Introduction
Meningiomas are one of the most common types of
intracranial tumor, with the incidence rate of meningiomas (∼19.2%)
ranking second among intracranial tumors worldwide (1). At an early stage, meningiomas are
benign tumors. However, previous studies (2,3) have
demonstrated that meningiomas exhibit several metastatic malignant
forms, with characteristics of fast-growth, invasion, metastasis
and easy to relapse. The malignancy degree of meningioma increases
with the increasing tumor grade (4).
Methylation is involved in the modification of heavy
metals and the regulation of gene expression. DNA methylation in
vertebrates includes global hypomethylation and regional
hypermethylation, which typically occurs at
cytosine-phosphate-guanine (CpG) sites. DNA methylation is
associated with the activation and expression of cancer genes
(5), and the process of DNA
methylation has been demonstrated to play an important role in the
occurrence and development of tumors at an early stage (6–8).
Werner syndrome protein (WRN) is a protein-coding
gene that is associated with a variety of diseases, including
Werner syndrome and Rothmund-Thomson syndrome (9). The WRN gene is located on the short arm
of chromosome 8 between positions 12 and 11.2, and the promoter of
the WRN gene has been shown to be methylated in numerous types of
malignant tumor (10,11). A previous study demonstrated that a
polymorphism of the WRN gene was associated with the risk of
developing a meningioma (12).
However, the association between WRN methylation and the occurrence
of meningiomas remains unclear, despite the incidence of
meningiomas being associated with the abnormal expression of a
variety of genes (13).
In the present study, the methylation status of the
WRN promoter was detected in the peripheral blood and tissue
samples of patients with meningioma using a methylation-specific
polymerase chain reaction (PCR) assay. Thus, the association
between WRN methylation and the incidence of meningioma was further
investigated.
Materials and methods
Reagents
A DNA extraction kit and a total protein extraction
kit were purchased from TIANGEN Biotech Co., Ltd. (Beijing, China).
An EZ DNA Methylation-Direct™ kit was purchased from Zymo Research
Corporation (Irvine, CA, USA). A rabbit anti-human WRN polyclonal
antibody (ab200), a rabbit anti-human Myc polyclonal antibody
(ab9106) and a mouse anti-human p53 monoclonal antibody (ab26) were
purchased from Abcam (Cambridge, MA, USA). In addition, a
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
polyclonal secondary antibody (ab6789) and a goat anti-rabbit IgG
polyclonal antibody (ab6721) were purchased from Abcam. BeyoECL
Plus enhanced chemiluminescence reagent was obtained from the
Beyotime Institute of Biotechnology (Suzhou, China).
Patients
A total of 56 consecutive patients with meningioma
were enrolled in the study (meningioma group), of which 31
individuals were male and 25 patients were female. The age of the
56 patients ranged between 9 and 76 years, with an average age of
47.6 years. The course of the disease ranged between 2 and 51
months, with an average disease course of 21.3 months. With regard
to the control group, 26 healthy individuals were enrolled in the
study (control group). Peripheral blood samples were collected from
the meningioma patients and the healthy individuals. In addition,
meningioma tissues were collected from the meningioma patients,
while healthy arachnoid tissues were collected from the healthy
individuals in the control group. Prior written and informed
consent was obtained from every patient, and the study was approved
by the Ethics Review Board of the Capital Medical University
(Beijing, China).
Detection of DNA methylation
DNA from the peripheral blood and tissue samples was
extracted using the DNA extraction kit, in accordance with the
manufacturer's instructions. The detection of the DNA methylation
status was performed using the EZ DNA Methylation-Direct™ kit, in
accordance with the manufacturer's instructions. DNA methylation
was detected using a methylation-specific PCR assay, where DNA
without the presence of methylation was used as a control. The
primer sequences for positive WRN gene methylation were as follows:
Forward, 5′-CGGGTAGGGGTATCGTTCGC-3′ and reverse,
5′-CGATATCCGAAATCAAACGACG-3′. The primer sequences for negative WRN
gene methylation were as follows: Forward,
5′-GTAGTTGGGTAGTAGGGGTATTGTTTGT-3′ and reverse,
5′-CCAATATCCAAAATCAAACAACAAC-3′.
