Introduction
Total hip arthroplasty (THA) is an effective
therapeutic intervention for pain relief in elderly patients with
hip degeneration (1). Although the
treatment can radically resolve the chronic pain that results from
hip lesions, the acute pain derived from hip surgery continues to
afflict patients post-operatively, particularly in elderly
patients, and this may cause physiological and psychological
complications (2,3). Unrelieved post-operative pain in
elderly individuals may lead to delayed mobilization and
rehabilitation, poor surgical outcomes and prolonged hospital stay
(4,5). Consequently, there is a clear
requirement for post-operative pain relief that is able to not only
reduce pain-related complications but also achieve rapid
rehabilitation following THA.
Non-steroidal anti-inflammatory drugs (NSAIDs) are
universally utilized as an assistant approach to acute pain
management (6). However, due to
non-selective inhibitory effects on cyclooxygenase (COX)-1 and
COX-2, several side-effects of conventional NSAIDs have emerged
(7). Celecoxib, as a representative
of the class of highly selective COX-2-specific inhibitors, can
specifically inhibit the functioning of COX-2 in vivo
without affecting the protective action of COX-1. In addition, it
suppresses the synthesis of prostaglandins (PGs) and thereby
reduces their effects, which include reduction of the pain
threshold and enhancement of the transduction and transmission of
nociceptive information in the central and peripheral nociceptors
(8). Several studies (9–11) have
reported the use of celecoxib as a pre-emptive approach to realize
satisfactory peri- or post-operative clinical pain management.
Patient-controlled opioid analgesia pumps have
commonly been used for post-operative acute pain management.
However, certain complications, such as post-operative nausea and
vomiting (PONV) and urinary retention, are attributable to the
usage of opioid narcotic when given at increasing concentrations
(12). In order to achieve a
compromise that not only decreases opioid consumption but also
achieves favorable pain relief, a combination of COX-2 inhibitor
and patient-controlled analgesia (PCA) may be administered.
In the Affiliated Taizhou People's Hospital of
Nantong University (Taizhou, China), a team has focused on studying
the efficacy of celecoxib following hip arthroscopic surgery
(13). However, there have been few
studies evaluating the use of oral celecoxib in the elderly
population undergoing THA when combined with PCA post-operatively
in clinical practice. The aim of the present study was to evaluate
the efficacy of celecoxib following THA in a multimodal analgesic
strategy. The hypothesis that peri-operative pain management using
oral celecoxib in combination with PCA can improve pain intensity,
achieve a narcotic-sparing effect, early ambulation, and better
rehabilitation after THA in elderly patients was investigated.
Patients and methods
Patient selection
Between June 2011 and June 2013, a total of 64
patients underwent THA in the Affiliated Taizhou People's Hospital
of Nantong University were considered eligible to participate in
the prospective randomized placebo-controlled study. Informed
consent was obtained from all patients who participated in this
study. The study was approved by the Regional Ethics Committee of
Taizhou People's Hospital.
Inclusion and exclusion criteria
Patients who were >65 years old, with
osteoarthritis and aseptic necrosis of femoral head met the
inclusion criteria. The exclusion criteria for this study were
allergy to celecoxib, sulfa drugs, acetylsalicylic acid or NSAIDs;
existence of blood coagulation dysfunction, or severe hepatic and
renal dysfunction; evidence of active peptic ulcer or recent
cardio-cerebrovascular events; relevant contraindications of THA,
such as active or chronic infection in the target hip or other
region of the body and chronic osteomyelitis; active joint
tuberculosis or blood disease; and inability to undergo lumbar
anesthesia due to previous lumbar diseases or surgeries. Patients
who experienced acute injury were excluded in order to establish a
unified group of patients that were in good physiological and
psychological condition prior to the surgery. Patients who refused
to anticipate in the study or who had inadequate training for PCA
usage were also excluded.
