Introduction
Acute ischemic stroke (AIS) is a common cause of
morbidity and mortality worldwide. Thrombolysis with recombinant
tissue plasminogen activator (rtPA) is the only proven beneficial
therapy in AIS, and this is received by <2% of patients
(1). The inaccessibility of this
treatment to the majority of patients is due to a number of
factors: A lack of adequate transport facilities and
infrastructure, including facilities for thrombolysis in most
centers; the high cost of tPA; and a lack of awareness among the
public and doctors (2). Furthermore,
there has been a slight increase in the incidence of hemorrhagic
complications, such as intracranial hemorrhage and gastrointestinal
bleeding associated with thrombolysis (2,3).
Glycoprotein IIb/IIIa inhibitors, following their
initial success in patients with acute coronary syndromes, have led
to an increasing interest to treat AIS over the past decade
(4–8). Highly selective platelet antagonists,
the glycoprotein (gp) IIb/IIIa inhibitors, block the fibrin binding
receptors reversibly and effectively prevent platelet
aggregation.
Bridging therapy is the combination of intravenous
(IV) and intra-arterial (IA) thrombolysis, and is part of the
therapeutic armamentarium in the daily practice of several stroke
centers (9). Some clinicians
recommend the use of tirofiban as a bridging therapy during the
perioperative period (10).
Tirofiban is a fast-acting, highly selective nonpeptide gpIIb/IIIa
antagonist used for the treatment of acute coronary syndrome up to
48 h after onset (6); however, the
risk factors associated with tirofiban administration, the impacts
of stroke severity and the type of treatment regimen on the
efficacy of the drug and the exact effect of tirofiban on patients
with AIS, including complications, incidence of symptomatic or
asymptomatic hemorrhage and long-term outcomes, are currently
unclear. The aim of the present study was to investigate the safety
of tirofiban alone and in combination with various treatments in
AIS.
Patients and methods
Patients
A total of 120 patients with AIS were included in
this study for treatment with tirofiban in the context of an
individual treatment trial. The study was approved by the Ethics
Committee of the People's Hospital of Zhengzhou (Zhengzhou, China)
in accordance with the Declaration of Helsinki. Written informed
consent was obtained from all participants.
Treatment
Patients received an initial dose of 0.4 mg over a
30 min period, and tirofiban administration was then continued at a
dosage of 0.1 mg/kg body weight/h. The recommended infusion
duration was 24 h. Patients receiving tirofiban alone and those
receiving tirofiban and other recanalization methods were included.
The patients were divided into three groups: Group A (n=68;
tirofiban monotherapy; Hangzhou Zhongmei Huadong Pharmaceutical
Co., Ltd., Hangzhou, China), group B (n=26; tirofiban in
combination with IV or intra-arterial IA thrombolysis) and group C
[n=26; tirofiban as a ‘bridging therapy’, i.e. an additional
recanalization measure started prior to and continued during and
subsequent to the intervention, known as percutaneous transluminal
coronary angioplasty (PTCA) and coronary stent implantation].
Data collection
Vascular risk profile, stroke localization,
treatment duration, cumulative dose and the time of tirofiban doses
were recorded. The modified Rankin Scale (mRS) (11) was used to describe the severity of
the stroke.
Pretreatment imaging, including cerebral computed
tomography (CCT) and stroke magnetic resonance imaging (MRI), was
performed to evaluate infarct demarcation and any early signs of
infarction. In patients who had completed an angiography prior to
treatment, it was noted whether a vascular occlusion had
occurred.
CCT and MRI images were scored for bleeding
complications during and subsequent to tirofiban application. To
determine the severity of bleeding, the European Cooperative Acute
Stroke Study II criteria (12), as
follows, were used: Hemorrhagic infarction 1 (HI1), small
hemorrhages along the infarct margins; HI2, confluent hemorrhage
within the infarct area but without a space-occupying effect;
parenchymal hematoma (PH) 1, bleeding affects ≤30% of the infarct
area and may show a small space-occupying effect; PH 2, blood flow
affecting >30% of the infarct area and with a significant
space-occupying effect; symptomatic intracerebral hemorrhage,
bleeding in any region of the brain accompanied by a significant
clinical deterioration (12). Those
patients in whom an initial vascular occlusion was documented were
evaluated for recanalization by means of representation-guided
vascular CT angiography (CTA), magnetic resonance angiography (MRA)
or digital subtraction angiography (DSA) following treatment. The
National Institutes of Health Stroke Scale (NIHSS), mRS and Barthel
index (BI) were used to assess the outcomes at discharge.