Western blot analysis
Total proteins from the peripheral blood and tissue
samples were extracted using the total protein extraction kit. The
total proteins were separated by 12% SDS-PAGE, and subsequently
analyzed by immunoblotting, where β-actin was used as an internal
control. Briefly, the membrane was blocked with 5% non-fat milk at
room temperature for 1 h. The membrane was then incubated with
primary antibodies at 4°C overnight, prior to washing. The membrane
was incubated with secondary antibodies at room temperature for 1
h,. The primary antibodies used included a rabbit anti-human WRN
polyclonal antibody (1:2,000), a rabbit anti-human Myc polyclonal
antibody (1:500) and a mouse anti-human p53 monoclonal antibody
(1:1,000). The secondary antibodies used in the experiment were a
HRP-conjugated goat anti-rabbit IgG polyclonal antibody (1:3,000)
and a HRP-conjugated goat anti-mouse IgG polyclonal antibody
(1:5,000). The blots were detected using BeyoECL Plus enhanced
chemiluminescence reagent, and image quantifications were performed
using ImageLab software, version 4.1 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The experiments were repeated a minimum of
three times.
Statistical analysis
All results are expressed as the mean ± standard
deviation. The statistical analyses were performed using SPSS
software, version 17.0 for Windows (SPSS, Inc., Chicago, IL, USA).
The least significant difference t-test was used to analyze the
comparisons between groups and for the analysis of paired data,
where P<0.05 was considered to indicate a statistically
significant difference.
Results
Positive rate of WRN methylation is
increased in the peripheral blood and tissues of meningioma
patients
To investigate the levels of WRN methylation in the
peripheral blood and tissues, a methylation-specific PCR assay was
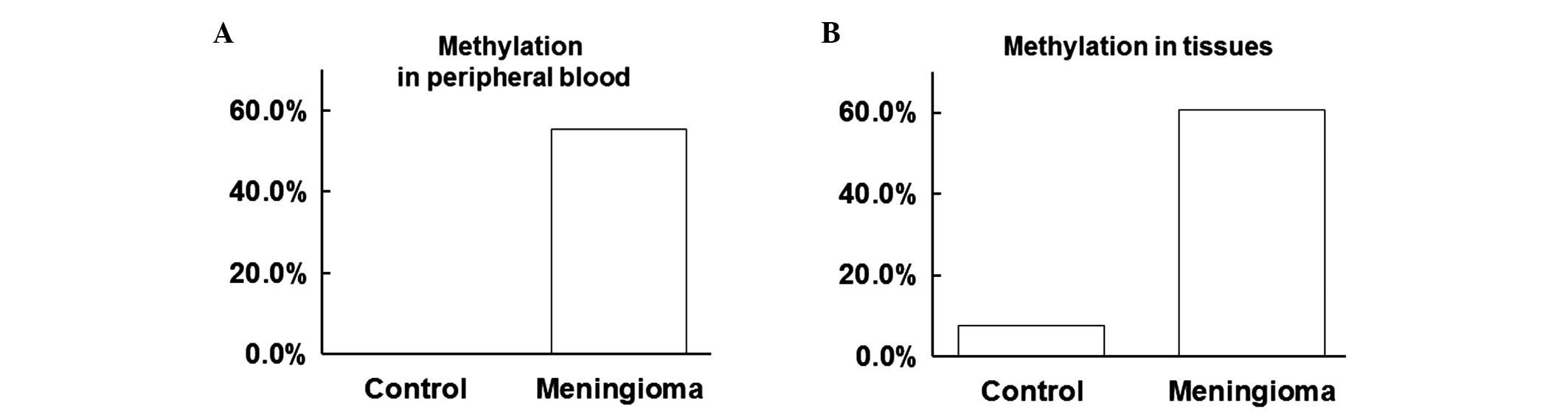
performed. As shown in Table I, the
positive rate of WRN methylation was 55.36 and 0.00% in the
peripheral blood of the meningioma and control groups,
respectively. Furthermore, the positive rate of WRN methylation was
60.71 and 7.69% in the tissues of the meningioma and control
groups, respectively. Thus, the positive rate of WRN methylation in
the peripheral blood of the meningioma group was significantly
increased when compared with the control group (P<0.05; Fig. 1A). In addition, the positive rate of
WRN methylation in the tissues of the meningioma group was
significantly increased when compared with the control group
(P<0.05; Fig. 1B). These results
indicated that the positive rate of WRN methylation was increased
in the peripheral blood and tissues of meningioma patients.
 | Table I.Positive rate of WRN methylation in
the peripheral blood and tissues. |
Table I.