Study design and procedure
A total of 64 consecutive patients who met the
requirements of the study were given sealed, opaque and
consecutively numbered envelopes, and then randomly allocated to
either the celecoxib or the control group by means of a randomly
generated number pattern that had been designed prior to
recruitment. The anesthesiologist and surgeon caring for the
patient were blind to the patient's group assignment and outcome
data. All relevant data were documented by an independent observing
surgeon (Jia Chen). The posterolateral approach between 10 and 14
cm was used for all procedures with repair of the capsule and
external rotators. Press-fit components (Pinnacle; DePuy Synthes,
Warsaw, IN, USA), and cement-free hydroxyapatite-coated stems
(Corail; DePuy Synthes) were available for THA in the present
study.
All surgeries were performed under combined spinal
and epidural anesthetics. Patients in the study accepted a
multimodal analgesic technique, which comprised the use of oral
celecoxib (Celebrex; Pfizer, Inc., New York, NY, USA) or placebo
capsule combined with an intravenous PCA morphine pump (ACE Medical
Industry Co., Ltd., Goyang, Korea) for peri-operative and
post-operative pain management. A PCA morphine (1 mg/ml) pump was
set to deliver a 2-ml bolus dose with a lockout interval of 10 min.
The total amount of morphine consumption was recorded. An
experienced nurse (Yaqing Du) had trained all patients how to use
the PCA device prior to surgery.
The celecoxib group received an initial 400-mg dose
1 h pre-operatively, and a 200 mg dose was given each 12 h
post-operatively for the first 5 days. Similarly, the control group
took a capsule of the same appearance at identical intervals pre-
and post-operatively.
Outcome assessments
Baseline demographic variables including the number
of patients, mean age, proportion of each gender, body mass index
(BMI) and surgery duration in each group are shown in Table I. The primary outcome was pain score,
as measured by a visual analog scale (VAS) of 0–10 (with 0
representing no pain and 10 representing the worst pain
imaginable). The secondary outcomes included PCA morphine
consumption; post-operative Harris hip score (HHS); time interval
until initial ambulation; rates of urinary retention and PONV
within 72 h; and intra- and post-operative blood loss. Pain
assessments were conducted at 6,12, 24,48 and 72 h, and 7 and 14
days after THA. The amounts of PCA morphine consumption were