Statistical analysis
All experiments were performed at least three times.
Data are expressed as the mean ± standard deviation. One-way
analysis of variance was used to analyze the data. Statistical
analyses were carried out using SPSS 17.0 software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
General characteristics
The study included a total of 120 patients (70 males
and 50 females), who were treated with tirofiban in the context of
an individual treatment trial. The vascular risk profile of the
patients is summarized in Table
I.
 | Table I.Vascular risk profile of patients. |
Table I.
Vascular risk profile of patients.
| Risk factors | n (%) |
|---|
| Diabetes
mellitus | 16 (13.3) |
| Arterial
hypertension | 74 (61.7) |
| Arteriosclerosis | 68 (56.7) |
| Heart rhythm
disorders | 30 (25.0) |
| Other cardiac source
of embolism | 26 (21.7) |
| Hyperlipidemia | 52 (43.3) |
| Obesity | 52 (43.3) |
| Previous stroke | 28 (23.3) |
| Smoking (current and
former) | 30 (25.0) |
Patient groups
The majority of the patients (n=68) received
tirofiban as a monotherapy and were assigned to group A. In these
patients, clot lysis by tPA was no longer a viable option,
primarily due to a lapsed three-hour time window. A total of 18
patients were treated within the time window, meaning that
different exclusion criteria for the lysis treatment were
applicable. In addition, two patients suffered from ulcerative
colitis and alcohol abuse, respectively, and another four patients
had already received the tirofiban treatment. These 26 patients
were thus assigned to group B and further divided into subgroups
according to whether tirofiban was administered following systemic
or IA thrombolysis.
The remaining 26 patients were assigned to group C.
In these patients, other recanalization or bridging IV therapy was
initiated during the tirofiban therapy. In one case, the bridge was
combined with a carotid endarterectomy. The young patient had
previously been treated systemically due to a medium infarction.
Since the symptoms did not improve, the patient was brought to the
People's Hospital of Zhengzhou, where a stenosis of the internal
carotid artery and a thrombus just distal to the stenosis were
found the day after admission. Eight patients received IA
thrombolysis, which was performed from the onset of symptoms until
2.5 h after the start of treatment. In three patients, however, the
time of symptom onset was unclear; IA embolectomy was performed in
these three patients. The mean time to the initiation of treatment
with tirofiban was 6.25 h, with another 35 min until the beginning
of the intervention. In one patient receiving IV lysis and
tirofiban simultaneously, the time of treatment was 2.5 h. In all
patients undergoing neuro-interventional radiology measures, the
infusion was maintained during and following treatment by coronary
angiography or by PTCA in the presence or absence of stent
implantation in patients with acute coronary syndrome. The
distribution of stroke localization in the different groups is
shown in Table II.
 | Table II.Distribution of stroke in the
different groups. |
Table II.
Distribution of stroke in the
different groups.
| Infarct | Total, n | Group A, n | Group B, n | Group C, n |
|---|
| Anterior circulation
infarct | 36 | 20 | 2 | 14 |
| Posterior circulation
infarct | 80 | 46 | 22 | 12 |
| Watershed
infarct | 4 | 2 | 2 | – |
Imaging findings
Prior to treatment, all of the patients received
CCT; in two cases, MRI was performed to rule out intracerebral
hemorrhage. Demarcated infarcts were found in 32 patients in the
imaging studies, while another 24 patients had early infarct
signs.
A total of 93.3% of the patients underwent vascular
imaging with CTA, MRA or DSA. Transcranial duplex sonography was
carried out in two cases. Sixty-eight patients (56.7% of the total
population) had a complete or nearly complete vessel occlusion.
Clinical outcomes and
complications
The application of tirofiban was recommended for a
duration of 24 h; however, the infusions were continued until a
stable situation had been established in 12 cases. Furthermore, a
second dose was administered in cases of fluctuating symptoms,
which significantly extended the total application time. The mean
treatment duration was 30.5 h (range, 4–106 h). The mean cumulative
dose was 15.7 mg tirofiban (range, 2.5–71.9 mg). The infusion had
to be terminated prematurely in 18 patients due to complications.
