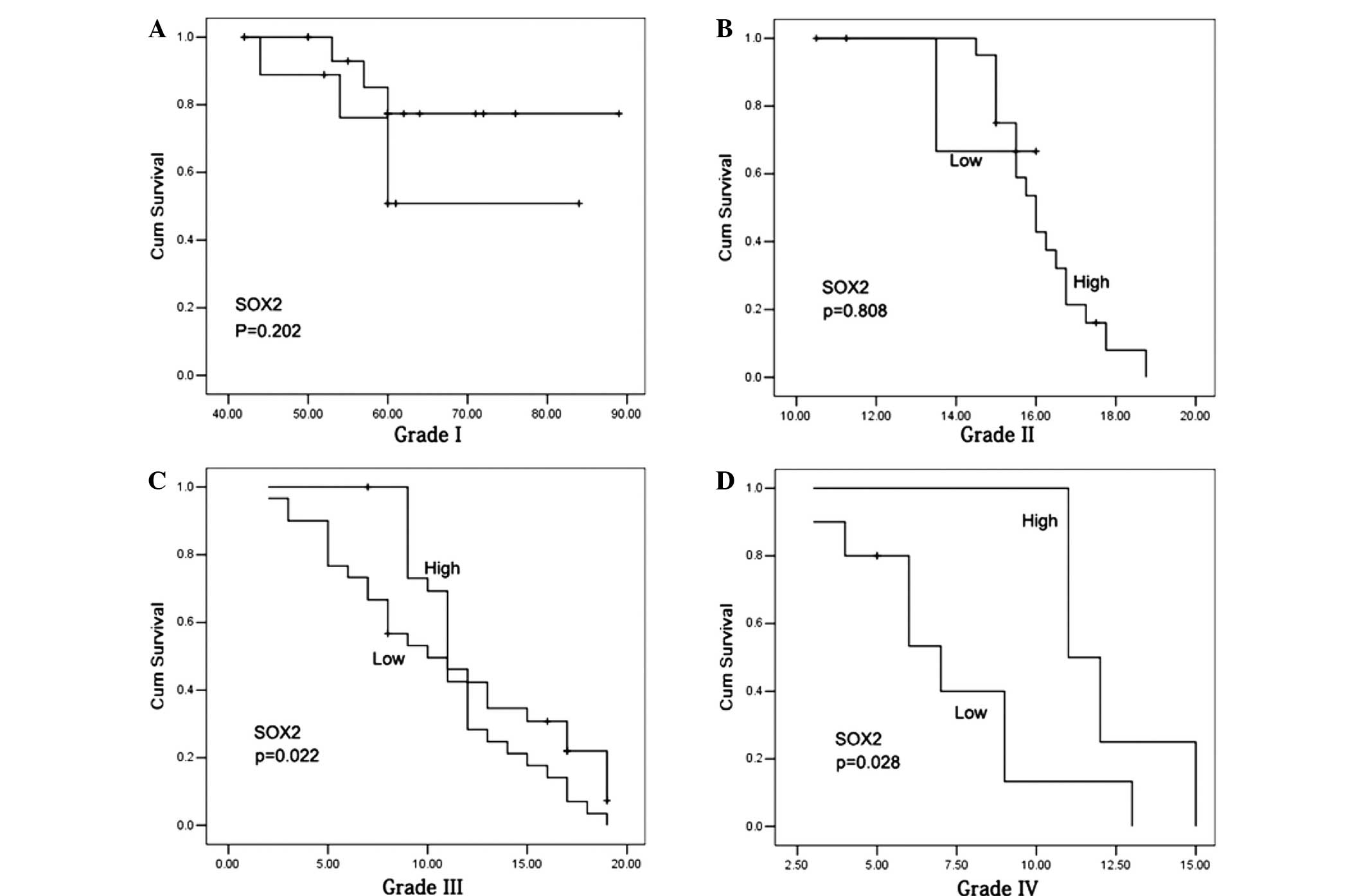
Fig. 3). In the patients with a
grade I–II glioma, no statistically significant differences were
observed in the 5-year survival rates between the cases with high
and low miR-200b expression levels (Fig.