recorded by an independent observer at 6, 12, 24 and 48 h
post-operatively. Patients were encouraged to ambulate with a
walker or two crutches as soon as possible and the time when
patients initially ambulated was recorded. The HHS at 3, 7 and 14
days was also documented. All patients received 5,000 IU
low-molecular-weight heparin subcutaneously daily for prophylaxis
of thromboembolism in the first 7 days post-operatively.
 | Table I.Patient demographics and baseline
characteristics. |
Table I.
Patient demographics and baseline
characteristics.
| Variable | Celecoxib group | Control group | P-value |
|---|
| Number of
patients | 34 | 30 | - |
| Age
(years)a | 73.6±4.8 | 74.5±4.5 | >0.05 |
| Male/female | 16/18 | 13/17 | - |
| BMI
(kg/m2)a | 32.3±5.6 | 34.4±6.2 | >0.05 |
| Surgery duration
(min)a | 98.2±13.7 | 96.5±14.9 | >0.05 |
Statistical analysis
Demographic variables, post-operative HHS and
intra-/post-operative blood loss were analyzed by independent
samples t-test. Rates of urinary retention and PONV were analyzed
by Chi-square test. Pain scores, morphine consumption and time
interval until initial ambulation were analyzed by Mann-Whitney U
test. Statistical analysis was performed using SPSS software,
version 19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline demographics and pain
assessments
There were no significant differences between the
two groups in terms of the number of patients, gender distribution,
age, BMI and duration of surgery (Table
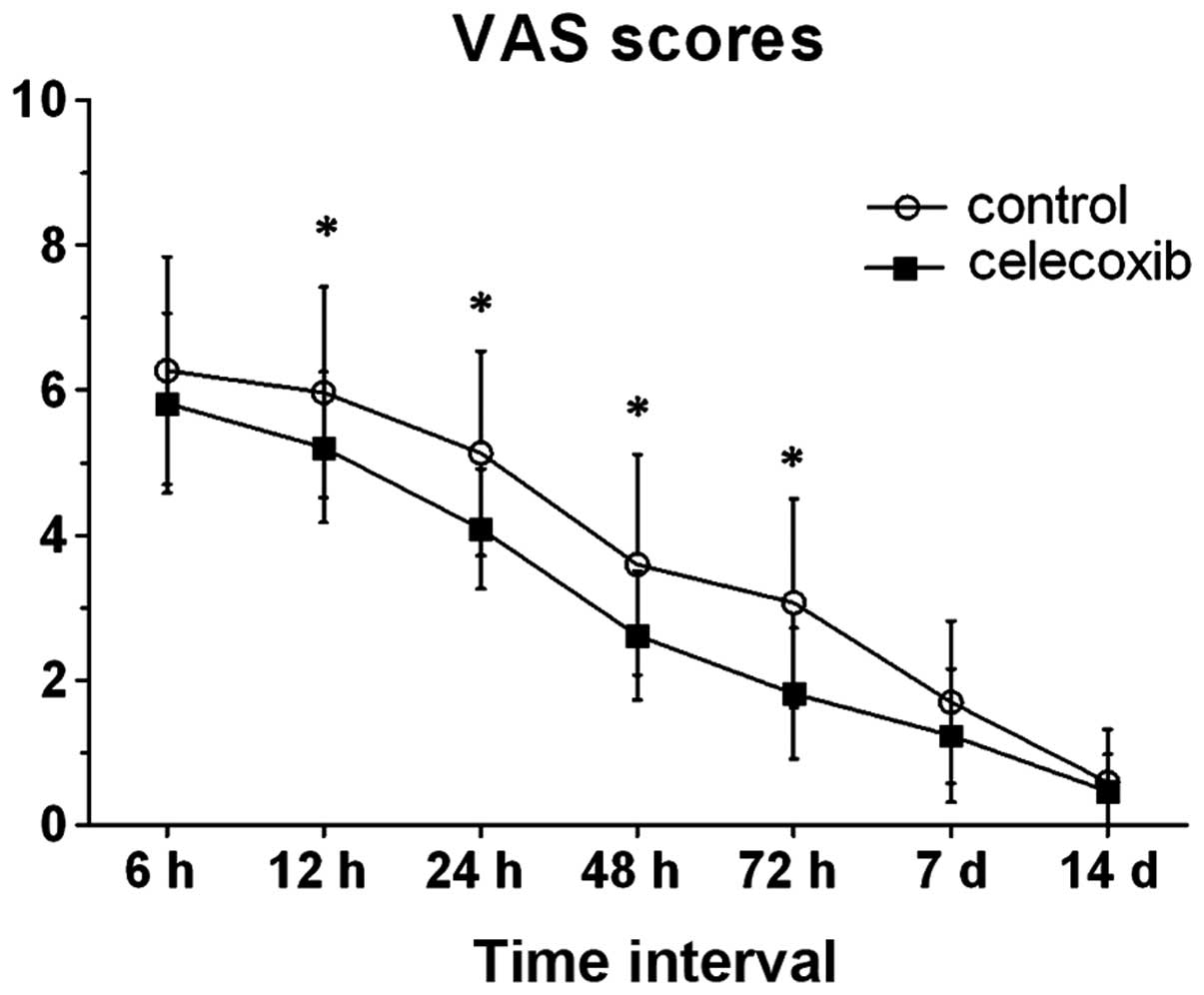
I). In comparison with the control group, the post-operative
VAS pain score was significantly lower at 12, 24, 48 and 72 h after
THA in the celecoxib group. When ambulating and accessing
rehabilitation until discharge, the two groups had no statistically
significant differences between them with regard to post-operative
VAS scores (7 days, P=0.105; 14 days, P=0.648), although patients
in the two groups had a decreasing perception of pain (Fig. 1).
HHS results and time of initial
ambulation
The post-operative HHS did not significantly differ
between the two groups at the time of ambulation and at 7 and 14
days after THA (P=0.081, 0.080 and 0.071, respectively; Table II). The time interval until initial
ambulation in the celecoxib group (4.5±1.2 days) was significantly
less than that in the control group (5.83±2.04 days; P<0.05;
Table II).
 | Table II.Intraoperative and postoperative
data. |
Table II.