Two patients exhibited a contrast-medium extravasation injury in
the basal ganglia by CT imaging following thrombolysis; in these
cases, the infusion was terminated due to the potential masking of
a bleed by the contrast agent. Four patients developed a large
space-occupying infarction and had to undergo a hemicraniectomy. In
two cases there were concerns whether the treatment should
continue, since the patients were suffering from colon cancer.
Thrombocytopenia occurred in two cases. Two patients suffered from
hematemesis and four patients had controlled parenchymal bleeding
at the end of the CT.
The time from symptom onset to tirofiban
administration showed considerable variation and ranged from 45 to
72 h; this was particularly due to the fact the patients in group A
were treated only with tirofiban and the fluctuation in the
symptoms was noticeable. In 10 patients, the time of symptom onset
could not be determined as the patient woke up with symptoms. The
median time represented the time that similar treatments were
started in groups A and C (Table
III).
 | Table III.Time to initiation of treatment with
tirofiban (hours). |
Table III.
Time to initiation of treatment with
tirofiban (hours).
| Statistic | Total population | Group A | Group B | Group C |
|---|
| Mean | 9.5 | 11.6 | 9.3 | 3.6 |
| Median | 4.5 | 4.3 | 7.0 | 2.5 |
In the total population, only eight (6.7%) out of
all the patients suffered hemorrhage. All hemorrhages were
classified as parenchymal bleeding without symptomatic bleeding.
Table IV shows the frequency of
bleeding in each group.
 | Table IV.Frequency of bleeding in the
groups. |
Table IV.
Frequency of bleeding in the
groups.
| Group | Total bleeding, n
(%) | HI1, n (%) | HI2, n (%) | PH1, n (%) | PH2, n (%) | SICH, n (%) |
|---|
| Group A | – | – | – | – | – | – |
| Group B | 6 (23.1) | – | – | 6 (23.1) | – | – |
| Group C | 2 (7.7) | – | – | 2 (7.7) | – | – |
| Total | 8 (6.7) | – | – | 8 (6.7) | – | – |
A total of 14 patients (11.7%) experienced
