Introduction
The efficacy of chemotherapeutic agents is largely
dependent on their ability to inhibit the growth of tumor cells
(1). A number of studies have
demonstrated that certain phytochemicals present in medicinal herbs
exert antitumor activity by inhibiting cancer cell growth (2). Trillium tschonoskii Maxim, also
known as ‘a pearl on head’, is predominantly distributed in
mid-Western China and has been used in traditional remedies for the
treatment of headache, hypertension, neurasthenia, giddiness and
cancer, as well as for the removal of carbuncles and the
amelioration of pain, for ≥1,000 years (3,4).
Previous studies have shown that a number of bioactive components,
such as steroidal saponins and glycosides, can be found in species
of the Trillium genus, including T. erectum (5,6), T.
kamtschaticum (7,8) and T. tschonoskii Maxim (9). Saponins are identified by their
structures which contain a steroidal or triterpenoid aglycone and
one or more sugar chains (10).
Previous studies have elucidated that the biological and
pharmacological properties of saponins are associated with their
structural diversity. These are exploited in a number of
traditional and industrial applications (11,12).
TTB2 is one of the steroidal saponins isolated from T.
tschonoskii Maxim (9); its
pharmacological effects and mechanisms remain unclear. The aim of
the present study was to evaluate the bioactive effect of TTB2 on
Ewing sarcoma (Rh1) cells.
Materials and methods
Reagents
Trypan blue, propidium iodide (PI) and RNase were
purchased from Sigma -Aldrich (St. Louis, MO, USA). RPMI-1640
culture media and fetal bovine serum (FBS) were supplied by
Gibco-BRL (Grand Island, NY, USA). Mouse polyclonal
anti-phosphorylated-(p-)ERK and anti-ERK, and rabbit polyclonal
anti-phosphorylated-(p-) AKT and anti-AKT were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal
anti-β-tubulin antibody and horseradish peroxidase-labeled
secondary anti-mouse and anti-rabbit antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Other
chemicals used in this study were special grade commercial
products. This study was approved by the Medicine Scientific
Research Ethics Committee of China Three Gorges University
(Yichang, China).
Plant material and extraction and
isolation of TTB2
The rhizomes of T. tschonoskii Maxim were
purchased from Muyu, a town of the Shennongjia Forest District of
Hubei (China). Professor Chen Faju, a botanist at the China Three
Gorges University (Yichang, China), identified the nature of the
collected T. tschonoskii Maxim. A voucher specimen (no.
2005ZW03128) was deposited in the Medicinal Plants Herbarium of the
College of Chemistry and Life Science (China Three Gorges
University). Air-dried powdered rhizomes (6.4 kg) were extracted
with methanol under reflux. The methanol extract (2,427 g) was
obtained. The extract was suspended in water (2.2 liters), and then
extracted with CHCl3, EtOAc and n-BuOH
successively. A portion of the n-BuOH extract (775 g) was
reduced in vacuo and dissolved in water to a volume small
enough to allow the drug to dissolve, and then subjected to
macroporous resin column chromatography (Guangfu Fine Chemical
Research Institute, Tianjin, China) in elution with gradient
solvents (100% water→100% methanol). A portion of 80% methanol
eluates (2.0 g) was separated by repeated reversed-phase
C18 silica gel column chromatography (Guangfu Fine
Chemical Research Institute) in elution with a gradient solvent
system (acetonitrile:water, between 35:65 and 0:100) to give rise
to 50 fractions. Fraction 37 (182 mg) was further separated by
semi-preparative high-performance liquid chromatography eluted with
43% acetonitrile (within 30 min, 2.0 ml/min, detection at 203 nm),
giving rise to the compound TTB2 (32 mg). TTB2 powder was dissolved
in distilled water. The filtered TTB2 stock solution was separated
into individual aliquots, which were kept at −20°C until further
use.
Cell culture
Rh1 cells (St. Jude Children's Research Hospital,
Memphis, TN, USA) were grown in antibiotic-free RPMI-1640 medium
supplemented with 10% FBS at 37°C and 5% CO2.
Cell viability assays
The viability of the cells was determined using the
trypan blue dye exclusion assay, in which the color changes
reflected the dead cells (13). In
brief, the cells (1×104/ml) were first seeded in the
cell culture flask (each concentration in triplicate). After 12 h,
the cells were treated with different concentrations of TTB2 (5,
7.5, 10, 12.5 and 15 μM) in medium for 12, 24 and 48 h,
respectively. Following trypsinization, the cells exposed to 0.2%
trypan blue were counted in an auto-hemocytometer (Invitrogen Life
Technologies, Carlsbad, CA, USA). Each experiment was repeated at
least three times.