Positive rate of WRN methylation in
the peripheral blood and tissues.
|
|
| Methylation in the
peripheral blood | Methylation in the
tissues |
|---|
|
|
|
|
|
|---|
| Groups | Cases (n) | Yes (n) | No (n) | Positive rate
(%) | P-value | Yes (n) | No (n) | Positive rate
(%) | P-value |
|---|
| Control | 26 | 0 | 26 | 0 |
| 2 | 24 | 7.69 |
|
| Meningioma | 56 | 31 | 25 | 55.36 | <0.001 | 32 | 24 | 60.71 | <0.001 |
Protein expression of WRN is inhibited
following WRN methylation in the peripheral blood and tissues
To determine the protein expression levels of WRN in
the peripheral blood and tissue samples, western blot analysis was
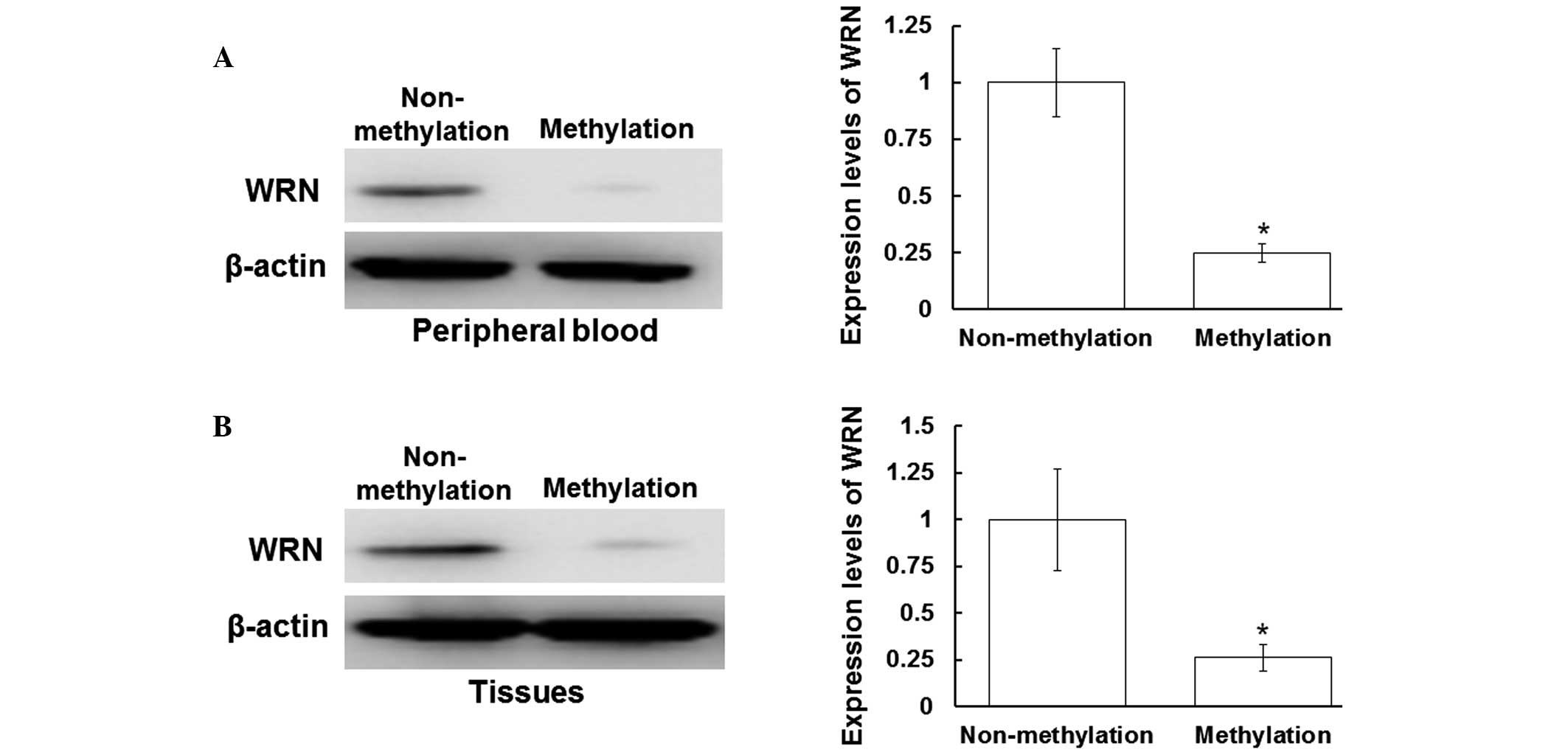
performed. As shown in Fig. 2A, the
protein expression levels of WRN in the peripheral blood samples
with positive WRN methylation were decreased when compared with
those without WRN methylation (P<0.05). In addition, the protein
expression levels of WRN in the tissues with WRN methylation were
decreased when compared with those without WRN methylation
(P<0.05). Therefore, in the peripheral blood and tissue samples
with positive WRN methylation, WRN protein expression levels were
significantly decreased. These results indicated that the protein
expression of WRN is inhibited following WRN methylation in the