extracerebral bleeding complications. Three, five and two patients
suffered from gastrointestinal hemorrhage, abdominal wall hematoma
and compartment syndrome following hemorrhage in the thigh,
respectively. None of the patients required a blood transfusion.
Two out of the 14 patients had received tirofiban following
systemic lysis therapy with tPA, while the remaining 12 patients
were treated in group A. One patient in group A was observed to
have clinically insignificant thrombocytopenia.
A complete or nearly complete vessel occlusion was
detected in 68 patients, 38 of whom were subjected to transcranial
duplex sonography. Twenty-four patients underwent recanalization of
the occluded vessel (Table V).
 | Table V.Arterial recanalization rate for
patients diagnosed with vascular occlusion. |
Table V.
Arterial recanalization rate for
patients diagnosed with vascular occlusion.
| Therapy | Recanalization, n
(%) |
|---|
| Tirofiban
monotherapy, n=8 | 2
(25) |
| IV thrombolysis,
n=8 | 4
(50) |
| IA thrombolysis,
n=16 | 14 (88) |
| IV and IA
thrombolysis, n=2 | 0
(0) |
| Embolectomy, n=4 | 2
(50) |
| Early CEA, n=2 | 2
(100) |
| Total, n=40 | 24 (60) |
Stoke severity
To assess the severity of the stroke, the patients
were assessed by the mRS and NIHSS at admission, as shown in
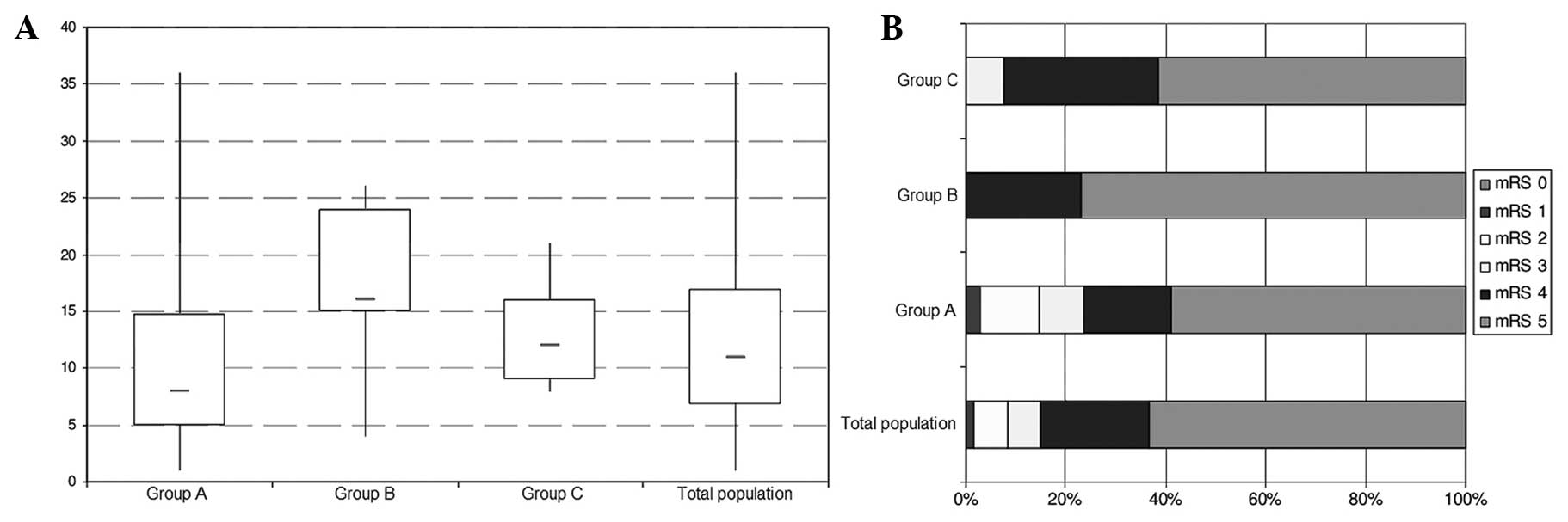
Fig. 1. The median NIHSS was 11
points in the overall patient population, eight points in group A,
16 points in group B and 12 points in group C. Overall, the
variation was large, but most cases were serious.
A total of 16 patients (six in group A, eight in
group B and two in group C) succumbed during the hospital stay; the
mortality rate was 13.3% (8.8% for group A, 30.7% for group B and
7.7% for group C) in the acute phase. These 16 patients had an mRS
of five at admission. Forty-eight of the 120 patients or their
relatives were interviewed. The follow-up period was between three
and 14 months after the stroke, and the BI and mRS were queried.
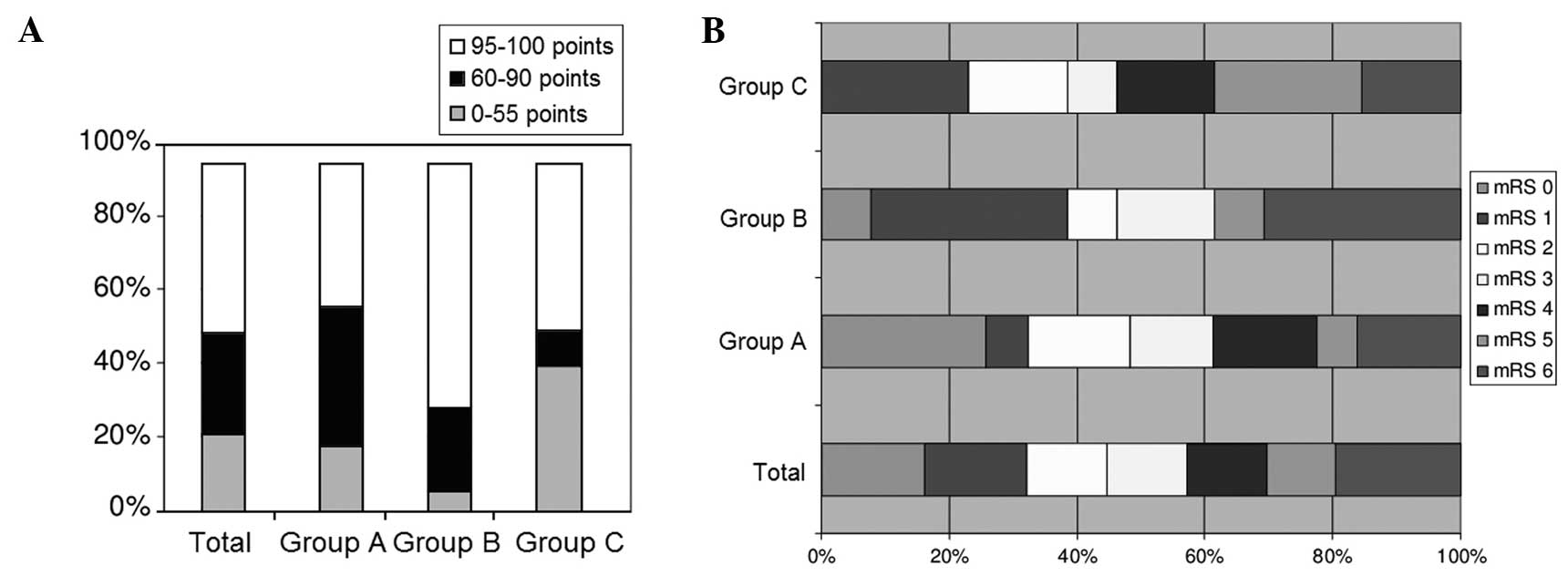
The results are shown in Fig. 2.