Cell cycle analysis
Cancer cells (5×106) were treated with
TTB2 at the indicated concentrations (5 and 10 μM) for 24 h. The
attached cells were then trypsinized and washed once with
phosphate-buffered saline (PBS). The cells were resuspended in 2 ml
70% ice-cold ethanol solution and fixed at 4°C overnight. The cells
were centrifuged (500 × g for 10 min) to remove ethanol and washed
again with PBS; the pellets were resuspended in 100 mg/ml PI
solution containing 100 mg/ml RNase, and then incubated at 37°C for
≥30 min. The stained cells were analyzed for DNA content by flow
cytometry (FCM; Beckman Coulter, Miami, FL, USA).
Western blot analysis
Protein expression was determined by western blot
analysis. Briefly, Rh1 cells (2×105) were seeded in
six-well plates for 12 h and then treated with the indicated
concentrations of TTB2 for 24 h. Following separation by SDS-PAGE,
the proteins were transferred to polyvinylidene difluoride
membranes and subjected to immunoblotting with antibodies against
p-ERK, ERK, p-Akt [serine (Ser)473], Akt and tubulin at 4°C
overnight. Subsequent to washing with 5% skimmed milk in
tris-buffered saline and Tween 20 (TBST) buffer (5 mM Tris-HCl, pH
7.6, 136 mM NaCl and 0.05% Tween-20), the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies and
visualized using the enhanced chemiluminescence system (Pierce,
Rockford, IL, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical evaluations were performed using SPSS 10.0
software (SPSS Inc., Chicago, IL, USA) using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
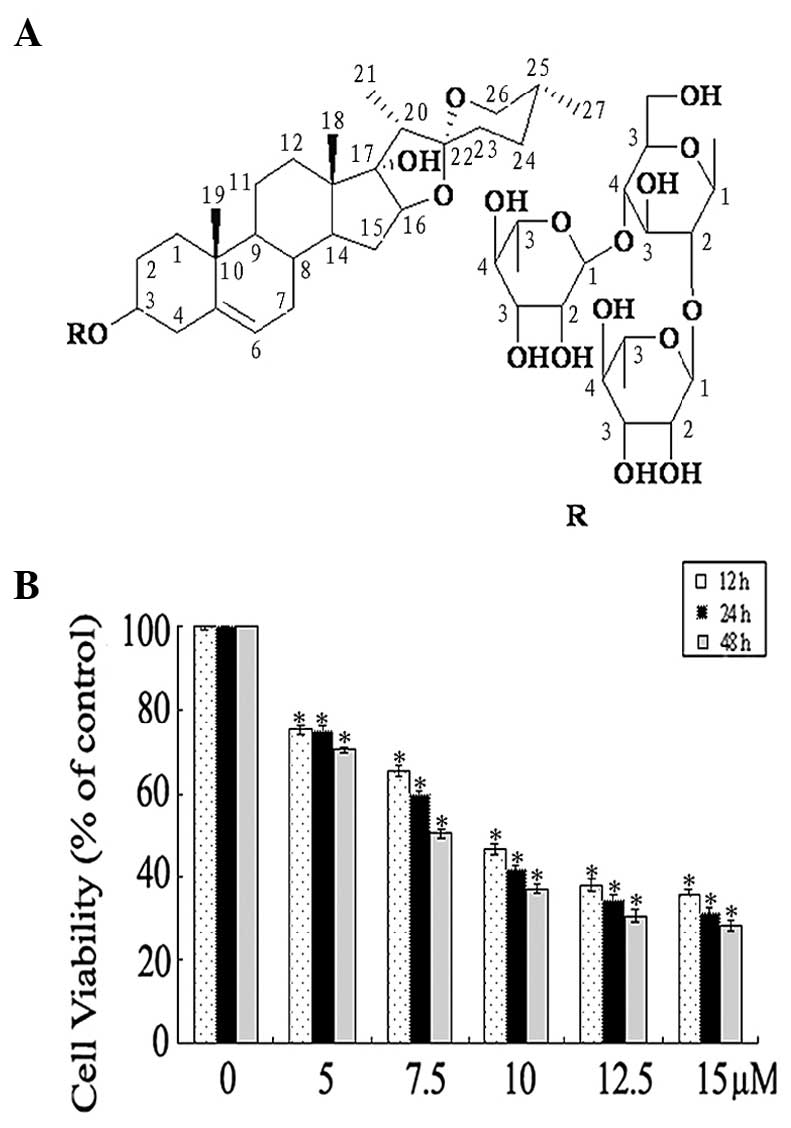
Chemical structure of TTB2
As shown in Fig. 1A,
the structure of TTB2 was pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)
[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside.