4A and B), or with positive and negative SOX2 expression
(P>0.05; Fig. 5A and B). However,
grade III–IV glioma patients with high miR-200b expression levels
exhibited a significantly higher 5-year survival rate compared with
patients with low miR-200b expression levels (P<0.05; Fig. 4C and D). Furthermore, grade III–IV
glioma patients with positive SOX2 expression exhibited a
significantly reduced 5-year survival rate compared with patients
with negative SOX2 expression (P<0.05; Fig. 5C and D).
Discussion
As the most common type of malignant brain tumor,
gliomas are characterized by high invasion, proliferation and
angiogenesis ability (1). Patients
with a glioma are estimated to have a median survival time of ~16
months following surgery, chemotherapy and radiotherapy (32,33). The
poor prognosis of glioma patients has been primarily attributed to
the presence of drug-resistant cells that have a low proliferative
capacity in the glioma, which are insensitive to current treatments
(34,35). There are a small number of cancer
stem cells with a self-renewal and unlimited differentiation
potential, termed glioma stem cells (GSCs), which are involved in
infiltration, metastasis, chemoresistance and tumor recurrence
(36). Therefore, demonstration of
the characteristics of the GSCs and the mechanisms underlying their
roles in tumorigenesis may facilitate the identification of novel
treatment approaches (37).
miRNAs serve crucial functions in tumorigenesis,
angiogenesis, invasion and apoptosis for various types of tumor
(10–12). Previous studies have identified the
dysregulation of specific miRNAs in malignant gliomas, and numerous
miRNAs are known to be involved in the pathogenesis of gliomas,
with a number useful for the diagnosis and treatment of gliomas
(13–18). miR-21, one of the most common miRNAs
identified by previous studies of gliomas, has been demonstrated to
function as an antiapoptotic factor, which targets a network of
p53, transforming growth factor-β and mitochondrial apoptosis tumor
suppressor genes in glioblastoma cells (38,39).
Plasma levels of miR-21, miR-128 and miR-342-3p have been
recognized as potential novel biomarkers for glioma, since the
expression levels of miR-128 and miR-342-3p have been shown to
positively correlate with the glioma histopathological grade
(40). In addition, miR-21 has been
detected in glioma cells and tumor blood vessels, and miR-21
expression in tumor cells indicates unfavorable prognostic value in
gliomas (41). miR-10b is known to
be overexpressed in the majority of glioblastoma cases, whereas the
miRNA is not detected in normal brain tissue (42). A further study indicated that miR-10b
induced glioma cell invasion by modulating the expression of the
tumor invasion factors, matrix metalloproteinase-14 and urokinase
receptor, via the direct target, homeobox D10. Glioma cells were
observed to lose their invasive ability in response to treatment
with specific antisense oligonucleotides (miR-10b inhibitors),
suggesting that miR-10b may be useful as a novel biological target
for the treatment of glioma (43).
Collectively, these results indicate that the coinhibition of
miR-21 and miR-10b may be an effective therapeutic strategy for
controlling the growth of human glioma cells by inhibiting oncogene
expression and overexpressing tumor suppressor genes (44). Furthermore, previous studies support
the hypothesis that miR-128 may exert a therapeutic effect by
suppressing proliferation and enhancing the differentiation of
glioma initiating cells (45–47).
However, to the best of our knowledge, the precise function of
miR-200b in gliomas remains unclear.