A favorable outcome (mRS, 0–2) during the first
three months after the stroke was only observed in 43.3% of the
patients (44.1% in group A, 46.7% in group B and 36.4% in group C).
The mean BI was 72.3 points in group A, 84.4 points in group B,
56.8 points in group C and 71.0 points in the total patient
population.
Discussion
In this retrospective study, it was shown that, in
certain cases, the gpIIb/IIIa antagonist tirofiban could be used
for the treatment of AIS. The present study was retrospective,
however, not controlled, and considered various treatment
strategies simultaneously; there is therefore an urgent need to
confirm the results with larger, prospective, controlled
studies.
In the present study, the outcomes of group A and
group C were better than group B. No patients in group A and only
two patients in group C had asymptomatic hemorrhages. A follow-up
study comparing rtPA (dosage, 0.6 mg/kg) plus eptifibatide with
standard lysis is in process. This may then become a phase III
efficacy study (13).
Notable results have also been found in experimental
stroke studies in rodents. In a study by Kleinschnitz et al
(14), a dose-dependent association
was found between the risk of intracerebral hemorrhage and the use
of anti-mouse gpIIb/IIIa F(ab')2 fragments at doses
resulting in a receptor blockade of >95%, but not at doses
resulting in a receptor blockade of 67.8%. Choudhri et al
(15) found significant bleeding
following the administration of the non-peptide substance SDZ GPI
562 at maximum doses in a mouse model of AIS. Subsequent to the
administration of lower doses, a significantly smaller infarct
volume than expected was observed by staining with
triphenyltetrazolium chloride. Other studies in experimental stroke
models in guinea pigs and squirrel monkeys with the non-peptide
gpIIb/IIIa blocker FK419 revealed no bleeding complications, but
showed reduced infarct volume as an indication of their
effectiveness (16,17).
The gpIIb/IIIa receptor (integrin aIIbb3) has the
same β3 subunit as the vitronectin receptor (integrin αvβ3), which
is present on resting endothelial cells in small numbers; however,
the expression of αvβ3 is upregulated in response to angiogenic
stimuli, such as hypoxia, transforming growth factor-β3 and
thrombin, as they occur in the context of regional cerebral
ischemia. The expression of the vitronectin receptor on endothelial
cells is responsible for the adhesion of monocytes to the
endothelium, conveys permeability to the blood-brain barrier and,
with vascular endothelial growth factor, contributes to the
proliferation and migration of inflammatory cells into the
perivascular tissue during angiogenesis (18,19). The
binding of gpIIb/IIIa receptor blockers to the vitronectin receptor
affects the permeability of the blood-brain barrier and thus
influences the occurrence of intracerebral hemorrhage. A
dose-dependent study of the effects of gpIIb/IIIa blockers on
activated endothelial cells may provide further insight.
While the link between fibronectin receptor
interference and the occurrence of intracranial bleeding (ICB) is
currently more of a theoretical nature, the favorable association
between vascular occlusion and reperfusion subsequent to ICB has
been previously shown (20). The use
of biomarkers in the blood-brain barrier enables the prediction of
intracranial hemorrhagic complications following stroke and
particularly subsequent to thrombolysis with the administration of
an additional therapeutic agent. Specifically, matrix
metalloproteinase-9, cellular fibronectin, S100β and glial
fibrillary acidic protein have been shown to facilitate the
prediction of intracranial hemorrhage (21).
