Introduction
Diabetes mellitus (DM) is a disease with one of the
fastest growing incidences worldwide. By 2030 developing countries
will face an increase of 69% and industrialized countries of 20% of
the number of patients with diabetes compared with 2010. For Africa
>18 million, according to some estimations even 24 million,
diabetic patients are predicted for the year 2030 (1). It has recently been suggested that
diabetes increases the risk of a variety of cancers, including
breast, pancreatic, colorectal and kidney cancer (2). Hepatitis B virus (HBV) infection is one
of the most severe infections and constitutes a major risk factor
for mortality from cirrhosis and liver cancer (3). A number of studies have identified an
association between HBV infection and the prevalence of DM;
however, the results of those studies were inconclusive. Certain
studies have supported the increased risk of DM in HBV-infected
patients when compared with non-HBV-infected controls (4–9) and
certain studies have had different results (10–17). To
the best of our knowledge, no meta-analysis has ever focused on
assessing the association between HBV infection and the risk of DM.
The present meta-analysis was conducted in order to gain an
enhanced understanding of the association between the
conditions.
Materials and methods
Identification and eligibility of
relevant studies
The PubMed, Embase, Chinese National Knowledge
Infrastructure and Wan Fang databases, as well as the Chinese
Science and Technology Journal Database, were used to perform a
comprehensive literature search for relevant articles published
until June 2014. The following keywords were used: ‘Hepatitis B
virus’ or ‘HBV’ or ‘hepatitis B’ and ‘diabetes’, ‘diabetes
mellitus’ or ‘DM’. The search was limited to human studies and
publication in either English or Chinese. The literature search was
conducted independently by two reviewers. An additional manual
search of the reference lists of all relevant articles for all
available review articles and primary studies was also
performed.
Study selection criteria
The inclusion criteria for this study were as
follows: i) Evaluation of the association between HBV infection and
the risk of DM; ii) presence of at least one comparison group
without HBV and hepatitis C virus (HCV) infection; iii) studies
with accessible full texts and iv) DM confirmed based on a)
self-reported DM (i.e., diagnosed by a physician), b) fasting
plasma glucose levels >7.0 mmol/l on two separate occasions or
c) impaired fasting glycemia of 6.1–7.0 mmol/l without insulin
medication. Where available, hepatitis B surface antigen (HBsAg)
and/or antibody against hepatitis B core antigen (anti-HBc) and/or
HBV DNA were detected to confirm HBV infection.
The exclusion criteria for this study were as
follows: i) Gestational DM; ii) observational studies without
control groups and case reports; iii) subset of a published article
with the same data and by the same authors; and iv) studies
involving patients with chronic liver disease with alternative
etiologies, such as autoimmune hepatitis, steatohepatitis,
cirrhosis, primary biliary cirrhosis, hepatocellular carcinoma and
primary cholangitis.
Data extraction
The necessary information was extracted from all
eligible studies by two independent investigators, according to the
aforementioned inclusion criteria. Disagreements were resolved by
consensus or with the assistance of a third reviewer. The following
information was collected: i) First author's name, ii) year of
publication, iii) country of origin, iv) age of the patients, v)
HBV detection method, vi) reported odds ratio (OR) with 95%
confidence interval (CI) and vi) number of cases and controls.
Statistical analysis
The fixed-effect or random-effects model was
selected as appropriate, depending on the heterogeneity among the
studies included in the present meta-analysis. The degree of
heterogeneity among the studies was assessed using the
I2 test. An I2 value >50% was considered
to represent substantial heterogeneity. The assumption that the OR
in a case-control study approximates the relative risk in a cohort
study was used. The fixed-effect model was used in the absence of
significant heterogeneity (I2<50%), whereas the
random-effects model was selected in the presence of significant
heterogeneity (I2>50%). P<0.05 was considered to
indicate a statistically significant difference for all included
studies. Subgroup analyses were performed based on study types and
ethnic groups. Begg's funnel plot and Egger's weighted regression
method were used to test for publication bias. All analyses listed
above were conducted using Stata software (version 12.0; StataCorp
LP, College Station, TX, USA).