SOX2 has been demonstrated to be involved in the
development and progression of various types of tumor, in which
aberrant SOX2 expression has been detected (22–26). In
addition, the abundant and glioma-restricted overexpression of
SOX2, as well as the generation of SOX2-specific and tumor-reactive
cytotoxic T lymphocytes, has identified this antigen as a potential
target for T-cell-based immunotherapy of glioma (30). SOX2-silenced glioblastoma
tumor-initiating cells have been shown to inhibit proliferation and
mitigate tumorigenicity in immunodeficient mice, indicating that
SOX2, or its immediate downstream effectors, may be an ideal target
for glioblastoma therapy (48). In
addition, SOX2 has been identified as a marker for undifferentiated
and proliferating cells, with its expression upregulated in the
most markedly anaplastic areas of glioblastomas and
oligodendrogliomas (28). In mouse
models (49), high expression of
miR-21 has been observed during embryonic and newborn brain
development, followed by a gradual reduction until undetectable at
postnatal day 7, which correlates with SOX2 expression. miR-21 and
SOX2 exhibited upregulation and an overlapping expression pattern
in RCAS/tv-a generated mouse brain glioma specimens. Following the
irreversible depletion of miR-21, the expression of SOX2 was
markedly reduced in mouse primary glioma cultures and human glioma
cell lines. Therefore, miR-21 and SOX2 were concluded to be
strongly regulated during embryogenesis, and define a distinct
population of putative tumor cells (49).
The results of the present study revealed higher
miR-200b expression levels in the glioma tissues, as compared with
the normal brain tissues. Furthermore, a reduction in miR-200b
expression was observed to correlate with an increased pathological
grade of the glioma, with the lowest expression observed in grade
IV glioma cases. These results indicate that miR-200b is involved
in glioma development and progression, and functions as a tumor
suppressor gene. Immunohistochemical analysis revealed a 53.7%
gross expression rate of SOX2 in the glioma tissues. SOX2 and
miR-200b expression levels were shown to significantly correlate
with the histological grading of the gliomas (P<0.05); however,
no associations were observed with regard to patient gender, age,
pathological classification or clinical staging of gliomas
(P>0.05). In patients with grade I and II gliomas, no
correlation was detected between miR-200b and SOX2 expression
levels, while a significant correlation was observed in grade III
and IV gliomas. A median 52-month follow-up revealed 1-, 3- and
5-year gross survival rates of 82.1, 50.0 and 30.7%, respectively,
in the 123 glioma patients. Univariate analysis revealed no
associations between the patient survival rate and the patient age
or gender, KPS score, histological grading and clinical staging
(P>0.05). However, univariate and multivariate analyses
suggested that miR-200b and SOX2 were independent prognostic
factors of gliomas (P<0.05), which is consistent with previous
studies (28,29).
Kaplan-Meier survival curve estimates and Cox
regression analysis indicated that the glioma patients with
positive SOX2 expression possessed a significantly reduced 5-year
survival rate compared with patients with negative SOX2 expression
(P<0.001). A significantly higher 5-year survival rate was
observed in the subjects with high miR-200b expression levels
compared with patients with low miR-200b expression levels
(P<0.001). In the patients with a grade I–II glioma, no
statistically significant differences were observed in the 5-year
survival rate between the cases with high and low miR-200b
expression levels, or with positive and negative SOX2 expression
(P>0.05). However, grade III–IV glioma patients with high
miR-200b expression exhibited a significantly increased 5-year
survival rate compared with the patients with low miR-200b
expression (P<0.05), and those with positive SOX2 expression
exhibited a significantly reduced 5-year survival rate when
compared with the patients with negative SOX2 expression
(P<0.05). These data indicate that, in a similar manner to other
miRNAs, miR-200b serves a critical function in the stemness of
glioma. Furthermore, miR-200b and SOX2 are independent indicators
for the prognosis of patients with a glioma.
In conclusion, SOX2 and miR-200b expression levels
are associated with the histological grading of glioma; however, no
associations were observed with the patient gender and age, or the
pathological classification and clinical staging of the glioma. In
addition, miR-200b and SOX2 may be used as independent prognostic
factors for glioma.
Acknowledgements
This study was supported by the People's Hospital of
Zhengzhou University. The authors thank the subjects that
participated in this study.
References
|
1
|
Ricard D, Idbaih A, Ducray F, Lahutte M,
Hoang-Xuan K and Delattre JY: Primary brain tumours in adults.