Intraoperative and postoperative
data.
| Variable | Celecoxib
group | Control group | P-value |
|---|
| Intra-operative
blood loss (ml) | 432.4±62.1 | 458.0±67.6 | 0.119 |
| Post-operative
blood loss (ml) | 233.7±25.9 | 242.3 ±27.5 | 0.201 |
| HHS (time of
ambulation) | 77.6±4.3 | 75.5±5.2 | 0.081 |
| HHS (7 days) | 81.5±5.1 | 79.4±4.6 | 0.080 |
| HHS (14 days) | 86.1±4.7 | 83.9±5.1 | 0.071 |
| Time interval until
first ambulation (days) | 4.5±1.2 | 5.83±2.04 | 0.008 |
| Urinary retention
rates (%) | 23.5 | 30 | 0.559 |
| PONV rates (%) | 35.3 | 47 | 0.355 |
Opioid usage and associated
complications
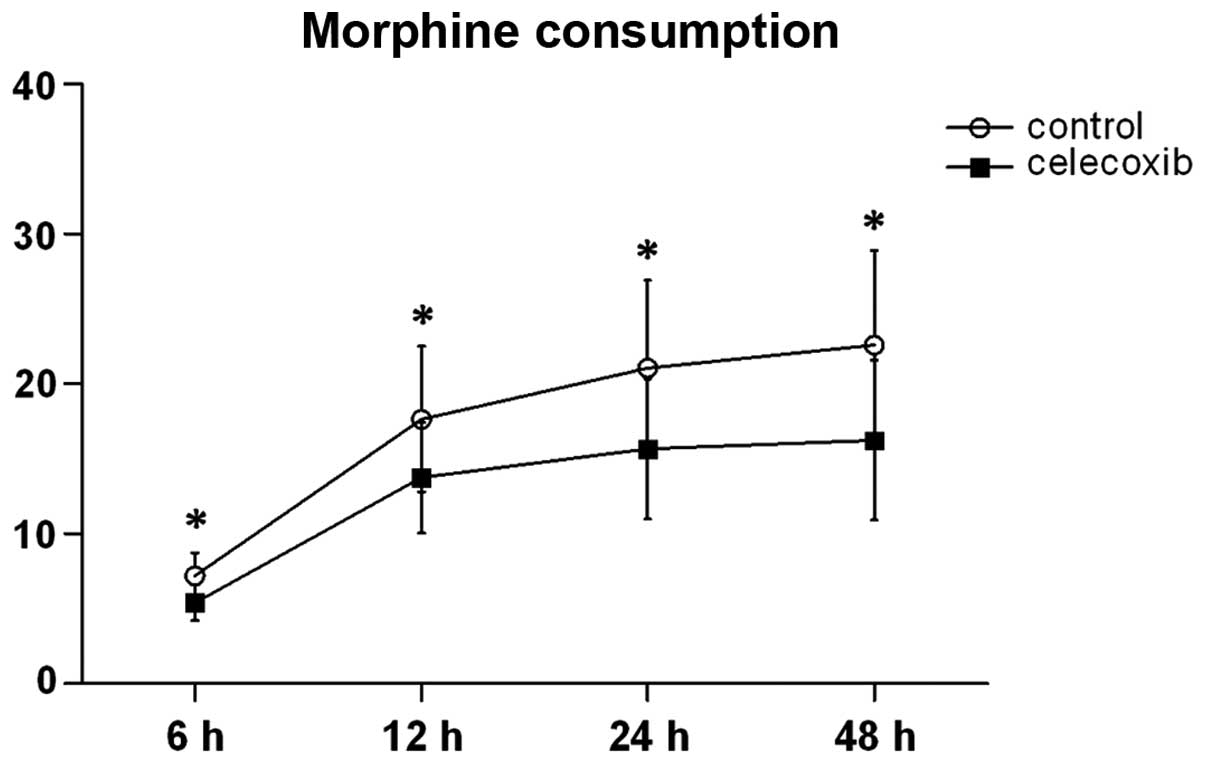
PCA morphine consumption was significantly decreased
in the celecoxib group when compared with the control group at 6,
12, 24 and 24 h (Fig. 2). Within 72
h after the surgery, the rates of urinary retention were 30 and
23.5% in the control and celecoxib groups, respectively.
Post-operative PONV rates were 47% in the control group and 35.3%
in the celecoxib group (Table II).