peripheral blood and tissues.
Myc and p53 protein expression levels
are increased in the peripheral blood and tissues with WRN
methylation
To investigate the effect of WRN methylation on the
regulation of tumor gene expression, western blot analysis was
performed to detect the protein expression levels of Myc and p53.
Myc and p53 are regulated by WRN in the process of tumor
development (14). As shown in
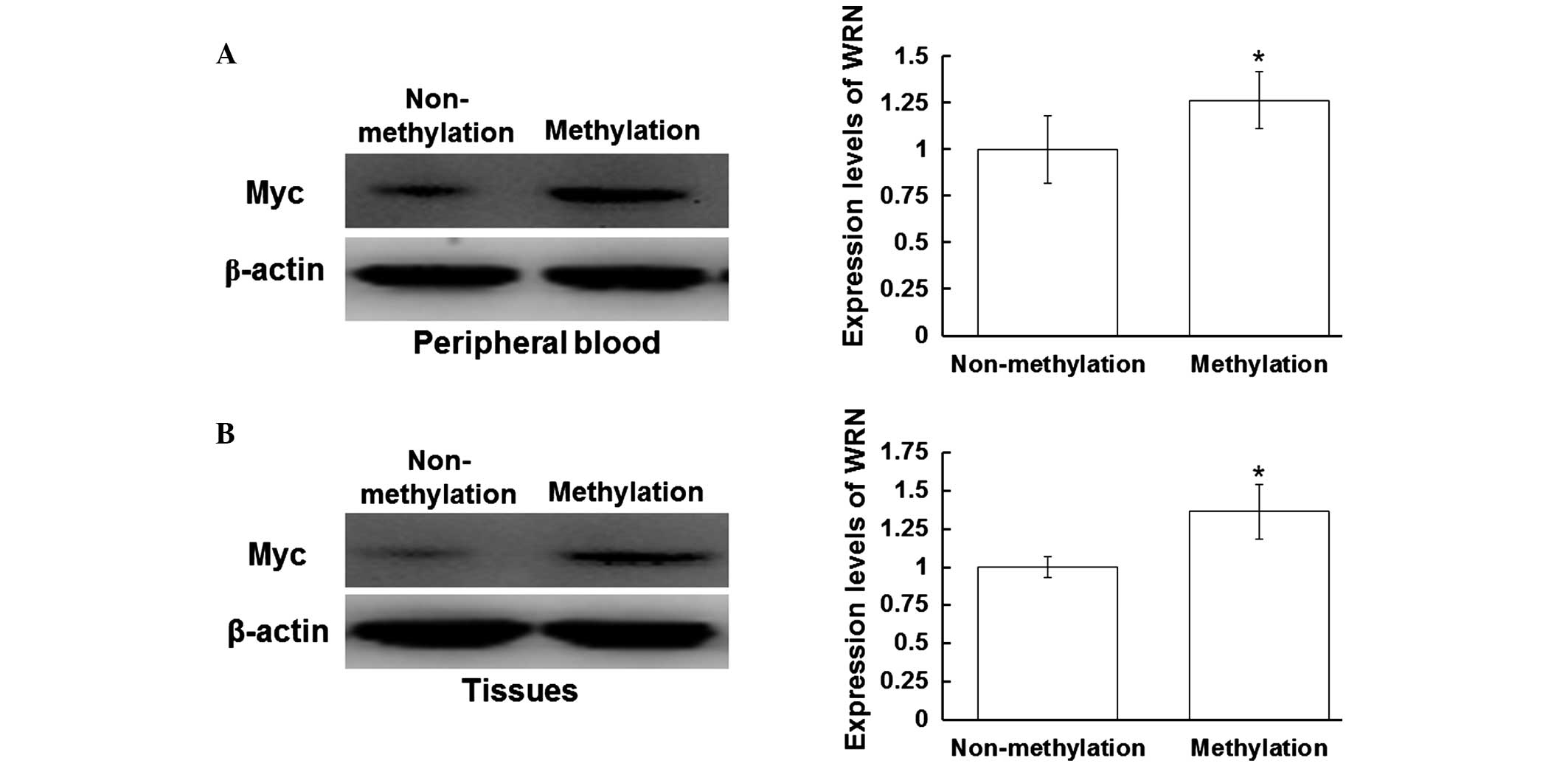
Fig. 3A, the expression levels of
Myc in the peripheral blood of the patients with positive WRN
methylation were increased when compared with those without WRN
methylation (P<0.05). In addition, the expression levels of Myc
in the tissue samples with WRN methylation were increased when
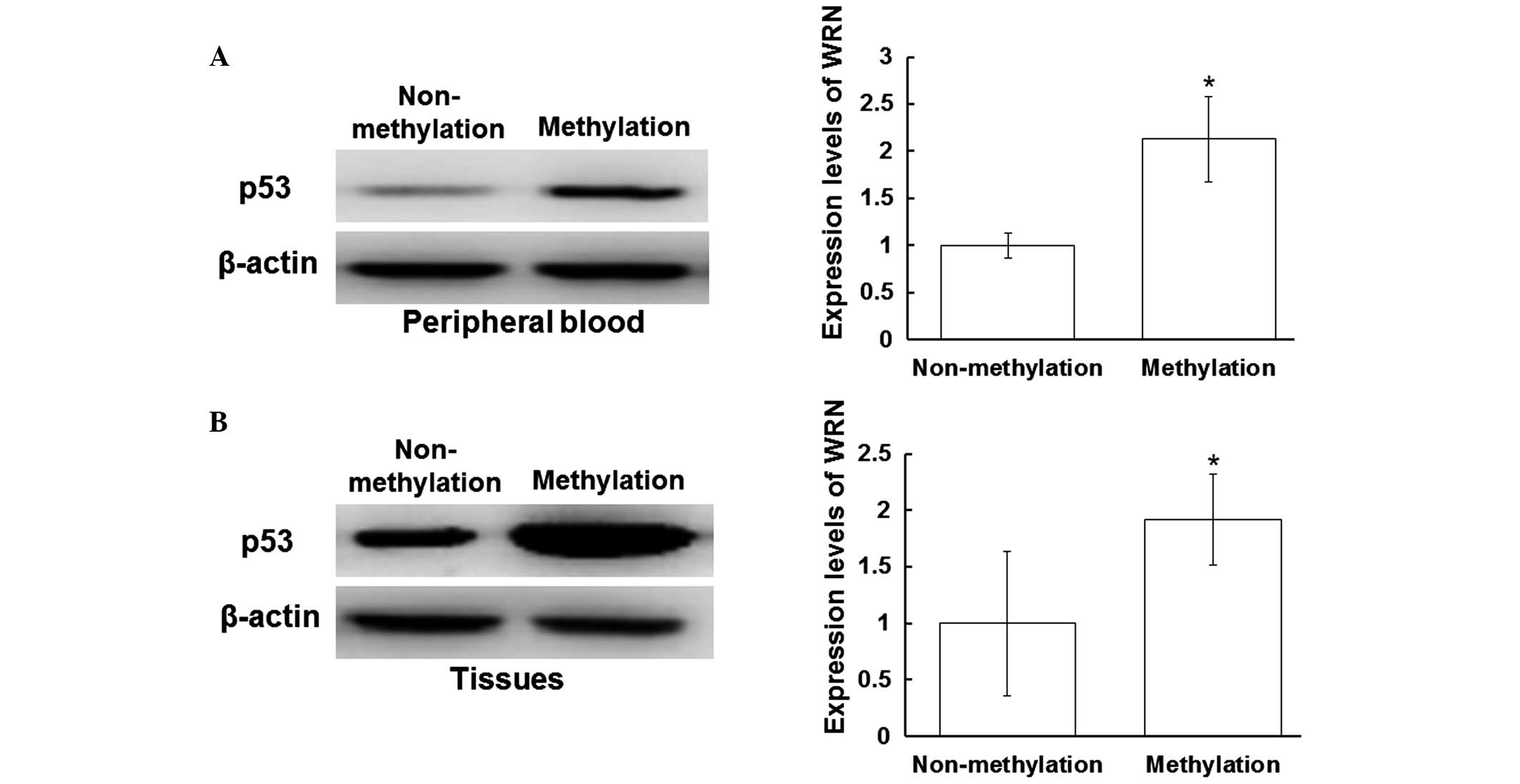
compared with those without WRN methylation (P<0.05) (Fig. 3B). As shown in Fig. 4A, the expression levels of p53 in the
peripheral blood samples with positive WRN methylation were
increased when compared with those without WRN methylation
(P<0.05). Furthermore, the expression levels of p53 in the
tissue samples with positive WRN methylation were increased when
compared with those without WRN methylation (P<0.05; Fig. 4B). These results indicated that the
protein expression levels of Myc and p53 were increased in the