Biomarkers could also be used to study the different
gpIIb/IIIa antagonists with regard to bleeding complications.
Mangiafico et al (22)
described 21 patients with AIS who underwent an aggressive
treatment regimen consisting of IV tirofiban for 24 to 48 h, IV
heparin, local lysis with urokinase and, in the majority of
patients, percutaneous transluminal angioplasty. It should be
noted, however, that the comparability is limited due to low
patient numbers. A previous study (23) investigated the combination of
tirofiban with unfractionated IV heparin (UFH) or with IV rtPA in
the treatment of acute stroke. Junghans et al (23) prospectively studied 18 patients
within 24 h after the onset of stroke symptoms; the patients were
initially treated with UFH, with a target activated partial
thromboplastin time of 50–70 sec, and then tirofiban at the dosage
recommended in the Platelet Receptor Inhibition in Ischemic
Syndrome Management in Patients Limited by Unstable Signs and
Symptoms study (24) for ~46 h.
Although no major intracerebral hemorrhage was observed in the
study, only a low recanalization rate of 25% was obtained.
Both tirofiban and heparin possess thrombolytic
properties. The rational behind the treatment is associated with
the time it takes for an endogenous mechanism mediated by the
endothelial cells to extend the effects of the thrombolytic therapy
to prevent the occurrence of further thrombi and the reocclusion of
re-opened vessels. In addition to the narrow time window, sole rtPA
administration causes easy vessel re-opening in ~1/3 re-occluded
cases (25). In group B of our work,
the vessel re-opening still exists despite the major tribes limit
the microcirculation.
In the present study, the long-term outcome of the
patients in group A was not as promising as that observed for the
SaTIS population; the SaTIS trial is the only previous study of
tirofiban in a large cohort of stroke patients (5). Furthermore, in the present study the
mean BI ≥3 months after stroke was 71 points (median, 90).
Overall, the present study has demonstrated the
safety of tirofiban in monotherapy or in combination with various
recanalization methods in the treatment of patients with AIS. Due
to the retrospective, non-controlled study design and the
relatively low numbers of patients with heterogeneous treatment
approaches, these data must be interpreted with caution; however,
they give a good insight into the safety of the use of tirofiban in
clinical practice.
References
|
1
|
Suwanwela NC, Phanthumchinda K and
Likitjaroen Y: Thrombolytic therapy in acute ischemic stroke in
Asia: The first prospective evaluation. Clin Neurol Neurosurg.
108:549–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nandigam K, Narayan SK, Elangovan S, Dutta
TK, Sethuraman KR and Das AK: Feasibility of acute thrombolytic
therapy for stroke. Neurol India. 51:470–473. 2003.PubMed/NCBI
|
|
3
|
Matsuo R, Kamouchi M, Fukuda H, et al: FSR
Investigators: Intravenous thrombolysis with recombinant tissue
plasminogen activator for ischemic stroke patients over 80 years
old: The Fukuoka Stroke Registry. PLoS One. 9:e1104442014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar S, Rajshekher G and Prabhakar S:
Platelet glycoprotein IIb/IIIa inhibitors in acute ischemic stroke.
Neurol India. 56:399–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siebler M, Hennerici MG, Schneider D, et
al: Safety of Tirofiban in acute Ischemic Stroke: the SaTIS trial.
Stroke. 42:2388–2392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciccone A, Abraha I and Santilli I:
Glycoprotein IIb-IIIa inhibitors for acute ischemic stroke. Stroke.
38:1113–1114. 2007. View Article : Google Scholar
|
|
7
|
Haerten K, Krabbe C and Raiber M: Efficacy
and safety of treatment of acute ischemic stroke with glycoprotein
IIb/IIIa receptor blocker in routine clinical practice. Dtsch Med
Wochenschr. 129:607–610. 2004.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bogousslavsky J and Leclerc JR: Platelet
glycoprotein IIb/IIIa antagonists for acute ischemic stroke.