Results
Description of studies
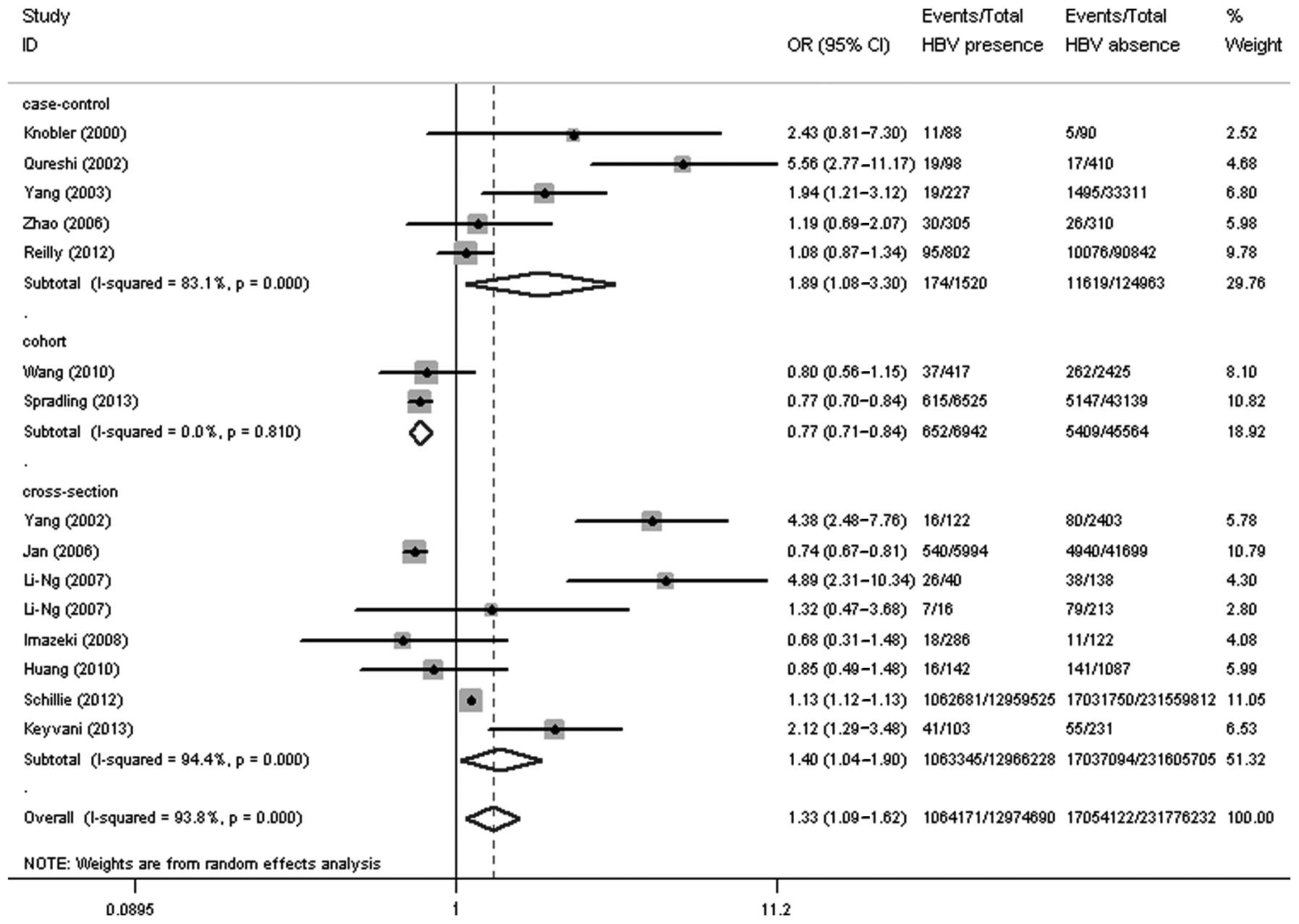
As summarized in Table
I, 14 articles (4–17), which were published between 2000 and
2013 and included 15 independent studies with 12,974,690
HBV-infected patients and 231,776,232 controls in total, were
assessed in the present meta-analysis. Among these 15 studies, 8
were cross-sectional studies, 5 were case-control studies and 2
were cohort studies. Six studies showed a significantly higher
prevalence of DM in HBV-infected patients than in the controls
(4–9), 7 studies lacked evidence of a
significant association between DM and HBV infection (6,12–17) and
2 studies revealed a lower prevalence of DM in patients with HBV
(10,11). The 15 studies involved 244,750,922
attendees and reported a total DM prevalence of 7.40%
(18,118,293/244,750,922). The cumulative sample size of
HBV-infected patients was 12,974,690, of which 1,064,171 also
suffered from DM (8.20%). Out of the 231,776,232 controls,
17,054,122 had DM (7.36%). The OR for the prevalence of DM in the
patients with HBV infection was 1.33 (95% CI, 1.09–1.62; P=0.005),
when compared with the controls (Fig.