peripheral blood and tissue samples that exhibited positive WRN
methylation.
Discussion
The incidence of meningiomas has increased in recent
years (15). The occurrence of
tumors is associated with the activation of cancer genes and the
inactivation of tumor suppressor genes (16,17).
Tumor suppressor genes exist in normal cells of every healthy
individual. When the tumor suppressor gene is activated, cell
proliferation is inhibited. However, when the tumor suppressor gene
is suppressed, the role of the tumor suppressor is eradicated
(18).
In the human genome, fragments that are rich in CpG
dinucleotides are known as CpG islands, and these are primarily
located in the promoter region and the first exon region of the
gene (17). Approximately 60% of the
promoter region is estimated to contain CpG islands (19). The occurrence and development of
tumors are associated with DNA methylation. A number of
tumor-associated tumor suppressor genes are methylated in the CpG
islands of the gene promoter, which has been shown to affect the
conformation and stability of DNA, and ultimately regulate gene
expression (20). WRN plays an
important role in DNA damage repair processes, which are involved
in the maintenance of gene stability (14,21).
High expression levels of WRN have been shown to inhibit the
regulatory function of Myc in the process of cell aging (14).
In the present study, the results demonstrated that
the positive rate of WRN methylation in the peripheral blood of the
meningioma group was increased when compared with the control
group. In addition, the protein expression levels of WRN were
significantly decreased in the peripheral blood and tissue samples
of the individuals exhibiting positive WRN methylation, indicating
that the expression levels of WRN may be inhibited by the
regulation of WRN methylation. Furthermore, the protein expression
levels of Myc and p53 were increased in the peripheral blood and
tissue samples from the patients with positive WRN methylation, as
compared with those that did not exhibit WRN methylation.
Therefore, these results indicate that WRN methylation may be
associated with tumorigenesis.
In conclusion, the methylation of WRN was
demonstrated to be associated with the occurrence and development
of invasive meningioma, possibly through the regulation of Myc and
p53 protein expression. Subsequently, the diagnosis of meningioma
may be predicted by the detection of WRN methylation. Therefore,
the detection of the WRN methylation status may play an important
role in the diagnosis and treatment of meningioma patients.