The data revealed no statistically significant difference between
the two groups in PONV rates (P=0.355); similar results were
exhibited in urinary retention rates in the two groups (P=0.559).
The intra- or post-operative blood loss also showed no
statistically significant difference between groups (Table II).
Discussion
This prospective randomized study demonstrates that
using oral celecoxib pre-emptively and post-operatively can achieve
effective clinical outcomes when compared with placebo for acute
pain management after THA in elderly patients. Celecoxib can
improve pain management, and the quality and function of
post-operative recovery, in addition to providing narcotic-sparing
effects in a multimodal analgesic strategy. Few side-effects were
found in the short time interval that was observed.
Several studies have applied celecoxib for acute
pain management in peri-operative multimodal analgesic strategies
(13–15). A previous study conducted in our
institution (13) demonstrated that
200 mg celecoxib administered in one dose pre-operatively was
effective in reducing post-operative pain and provided a
narcotic-sparing effect in patients undergoing arthroscopic hip
surgery. Reuben et al (15)
studied the peri-operative dose of celecoxib in pain management
following spinal fusion surgery, and concluded that an oral 400-mg
celecoxib capsule as pre-emptive analgesia followed by an
additional 200 mg dose 12 h later resulted in a 31% reduction in
morphine use and significantly lower pain scores when compared with
a pre-operative 200 mg dose, in which there was only a 9% reduction
in morphine use. Although these studies studied different celecoxib
doses, there were no differences in peri- and post-operative
complications associated with bleeding, wound hematoma and
gastrointestinal reactions between them. In the present study, oral
400 mg celecoxib was administered pre-operatively followed by 200
mg per 12 h post-operatively in combination with intravenous PCA
morphine pump for the first 5 days and this demonstrated the
favorable efficacy of celecoxib in acute pain management following
THA. Thus, it is considered that celecoxib can take an important
role in multimodal analgesic regimens.
There have been a number of studies demonstrating
that COX-2 inhibitors can contribute to reduced VAS pain scores in
peri- and post-operative pain management following total joint
arthroplasty (9,14,16), and
a meta-analysis (17) demonstrated
that reasonably used COX-2 inhibitors can reduce post-operative
pain and VAS scores when compared with placebo. In the current
study, the peri-operative VAS scores were significant lower in the
celecoxib group at 12,24,48 and 72 h after THA. When celecoxib
usage was stopped after the initial 5-day therapeutic period, the
two groups in the study had no significant difference in VAS
scores. In addition, the elderly patients in the celecoxib group
experienced an episode of mild pain relief, which might minimize
acute pain-related complication, such as delirium, hyperpiesia and
neuropsychological tension (18,19).
The quality and function of post-operative recovery
is an indication of pain management following THA. Patients who
were willing to walk or take exercise had less pain perception when
ambulating. The post-operative HHS was evaluated at the time of
ambulation, and at 7 and 14 days. In addition, the interval from
surgery until initial ambulation was evaluated. Kang et al
(14) supplemented intravenous PCA
with pre-emptive 200 mg celecoxib followed by intra-operative
periarticular injections and observed no significant difference in
early walking activity from patients treated with PCA alone;
however, patients treated with the multimodal analgesic regimen
using oral celecoxib started walking or taking exercise earlier. In
the present study, although the HHSs in the celecoxib group were
slightly higher than those in the control group, the scores
exhibited no statistically significant differences between the two
groups post-operatively until discharge. However, elderly patients
in the celecoxib group had a reduced time interval until auxiliary
ambulation. Therefore, it can be considered that peri-operative
pain management with adjuvant celecoxib can decrease the perception
of pain and improve pain relief when ambulating.
There are a variety of peri-operative complications
associated with opioid narcotics, such as PONV, urinary retention,
pruritus, drowsiness and sedation (20–23).