1).
 | Table I.Main characteristics of the 15 studies
included in the meta-analysis. |
Table I.
Main characteristics of the 15 studies
included in the meta-analysis.
| First author, year
(ref.) | Country | Type of study | Confirmation of
HBV | Method of
detection | Confirmation of
DM | Type of DM | Age (years) |
|---|
| Spradling, 2013
(10) | USA | Cohort | HBsAg |
| Clinically
diagnosed | T2DM | >18 |
| Keyvani, 2013
(4) | Iran | CS | Anti-HBc | ELISA | Patient reported | DM |
60.5±14.1a |
| Schillie, 2012
(5) | USA | CS | Anti-HBc | ELISA/CLIA | Patient reported | DM | 18–70b |
| Reilly, 2012
(12) | USA | CC | HBsAg |
| Patient reported | DM | 44
(23–88)c |
| Huang, 2010 (13) | Chinese Taipei | CS | HBsAg |
| FPG or 2-hPPG | T2DM |
52.7±0.73a |
| Wang, 2010 (14) | Chinese Taipei | Cohort | HBsAg | ELISA | Criteriad | T2DM | 40–70b |
| Imazeki, 2008
(15) | Japan | CS | HBsAg | ELISA | FPG | T2DM |
45.1±13.6a |
| Li-Ng, 2007 (6) | USA (Asian
Americans) | CS | HBsAg |
| Random blood
glucose | T2DM | 58
(30–79)c |
| Li-Ng, 2007 (6) | USA (Pacific
Islanders) | CS | HBsAg |
| Random blood
glucose | T2DM | 61
(52–76)c |
| Jan, 2006 (11) | Chinese Taipei | CS | HBsAg | RIA | FPG | T2DM |
|
| Zhao, 2006
(16) | China | CC | HBV DNA | PCR | WHO | DM |
45.68±25.38a |
| Yang, 2003
(7) | China | CC | HBsAg | ELISA |
Criteriad | T2DM |
45.7±11.9a |
| Yang, 2002
(9) | China | CS | HBsAg | ELISA | WHO | T2DM | 30–50b |
| Qureshi, 2002