Acknowledgements
The study was supported by grants from the Natural
Science Foundation of China (no. 81341059) and the Beijing Nova
Program (no. 2012033). The authors thank Professor Hong Wan from
the Beijing Neurosurgery Institute of the Capital Medical
University for the support provided during the study.
References
|
1
|
Chamberlain MC, Glantz MJ and Fadul CE:
Recurrent meningioma: Salvage therapy with long-acting somatostatin
analogue. Neurology. 69:969–973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park KJ, Yu MO, Song NH, et al: Expression
of astrocyte elevated gene-1 (AEG-1) in human meningiomas and its
role in cell proliferation and survival. J Neurooncol. 121:31–39.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erkutlu I, Buyukhatipoglu H, Alptekin M,
et al: Spinal drop metastases from a papillary meningioma: A case
report and review of the literature: Utility of CSF sampling. Med
Oncol. 26:242–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Ohgaki H and Wiestler OD: The
2007 WHO classification of tumours of the central nervous system.
Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hatziapostolou M and Iliopoulos D:
Epigenetic aberrations during oncogenesis. Cell Mol Life Sci.
68:1681–1702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarez H, Opalinska J, Zhou L, et al:
Widespread hypomethylation occurs early and synergizes with gene
amplification during esophageal carcino-genesis. PLoS Genet.
7:e10013562011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simmer F, Brinkman AB, Assenov Y, et al:
Comparative genome-wide DNA methylation analysis of colorectal
tumor and matched normal tissues. Epigenetics. 7:1355–1367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordà M and Peinado MA: Methods for DNA
methylation analysis and applications in colon cancer. Mutat Res.
693:84–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furuichi Y: Premature aging and
predisposition to cancers caused by mutations in RecQ family
helicases. Ann NY Acad Sci. 928:121–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Xie L, Wang J, et al: Correlation
between the methylation of SULF2 and WRN promoter and the
irinotecan chemosensitivity in gastric cancer. BMC Gastroenterol.
13:1732013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawasaki T, Ohnishi M, Suemoto Y, et al:
WRN promoter methylation possibly connects mucinous
differentiation, microsatellite instability and CpG island
methylator phenotype in colorectal cancer. Mod Pathol. 21:150–158.
2008.PubMed/NCBI
|
|
12
|
Wang K, Hao SY, Huang GY, et al:
Association study between polymorphism of WRN gene and meningioma
in Chinese population. Zhong Guo Wei Qin Xi Shen Jing Wai Ke Za
Zhi. 17:248–251. 2012.(In Chinese).
|
|
13
|
He S, Pham MH, Pease M, et al: A review of
epigenetic and gene expression alterations associated with
intracranial meningiomas. Neurosurg Focus. 35:E52013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Opresko PL, Calvo JP and von Kobbe C:
Roles for Werner syndrome protein in the promotion of tumor cell
growth. Mech Ageing Dev. 128:423–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campbell BA, Jhamb A, Maguire JA, et al:
Meningiomas in 2009: Controversies and future challenges. Am J Clin
Oncol. 32:73–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vranic A, Peyre M and Kalamarides M: New
insights into meningioma: From genetics to trials. Curr Opin Oncol.
24:660–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Curtin K, Slattery ML and Samowitz WS: CpG
island methylation in colorectal cancer: Past, present and future.
Patholog Res Int. 2011:9026742011.PubMed/NCBI
|
|
18
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feltus FA, Lee EK, Costello JF, et al:
Predicting aberrant CpG island methylation. Proc Natl Acad Sci USA.
100:12253–12258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takai D and Jones PA: Comprehensive
analysis of CpG islands in human chromosomes 21 and 22. Proc Natl
Acad Sci USA. 99:3740–3745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bacolla A, Wang G, Jain A, et al: Non-B
DNA-forming sequences and WRN deficiency independently increase the
frequency of base substitution in human cells. J Biol Chem.
286:10017–10026. 2011. View Article : Google Scholar : PubMed/NCBI
|