Utilizing celecoxib plus opioid medication is a prevalent approach
to multimodal analgesic strategy in acute pain management
post-operatively, which can decrease the consumption of opioid. In
the present study, the post-operative opioid consumption in the
celecoxib group was 38% less than in control group; however, there
were no statistically significant differences in urinary retention
rates or PONV rates when compared with the placebo group. Huang
et al (9) compared celecoxib
with placebo in pain management following total knee arthroplasty,
and concluded that peri-operative celecoxib can contribute to a
reduction in post-operative opioid consumption of 40%. However,
although the reduced opioid use led to a decline in the rate of
PONV incidence (28 vs. 43%), there were no difference in PONV
incidence in the two groups. This observation was consistent with
the present study, which showed 35.3 and 47% PONV rates in the
celecoxib and control groups, respectively. No statistically
significant difference in PONV rates was identified between the two
groups. The rates of urinary retention incidence in the present
study showed an analogous effect (23.5 vs. 30%). As the number of
patients in this study was limited, a study with a larger sample
size is required to verify the correlation between declined opioid
consumption and opioid-related side-effects.
Celecoxib, as a representative of the class of
selective COX-2 inhibitors, has the advantage of selective effects
on COX-2 when compared with conventional NSAIDs. To the best of our
knowledge, several studies have demonstrated an association between
NSAIDs and the functioning of platelets (24–28).
Selective COX-2 inhibitors, however, have no ability to inhibit
platelet aggregation or coagulation function (29–31).
They specifically inhibit the effect of COX-2, which takes an
important role in catalyzing PG synthesis and the subsequent
inflammatory response (32). Thus,
COX-2 inhibitors avoid the side-effects of NSAIDs, which are
primarily actualized by the inhibition of COX-1, resulting in
gastrointestinal adverse reactions, the inhibition of platelet
aggregation and impairment of renal function. Ekman et al
(10) administered oral 400 mg
celecoxib 1 h prior to arthroscopic knee surgery. The results
suggested that celecoxib did not interfere with normal hematologic
function. A retrospective cohort study (33) based on databases covering >1.3
million patients aged >66 years demonstrated lower rates of
upper gastrointestinal hemorrhage for celecoxib in comparison with
conventional NSAIDs. In the present study, the volumes of peri- and
post-operative blood loss had no significant difference between the
two observed groups, and no antiplatelet activity-related
complications, such as incision hematoma, bleeding and ulcer
bleeding, were exhibited in the observed interval. The period of
acute pain management is relatively short, and the observation of
the side-effects of celecoxib associated with the inhibition of
platelet function may require a prolonged observation period.
There are certain limitations to the present study.
First, due to the rigid inclusion and exclusion criteria, there
were limited numbers of patients who were eligible for this study;
this resulted in a small sample size and may have introduced
potential selection bias. Secondly, patients who suffered from
fresh fracture of the femur neck were excluded, although this is a
preferable indication for elderly patients undergoing THA. However,
the pain derived from acute hip trauma, post-traumatic
transportation and consequent pre-operative treatment, such as limb
skeletal traction, may lead to a higher incidence of acute
confusional states and greater pain intensity, which may influence
the pre-operative psychological state and post-operative recovery
(34,35). In order to establish a unified group
of patients who were in a good physiological and psychological
state prior to the surgery, patients who experienced acute injury
were excluded and another study may be conducted to focus on the
population with traumatic hip injury. Finally, the safety of
celecoxib has not been verified in this study, since the
observation period was short. To the best of our knowledge,
coxib-type drugs are associated with a potential increased risk for
thrombosis, and renal and cardiovascular (CV) adverse events
(36–39). The safety of drugs of the coxib class
continues to require further evaluation, particularly in elderly
patients who are more likely to suffer from CV disease and renal
function disorders. Larger observation periods for celecoxib safety
verification are planned in our future study.
In conclusion, the present study demonstrates that
using celecoxib in a multimodal analgesic strategy pre-and
post-operatively can achieve favorable efficacy in the management
of pain. The use of a treatment regimen comprising oral treatment
with celecoxib at a dose of 400 mg pre-emptively and 200 mg per 12
h post-operatively in combination with PCA morphine pump should
improve pain intensity, reduce opioid consumption, and achieve
early ambulation and improved rehabilitation after THA in elderly
patients.