(8) | Pakistan | CC | HBsAg | ELISA | Clinically
diagnosed | T2DM | 42±13a |
| Knobler, 2000
(17) | Israel | CC | HbsAg and
anti-HBc | ELISA |
Criteriad | T2DM | 45±15a |
Subgroup analysis
The results of the present meta-analysis
demonstrated that the HBV-infected patients had a higher risk of
developing DM when compared with the uninfected patients (OR, 1.33;
95% CI, 1.09–1.62; P=0.005); however, the heterogeneity between the
two groups was high (I2=93.8%). Subgroup analyses based
on study type and region were performed to investigate the factors
that could impact the overall results. The results of the study
type-based subgroup analysis indicated that the prevalence of DM in
the HBV group was significantly higher than that in the control
group, both in case-control (11.45 vs. 9.30%; OR, 1.89; 95% CI,
1.08–3.30; P=0.025) and cross-sectional (8.20 vs. 7.36%; OR, 1.40;
95% CI, 1.04–1.90; P=0.027) studies; however, the prevalence of DM
in the case group was lower than that in the control group in the
cohort studies (9.40 vs. 11.87%; OR, 0.77; 95% CI, 0.71–0.84;
P<0.001) (Fig. 1). The results of
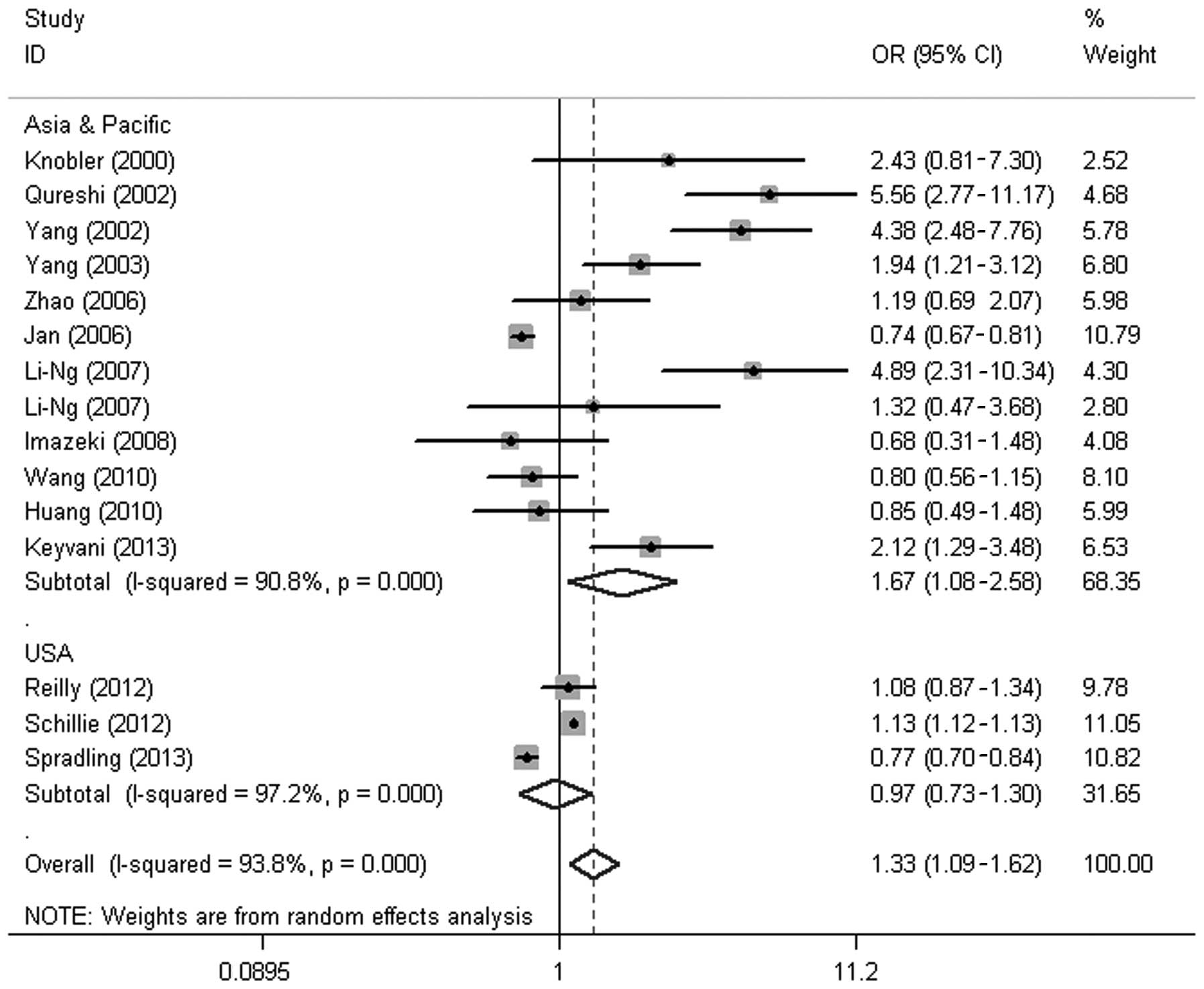
the region-based subgroup analysis showed that the prevalence of DM
in the HBV group was significantly higher than that in the control
group in the Asia-Pacific region (OR, 1.67; 95% CI, 1.08–2.58;
P=0.022); however, no significant difference was identified between
the two groups in the USA (OR, 0.97; 95% CI, 0.73–1.30; P=0.86)
(Fig. 2).