Lancet. 379:1984–1996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohler BA, Ward E, McCarthy BJ, Schymura
MJ, Ries LA, Eheman C, et al: Annual report to the nation on the
status of cancer, 1975–2007, featuring tumors of the brain and
other nervous system. J Natl Cancer Inst. 103:714–736. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fisher JL, Schwartzbaum JA, Wrensch M and
Wiemels JL: Epidemiology of brain tumors. Neurol Clin. 25:867–890.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cordner R, Black KL and Wheeler CJ:
Exploitation of adaptive evolution in glioma treatment. CNS Oncol.
2:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith C and Ironside JW: Diagnosis and
pathogenesis of gliomas. Curr Diagn Pathol. 13:180–192. 2007.
View Article : Google Scholar
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions'. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, et al: MicroRNA expression profiles classify
human cancers. Nature. 435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Li J, Liu L, Li W, Yang Y and Yuan
J: MicroRNA in human glioma. Cancers (Basel). 5:1306–1331. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun B, Pu B, Chu D, Chu X, Li W and Wei D:
MicroRNA-650 expression in glioma is associated with prognosis of
patients. J Neurooncol. 115:375–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou SX, Ding BJ, Li HZ, Wang L, Xia F, Du
F, et al: Identification of microRNA-205 as a potential prognostic
indicator for human glioma. J Clin Neurosci. 20:933–937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu E, Wang D, Zhang X, Li J, Hu Y, Gong H,
et al: Four common polymorphisms in microRNAs and the risk of adult
glioma in a Chinese case-control study. J Mol Neurosci. 51:933–940.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Li P, Li A, Jiang W, Wang H, Wang
J and Xie K: Plasma specific miRNAs as predictive biomarkers for
diagnosis and prognosis of glioma. J Exp Clin Cancer Res.
31:972012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lavon I: The role of microRNAs in gliomas
and their potential applications for diagnosis and treatmentNovel
Therapeutic Concepts in Targeting Glioma. Farassati F: InTech; pp.
75–88. 2012
|
|
19
|
Sarkar A and Hochedlinger K: The sox
family of transcription factors: Versatile regulators of stem and
progenitor cell fate. Cell Stem Cell. 12:15–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masui S, Nakatake Y, Toyooka Y, Shimosato
D, Yagi R, Takahashi K, et al: Pluripotency governed by Sox2 via
regulation of Oct3/4 expression in mouse embryonic stem cells. Nat
Cell Biol. 9:625–635. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao
A, Liu F, Que J and Lan X: The multiple roles for Sox2 in stem cell
maintenance and tumorigenesis. Cell Signal. 25:1264–1271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
González-Márquez R, Llorente JL, Rodrigo
JP, García-Pedrero JM, Álvarez-Marcos C, Suárez C and Hermsen MA:
SOX2 expression in hypopharyngeal, laryngeal and sinonasal squamous
cell carcinoma. Hum Pathol. 45:851–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiao B, He B, Cai J and Yang W: The
expression profile of Oct4 and Sox2 in the carcinogenesis of oral
mucosa. Int J Clin Exp Pathol. 7:28–37. 2013.PubMed/NCBI
|
|
24
|
Huang YH, Luo MH, Ni YB, Tsang JY, Chan
SK, Lui PC, Yu AM, Tan PH and Tse GM: Increased SOX2 expression in
less differentiated breast carcinomas and their lymph node
metastases. Histopathology. 64:494–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J
and Chen G: Elevated expression of SOX2 and FGFR1 in correlation
with poor prognosis in patients with small cell lung cancer. Int J
Clin Exp Pathol. 6:2846–2854. 2013.PubMed/NCBI
|
|
26
|
Chen Y, Huang Y, Huang Y, Chen J, Wang S
and Zhou J: The prognostic value of SOX2 expression in non-small
cell lung cancer: A meta-analysis. PLoS One. 8:e711402013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dahlrot RH, Hermansen SK, Hansen S and
Kristensen BW: What is the clinical value of cancer stem cell
markers in gliomas? Int J Clin Exp Pathol. 6:334–348.
2013.PubMed/NCBI
|
|
28
|
Annovazzi L, Mellai M, Caldera V, Valente
G and Schiffer D: SOX2 expression and amplification in gliomas and
glioma cell lines. Cancer Genomics Proteomics. 8:139–147.