Abbreviations:
|
PCA
|
patient-controlled analgesia
|
|
THA
|
total hip arthroplasty
|
|
VAS
|
visual analog scale
|
|
HHS
|
Harris hip score
|
|
PONV
|
postoperative nausea and vomiting
|
|
NSAIDs
|
non-steroidal anti-inflammatory
drugs
|
|
COX
|
cyclooxygenase
|
|
PG
|
prostaglandin
|
|
CV
|
cardiovascular
|
References
|
1
|
Kennedy JW, Johnston L, Cochrane L and
Boscainos PJ: Outcomes of total hip arthroplasty in the
octogenarian population. Surgeon. 11:199–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrell BA: Pain management in elderly
people. J Am Geriatr Soc. 39:64–73. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eid T and Bucknall T: Documenting and
implementing evidence-based post-operative pain management in older
patients with hip fractures. J Orthop Nurs. 12:90–98. 2008.
View Article : Google Scholar
|
|
4
|
Illgen RL, Pellino TA, Gordon DB, Butts S
and Heiner JP: Prospective analysis of a novel long-acting oral
opioid analgesic regimen for pain control after total hip and knee
arthroplasty. J Arthroplasty. 21:814–820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morrison RS, Magaziner J, McLaughlin MA,
et al: The impact of post-operative pain on outcomes following hip
fracture. Pain. 103:303–311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore ND: In search of an ideal analgesic
for common acute pain. Acute Pain. 11:129–137. 2009. View Article : Google Scholar
|
|
7
|
Sostres C, Gargallo CJ, Arroyo MT and
Lanas A: Adverse effects of non-steroidal anti-inflammatory drugs
(NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best
Pract Res Clin Gastroenterol. 24:121–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burian M and Geisslinger G: COX-dependent
mechanisms involved in the antinociceptive action of NSAIDs at
central and peripheral sites. Pharmacol Ther. 107:139–154. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YM, Wang CM, Wang CT, Lin WP, Horng
LC and Jiang CC: Perioperative celecoxib administration for pain
management after total knee arthroplasty - A randomized, controlled
study. BMC Musculoskelet Disord. 9:772008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ekman EF, Wahba M and Ancona F: Analgesic
efficacy of perioperative celecoxib in ambulatory arthroscopic knee
surgery: A double-blind, placebo-controlled study. Arthroscopy.
22:635–642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinatra R: Role of COX-2 inhibitors in the
evolution of acute pain management. J Pain Symptom Manage. 24
(Suppl):S18–S27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Remy C, Marret E and Bonnet F: Effects of
acetaminophen on morphine side-effects and consumption after major
surgery: Meta-analysis of randomized controlled trials. Br J
Anaesth. 94:505–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Zhu W, Zhu L and Du Y: Efficacy
of celecoxib for pain management after arthroscopic surgery of hip:
A prospective randomized placebo-controlled study. Eur J Orthop
Surg Traumatol. 24:919–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang H, Ha YC, Kim JY, Woo YC, Lee JS and
Jang EC: Effectiveness of multimodal pain management after bipolar
hemiarthroplasty for hip fracture: A randomized, controlled study.
J Bone Joint Surg Am. 95:291–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reuben SS and Ekman EF: The effect of
cyclooxygenase-2 inhibition on analgesia and spinal fusion. J Bone
Joint Surg Am. 87:536–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schroer WC, Diesfeld PJ, LeMarr AR and
Reedy ME: Benefits of prolonged postoperative cyclooxygenase-2
inhibitor administration on total knee arthroplasty recovery: A
double-blind, placebo-controlled study. J Arthroplasty. 26
(Suppl):2–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin J, Zhang L and Yang H: Perioperative
administration of selective cyclooxygenase-2 inhibitors for
postoperative pain management in patients after total knee
arthroplasty. J Arthroplasty. 28:207–213.e2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radinovic K, Milan Z, Markovic-Denic L,
Dubljanin-Raspopovic E, Jovanovic B and Bumbasirevic V: Predictors
of severe pain in the immediate postoperative period in elderly
patients following hip fracture surgery. Injury. 45:1246–1250.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feldt KS and Oh HL: Pain and hip fracture
outcomes for older adults. Orthop Nurs. 19:35–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keïta H, Geachan N, Dahmani S, et al:
Comparison between patient-controlled analgesia and subcutaneous
morphine in elderly patients after total hip replacement. Br J
Anaesth. 90:53–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dorr LD, Raya J, Long WT, Boutary M and
Sirianni LE: Multimodal analgesia without parenteral narcotics for
total knee arthroplasty. J Arthroplasty. 23:502–508. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hudcova J, McNicol E, Quah C, Lau J and
Carr DB: Patient controlled intravenous opioid analgesia versus
conventional opioid analgesia for postoperative pain control: A
quantitative systematic review. Acute Pain. 7:115–132. 2005.