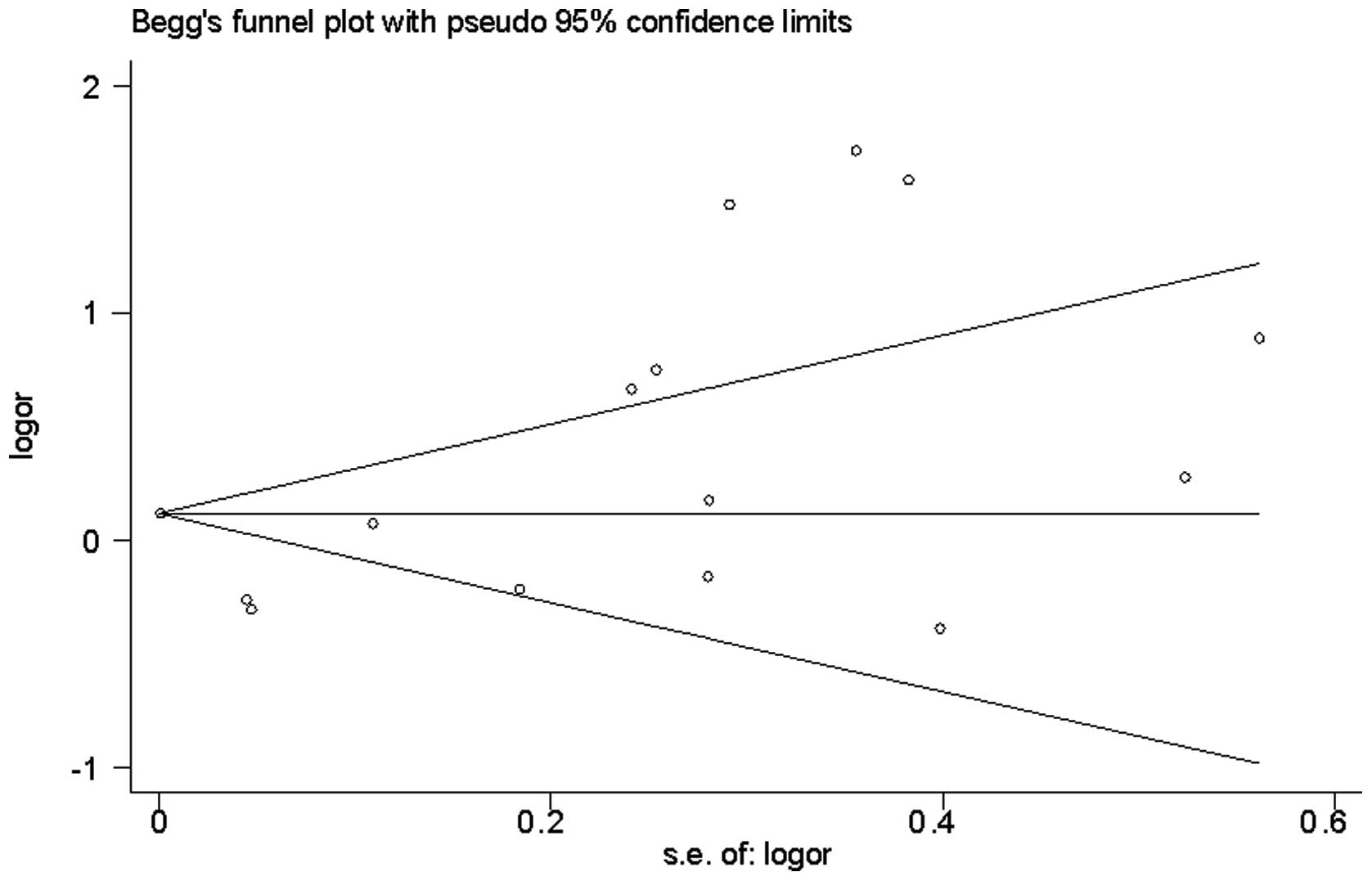
Publication bias
Funnel plot analysis did not reveal any evidence of
publication bias in the 15 studies [Begg's test z=0.99 (continuity
corrected); P=0.322] (Fig. 3). The
Egger's test also indicated a lack of significant publication bias
(P=0.906).