2011.PubMed/NCBI
|
|
29
|
Schmitz M, Temme A, Senner V, Ebner R,
Schwind S, Stevanovic S, et al: Identification of SOX2 as a novel
glioma-associated antigen and potential target for T cell-based
immunotherapy. Br J Cancer. 96:1293–1301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karnofsky DA and Burchenal JH: The
clinical evaluation of chemotherapeutic agents in cancerEvaluation
of Chemotherapeutic Agents. MacLeod CM: Columbia University Press;
New York: pp. 191–205. 1949
|
|
31
|
Mur P, Mollejo M, Hernández-Iglesias T, de
Lope ÁR, Castresana JS, García JF, et al: Molecular classification
defines 4 prognostically distinct glioma groups irrespective of
diagnosis and grade. J Neuropathol Exp Neurol. 74:241–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noda SE, El-Jawahri A, Patel D,
Lautenschlaeger T, Siedow M and Chakravarti A: Molecular advances
of brain tumors in radiation oncology. Semin Radiat Oncol.
19:171–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Desjardins A, Rich JN, Quinn JA,
Vredenburgh J, Gururangan S, Sathornsumetee S, et al: Chemotherapy
and novel therapeutic approaches in malignant glioma. Front Biosci.
10:2645–2668. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
35
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, McKay RM and Parada LF: Malignant
glioma: Lessons from genomics, mouse models and stem cells. Cell.
149:36–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bier A, Giladi N, Kronfeld N, Lee HK,
Cazacu S, Finniss S, et al: MicroRNA-137 is downregulated in
glioblastoma and inhibits the stemness of glioma stem cells by
targeting RTVP-1. Oncotarget. 4:665–676. 2013.PubMed/NCBI
|
|
38
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Papagiannakopoulos T, Shapiro A and Kosik
KS: MicroRNA-21 targets a network of key tumor-suppressive pathways
in glioblastoma cells. Cancer Res. 68:8164–8172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Q, Li P, Li A, Jiang W, Wang H, Wang
J and Xie K: Plasma specific miRNAs as predictive biomarkers for
diagnosis and prognosis of glioma. J Exp Clin Cancer Res.
31:972012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hermansen SK, Dahlrot RH, Nielsen BS,
Hansen S and Kristensen BW: MiR-21 expression in the tumor cell
compartment holds unfavorable prognostic value in gliomas. J
Neurooncol. 111:71–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang
J, et al: MicroRNA-10b induces glioma cell invasion by modulating
MMP-14 and uPAR expression via HOXD10. Brain Res. 1389:9–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong CG, Wu WK, Feng SY, Wang XJ, Shao JF
and Qiao J: Co-inhibition of microRNA-10b and microRNA-21 exerts
synergistic inhibition on the proliferation and invasion of human
glioma cells. Int J Oncol. 41:1005–1012. 2012.PubMed/NCBI
|
|
45
|
Zhang Y, Chao T, Li R, Liu W, Chen Y, Yan
X, et al: MicroRNA-128 inhibits glioma cells proliferation by
targeting transcription factor E2F3a. J Mol Med (Berl). 87:43–51.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, et al: Targeting of the Bmi-1
oncogene/stem cell renewal factor by microRNA-128 inhibits glioma
proliferation and self-renewal. Cancer Res. 68:9125–9130. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi ZM, Wang J, Yan Z, You YP, Li CY, Qian
X, et al: MiR-128 inhibits tumor growth and angiogenesis by
targeting p70S6K1. PLoS One. 7:e327092012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gangemi RM, Griffero F, Marubbi D, Perera
M, Capra MC, Malatesta P, et al: SOX2 silencing in glioblastoma
tumor-initiating cells causes stop of proliferation and loss of
tumorigenicity. Stem Cells. 27:40–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Põlajeva J, Swartling FJ, Jiang Y, Singh
U, Pietras K, Uhrbom L, Westermark B and Roswall P: miRNA-21 is
developmentally regulated in mouse brain and is co-expressed with
SOX2 in glioma. BMC Cancer. 12:3782012. View Article : Google Scholar : PubMed/NCBI
|