View Article : Google Scholar
|
|
23
|
Wheeler M, Oderda GM, Ashburn MA and
Lipman AG: Adverse events associated with postoperative opioid
analgesia: A systematic review. J Pain. 3:159–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brooks P, Emery P, Evans JF, et al:
Interpreting the clinical significance of the differential
inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology
(Oxford). 38:779–788. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng JC, Siegel LB, Katari B, Traynoff SA
and Ro JO: Nonsteroidal anti-inflammatory drugs and aspirin: A
comparison of the antiplatelet effects. Am J Ther. 4:62–65. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lanas A, García-Rodríguez LA, Arroyo MT,
et al: Investigators of the Asociación Española de
Gastroenterología (AEG): Effect of antisecretory drugs and nitrates
on the risk of ulcer bleeding associated with nonsteroidal
anti-inflammatory drugs, antiplatelet agents, and anticoagulants.
Am J Gastroenterol. 102:507–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carson JL, Strom BL, Soper KA, West SL and
Morse ML: The association of nonsteroidal anti-inflammatory drugs
with upper gastrointestinal tract bleeding. Arch Intern Med.
147:85–88. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gladding PA, Webster MW, Farrell HB, Zeng
IS, Park R and Ruijne N: The antiplatelet effect of six
non-steroidal anti-inflammatory drugs and their pharmacodynamic
interaction with aspirin in healthy volunteers. Am J Cardiol.
101:1060–1063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leese PT, Hubbard RC, Karim A, Isakson PC,
Yu SS and Geis GS: Effects of celecoxib, a novel cyclooxygenase-2
inhibitor, on platelet function in healthy adults: A randomized,
controlled trial. J Clin Pharmacol. 40:124–132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graff J, Arabmotlagh M, Cheung R,
Geisslinger G and Harder S: Effects of parecoxib and dipyrone on
platelet aggregation in patients undergoing meniscectomy: A
double-blind, randomized, parallel-group study. Clin Ther.
29:438–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leese PT, Talwalker S, Kent JD and Recker
DP: Valdecoxib does not impair platelet function. Am J Emerg Med.
20:275–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buttar NS and Wang KK: The ‘aspirin’ of
the new millennium: Cyclooxygenase-2 inhibitors. Mayo Clin Proc.
75:1027–1038. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mamdani M, Rochon PA, Juurlink DN, et al:
Observational study of upper gastrointestinal haemorrhage in
elderly patients given selective cyclo-oxygenase-2 inhibitors or
conventional non-steroidal anti-inflammatory drugs. BMJ.
325:6242002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johansson I, Bååth C, Wilde-Larsson B and
Hall-Lord ML: Acute confusion states, pain, health, functional
status and quality of care among patients with hip fracture during
hospital stay. Int J Orthop Trauma Nurs. 17:120–130. 2013.
View Article : Google Scholar
|
|
35
|
Gustafson Y, Berggren D, Brännström B, et
al: Acute confusional states in elderly patients treated for
femoral neck fracture. J Am Geriatr Soc. 36:525–530. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi S and Klotz U: Clinical use and
pharmacological properties of selective COX-2 inhibitors. Eur J
Clin Pharmacol. 64:233–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bombardier C, Laine L, Reicin A, et al:
VIGOR Study Group: Comparison of upper gastrointestinal toxicity of
rofecoxib and naproxen in patients with rheumatoid arthritis. N
Engl J Med. 343:1520–1528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brater DC, Harris C, Redfern JS and Gertz
BJ: Renal effects of COX-2-selective inhibitors. Am J Nephrol.
21:1–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brater DC: Effects of nonsteroidal
anti-inflammatory drugs on renal function: Focus on
cyclooxygenase-2-selective inhibition. Am J Med. 107 (Suppl
1):65S–71S. 1999. View Article : Google Scholar : PubMed/NCBI
|