Discussion
Liver disease of various etiologies, including HCV
infection, has been implicated as a cause of DM in several previous
studies (18–20). A hypothesis that there may be an
association between HBV infection and DM incidence has been
proposed (8,9); however, the results remain
controversial. In order to resolve this controversy, 15 studies
were selected for the present meta-analysis, the aim of which was
to comprehensively evaluate the association between HBV infection
and the prevalence of DM.
The present study indicates that patients with HBV
infection are at higher risk of developing DM when compared with
patients without HBV infection. The findings of this study are in
accordance with those of a large-sample, cross-sectional study in
the USA (5). According to the
aforementioned results, HBV infection could comprise a potential
risk factor for the development of DM. Several mechanisms may be
involved in the association between HBV infection and the
prevalence of DM. First, the liver is an organ that plays a key
role in the regulation of glucose homeostasis by balancing the
storage and output of glucose. Liver damage caused by HBV infection
may lead to a glycometabolism disorder (21,22), and
persistent inflammatory activities in the liver may cause defective
glucose homeostasis. Inflammatory mediators, such as tumor necrosis
factor-α and nitric oxide, have been shown to impair the metabolic
action of insulin in the liver, which results in hepatic
dysfunction and, in turn, leads to insulin resistance (23–26).
Furthermore, inducible nitric oxide synthase expression has been
shown to be elevated in the liver of patients suffering from
chronic HBV infection (27).
Secondly, several studies (28,29) have
found HBV infection in the pancreas. The replication of HBV in
extrahepatic sites, such as the pancreas, is responsible for β-cell
damage and may ultimately lead to diabetes (28,29). In
addition, insulin resistance may be involved in the pathogenesis of
hepatogenous diabetes. Ji et al (30) reported that the pre-S2 protein of HBV
decreased the expression of the insulin receptor gene, leading to
insulin resistance.
In the present meta-analysis, it was notable that
the subgroup analysis showed the prevalence of DM in the HBV group
to be significantly higher than that in the control group, both in
the case-control and cross-sectional studies; however, the
prevalence of DM in the case group was lower than that in the
control group in the cohort studies, which included a study
performed on subjects from the USA. Region-based subgroup analysis
revealed that HBV infection was associated with an increased risk
of DM in the Asia-Pacific region, while no significant difference
was found in the prevalence of DM between the HBV-infected patients
and the controls in the USA. The low incidence of HBV infection and
high prevalence rate of DM in the USA may explain these results.
The overall prevalence of chronic HBV infection in the USA is 0.4%,
whereas 10–15% of Asian Americans/Pacific Islanders suffer from
chronic HBV infection. In most Asian regions, the prevalence of HBV
infection is >8.0% (29). By
comparison, ~12.4% of the US population had been diagnosed with DM
up to 2010 (31), while the
prevalence of DM in Asia has been reported to be 8.7% (32).
Of note, the Keelung Community-Based Integrated
Screening Study (14), conducted in
Chinese Taipei, found that the DM prevalence was lower among
HBV-infected patients than that among uninfected patients. Two more
studies (11,12) from Chinese Taipei also showed a lower
prevalence of DM among HBV-infected patients, although the
differences were not statistically significant. The reason for this
finding is unclear but may be due to dietary, genetic or other
environmental differences among these ethnic groups.
Despite the considerable efforts made and resources
employed to test the association between HBV infection and the DM
prevalence, the present meta-analysis had certain limitations.