Neurology. 57:(5 Suppl 2). S53–S57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazighi M, Meseguer E, Labreuche J and
Amarenco P: Bridging therapy in acute ischemic stroke: A systematic
review and meta-analysis. Stroke. 43:1302–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brilakis ES, Banerjee S and Berger PB:
Perioperative management of patients with coronary stents. J Am
Coll Cardiol. 49:2145–2150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banks JL and Marotta CA: Outcomes validity
and reliability of the modified Rankin scale: Implications for
stroke clinical trials: A literature review and synthesis. Stroke.
38:1091–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hacke W, Kaste M, Fieschi C, et al:
Randomised double-blind placebo-controlled trial of thrombolytic
therapy with intravenous alteplase in acute ischaemic stroke (ECASS
II). Second European-Australasian Acute Stroke Study Investigators.
Lancet. 352:1245–1251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pancioli AM, Broderick T, Brott T, et al:
CLEAR Trial Investigators: The combined approach to lysis utilizing
eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke
trial. Stroke. 39:3268–3276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleinschnitz C, Pozgajova M, Pham M,
Bendszus M, Nieswandt B and Stoll G: Targeting platelets in acute
experimental stroke: Impact of glycoprotein Ib, VI, and IIb/IIIa
blockade on infarct size, functional outcome, and intracranial
bleeding. Circulation. 115:2323–2330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choudhri TF, Hoh BL, Zerwes HG, et al:
Reduced microvascular thrombosis and improved outcome in acute
murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet
aggregation. J Clin Invest. 102:1301–1310. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moriguchi A, Maeda M, Mihara K, Aoki T,
Matsuoka N and Mutoh S: FK419, a novel nonpeptide GPIIb/IIIa
antagonist, restores microvascular patency and improves outcome in
the guinea-pig middle cerebral artery thrombotic occlusion model:
comparison with tirofiban. J Cereb Blood Flow Metab. 25:75–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda M, Moriguchi A, Mihara K, et al:
FK419, a nonpeptide platelet glycoprotein IIb/IIIa antagonist,
ameliorates brain infarction associated with thrombotic focal
cerebral ischemia in monkeys: comparison with tissue plasminogen
activator. J Cereb Blood Flow Metab. 25:108–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coller BS: Binding of abciximab to alpha V
beta 3 and activated alpha M beta 2 receptors: with a review of
platelet-leukocyte interactions. Thromb Haemost. 82:326–336.
1999.PubMed/NCBI
|
|
19
|
Murphy JF, Bourdet JC, Wyler B, et al: The
vitronectin receptor (alpha v beta 3) is implicated, in
coorperation with P-selectin and platelet-activating factor, in the
adhesion of monocytes to activated endothelial cells. Biochem J.
304:537–542. 1994.PubMed/NCBI
|
|
20
|
Molina CA, Alvarez-Sabín J, Montaner J, et
al: Thrombolysis-related hemorrhagic infarction: a marker of early
reperfusion, reduced infarct size, and improved outcome in patients
with proximal middle cerebral artery occlusion. Stroke.
33:1551–1556. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montaner J, Molina CA, Monasterio J, et
al: Matrix metalloproteinase-9 pretreatment level predicts
intracranial hemorrhagic complications after thrombolysis in human
stroke. Circulation. 107:598–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mangiafico S, Cellerini M, Nencini P,
Gensini G and Inzitari D: Intravenous glycoprotein IIb/IIIa
inhibitor (tirofiban) followed by intra-arterial urokinase and
mechanical thrombolysis in stroke. AJNR Am J Neuroradiol.
26:2595–2601. 2005.PubMed/NCBI
|
|
23
|
Junghans U, Seitz RJ, Aulich A, Freund HJ
and Siebler M: Bleeding risk of tirofiban, a nonpeptide GPIIb/IIIa
platelet receptor antagonist in progressive stroke: an open pilot
study. Cerebrovasc Dis. 12:308–312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Platelet Receptor Inhibition in Ischemic
Syndrome Management in Patients Limited by Unstable Signs and
Symptoms (PRISM-PLUS) Study Investigators: Inhibition of the
platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable
angina and non-Q-wave myocardial infarction. N Engl J Med.
338:1488–1497. 1998.PubMed/NCBI
|
|
25
|
Alexandrov AV and Grotta JC: Arterial
reocclusion in stroke patients treated with intravenous tissue
plasminogen activator. Neurology. 59:862–867. 2002. View Article : Google Scholar : PubMed/NCBI
|