First, HBV infection was diagnosed by different serum markers
across the included studies. The markers included the HBsAg,
anti-HBc, HBV DNA or a combination of more than one serum marker,
making it impossible to distinguish between past and the ongoing
HBV infections. Furthermore, the severity of the hepatitis was not
known, since only a few studies had reported the aminotransferase
levels. Secondly, three different types of studies were involved in
the present meta-analysis: Case-control, cross-sectional and cohort
studies. The various types of study designs could have been
partially responsible for the heterogeneity across the studies. In
addition, the majority of the studies included were retrospective,
making them susceptible to recall and selection bias. No evidence
for an association between HBV infection and the prevalence of DM
was provided by the 2 cohort studies; however, the results should
be considered with caution since the number of patients included
was relatively small. Thirdly, the selection of the controls varied
among studies. Some studies used a healthy population as controls,
while others used hospitalized patients without HBV infection as
controls; therefore, it is possible that selection bias existed,
since these studies may have included controls at different risks
of developing DM. Furthermore, heterogeneity was significant across
studies, which decreased the reliability of the summary OR
estimates. Finally, data were only obtained from papers published
in English or Chinese, which could have also contributed to
selection bias.
In conclusion, the present study indicates that
patients with HBV infection are at higher risk of developing DM
compared with uninfected patients and that HBV infection may be a
potential risk factor for DM development. Due to the considerable
heterogeneity across the studies and the limitations of this
meta-analysis, further research is required to investigate the
possible association between HBV infection and the prevalence of
DM.
Acknowledgements
This study was supported by the Natural Science
Foundation of Guangdong (grant no. S2013010016631) and the
Guangdong Supporting Grant for Outstanding Talent (grant no.
C1030925).
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JY, Jeon I, Lee JM, et al: Diabetes
mellitus as an independent risk factor for lung cancer: A
meta-analysis of observational studies. Eur J Cancer. 49:2411–2423.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ott JJ, Stevens GA, Groeger J and Wiersma
ST: Global epidemiology of hepatitis B virus infection: New
estimates of age-specific HBsAg seroprevalence and endemicity.
Vaccine. 30:2212–2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keyvani H, Agah S, Kabir A and Alavian SM:
Prevalence and risk factors of isolated anti-HBc antibody and
occult hepatitis B infection in hemodialysis patients: A nationwide
study. Ann Hepatol. 12:213–219. 2013.PubMed/NCBI
|
|
5
|
Schillie SF, Xing J, Murphy TV and Hu DJ:
Prevalence of hepatitis B virus infection among persons with
diagnosed diabetes mellitus in the United States, 1999–2010. J
Viral Hepat. 19:674–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li-Ng M, Tropp S, Danoff A and Bini EJ:
Association between chronic hepatitis B virus infection and
diabetes among Asian Americans and Pacific Islanders. Dig Liver
Dis. 39:549–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang SQ, Chen HS, Jiang D, et al:
Relationship between chronic hepatitis C and type II diabetes
mellitus. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi.
17:46–49. 2003.(In Chinese). PubMed/NCBI
|
|
8
|
Qureshi H, Ahsan T, Mujeeb SA, et al:
Diabetes mellitus is equally frequent in chronic HCV and HBV
infection. J Pak Med Assoc. 52:280–283. 2002.PubMed/NCBI
|
|
9
|
Yang JH, Sun CM, Xu XB and Bao CX:
Susceptibility of diabetes in patients with positive hepatitis B
surface antigen. Zhongguo Wuzhen Xue Zazhi. 2:5642002.(In
Chinese).
|
|
10
|
Spradling PR, Simons B, Narayanan M, et
al: Incidence of diabetes mellitus in a population-based cohort of
persons with chronic hepatitis B virus infection. J Viral Hepat.
20:510–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jan CF, Chen CJ, Chiu YH, et al: A
population-based study investigating the association between
metabolic syndrome and hepatitis B/C infection (Keelung
Community-based Integrated Screening study No.10). Int J Obes
(Lond). 30:794–799. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reilly ML, Schillie SF, Smith E, et al:
Increased risk of acute hepatitis B among adults with diagnosed
diabetes mellitus. J Diabetes Sci Technol. 6:858–866. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang ZS, Huang TS, Wu TH, et al:
Asymptomatic chronic hepatitis B virus infection does not increase
the risk of diabetes mellitus: A ten-year observation. J
Gastroenterol Hepatol. 25:1420–1425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CS, Chang TT, Yao WJ, et al: The
impact of smoking on incident type 2 diabetes in a cohort with
hepatitis B but not hepatitis C infection. J Viral Hepat. Aug.
31–2010.(Epub ahead of print).
|
|
15
|
Imazeki F, Yokosuka O, Fukai K, et al:
Prevalence of diabetes mellitus and insulin resistance in patients
with chronic hepatitis C: Comparison with hepatitis B
virus-infected and hepatitis C virus-cleared patients. Liver Int.
28:355–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao P, Wang JB and Jiao J: Investigation
on the incidence of diabetes in chronic hepatitis C patients and
their HCV genotypes. Zhonghua Gan Zang Bing Za Zhi. 14:86–88.
2006.(In Chinese). PubMed/NCBI
|
|
17
|
Knobler H, Schihmanter R, Zifroni A, et
al: Increased risk of type 2 diabetes in noncirrhotic patients with
chronic hepatitis C virus infection. Mayo Clin Proc. 75:355–359.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naing C, Mak JW, Ahmed SI and Maung M:
Relationship between hepatitis C virus infection and type 2
diabetes mellitus: Meta-analysis. World J Gastroenterol.
18:1642–1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Picardi A, D'Avola D, Gentilucci UV, et
al: Diabetes in chronic liver disease: From old concepts to new
evidence. Diabetes Metab Res Rev. 22:274–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harrison SA: Liver disease in patients
with diabetes mellitus. J Clin Gastroenterol. 40:68–76. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raddatz D and Ramadori G: Carbohydrate
metabolism and the liver: Actual aspects from physiology and
disease. Z Gastroenterol. 45:51–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tappy L and Minehira K: New data and new
concepts on the role of the liver in glucose homeostasis. Curr Opin
Clin Nutr Metab Care. 4:273–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teli T, Xanthaki D and Karalis KP:
Regulation of appetite and insulin signaling in inflammatory
states. Ann NY Acad Sci. 1083:319–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Postic C, Dentin R and Girard J: Role of
the liver in the control of carbohydrate and lipid homeostasis.
Diabetes Metab. 30:398–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collier B, Dossett LA, May AK and Diaz JJ:
Glucose control and the inflammatory response. Nutr Clin Pract.
23:3–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leclercq IA, Da Silva Morais A, Schroyen
B, et al: Insulin resistance in hepatocytes and sinusoidal liver
cells: Mechanisms and consequences. J Hepatol. 47:142–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Majano PL, García-Monzón C, López-Cabrera
M, et al: Inducible nitric oxide synthase expression in chronic
viral hepatitis. Evidence for a virus-induced gene upregulation. J
Clin Invest. 101:1343–1352. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoffe B, Burns DK, Bhatt HS and Combes B:
Extrahepatic hepatitis B virus DNA sequences in patients with acute
hepatitis B infection. Hepatology. 12:187–192. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimoda T, Shikata T, Karasawa T, et al:
Light microscopic localization of hepatitis B virus antigens in the
human pancreas. Possibility of multiplication of hepatitis B virus
in the human pancreas. Gastroenterology. 81:998–1005.
1981.PubMed/NCBI
|
|
30
|
Ji D, Cheng J, Dong Z, et al: Screening
and identification of genes trans-regulated by HBV pre-S2 protein
with cDNA microarray. World Chin J Digestology. 12:1559–1563.
2004.(In Chinese).
|
|
31
|
Selvin E, Parrinello CM, Sacks DB, et al:
Trends in prevalence and control of diabetes in the United States,
1988–1994 and 1999–2010. Ann Intern Med. 160:517–525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guariguata L, Whiting DR, Hambleton I, et
al: Global estimates of diabetes prevalence for 2013 and
projections for 2035. Diabetes Res Clin Pract. 103:137–149. 2014.
View Article : Google Scholar : PubMed/NCBI
|