Inhibition of Rh1 cell line viability
by TTB2
The effect of TTB2 on cell viability was examined by
treating the Rh1 cells with five concentrations of TTB2 (5, 7.5,
10, 12.5 and 15 μM) in the presence of 10% serum medium. After 12,
24 and 48 h of treatment, the viability of the cells was determined
by the trypan blue assay. Untreated cells (control) were considered
as the baseline (100% viable) for the analysis. Fig. 1B shows that TTB2 appeared to be an
effective inhibitor of Rh1 cell viability, which was inhibited in a
dose- and time-dependent manner.
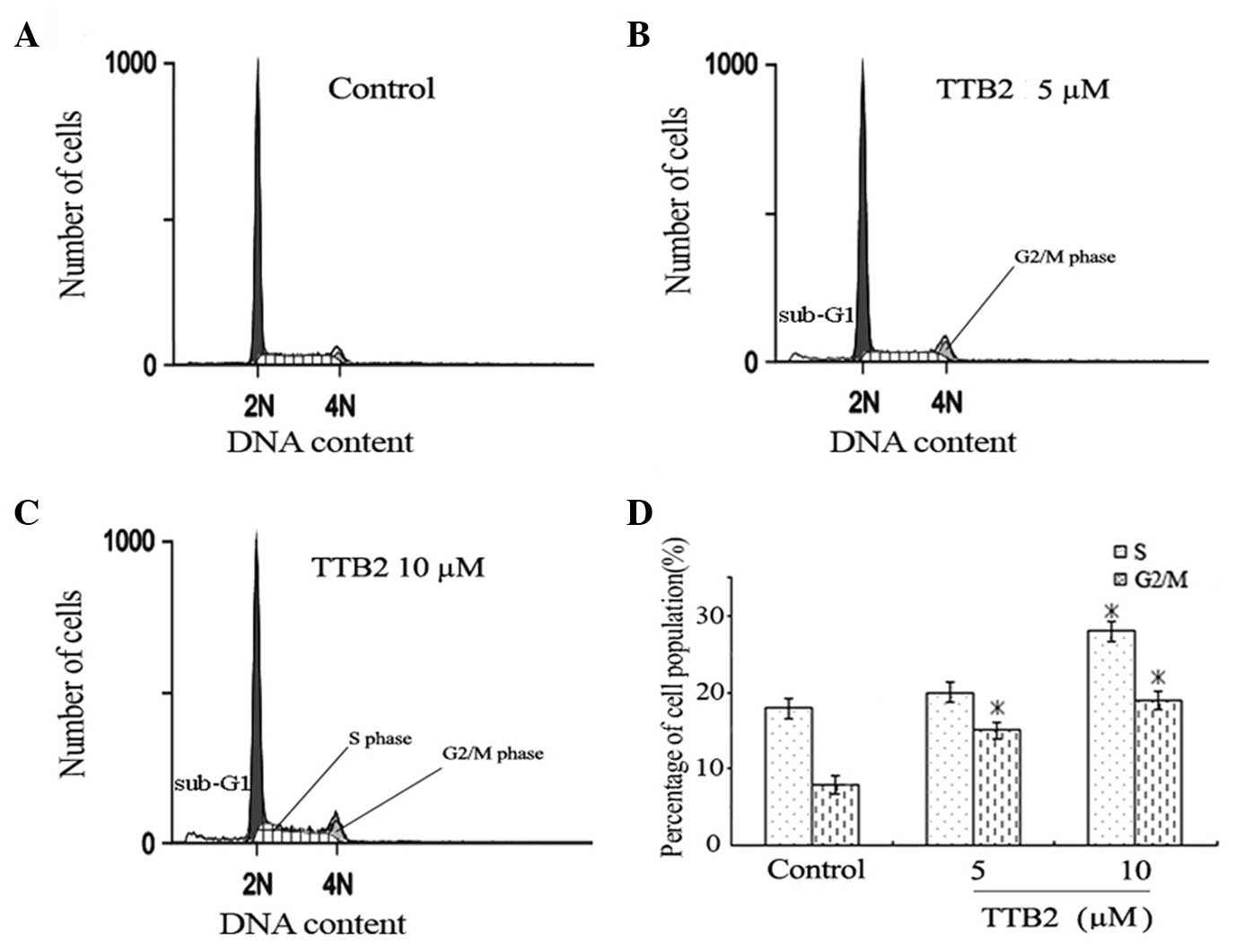
TTB2 induces G2/M and S
phase cell cycle arrest
To determine the effect of TTB2 on the cell cycle
progression of Rh1 cells, FCM analysis was performed on cells
treated with 5 and 10 μM TTB2 for 24 h (Fig. 2). The two concentrations of TTB2
caused a significant increase in the percentage of G2/M
phase cells (Fig. 2B and C), showing
that TTB2 arrests the cell cycle progression in the G2/M
phase when compared with the controls (Fig. 2A). The high concentration of TTB2 (10
μM) also led to arrest in the S phase. These results indicate that
TTB2 mediated a prolongation of cell cycle progression in the
G2/M and S phases.
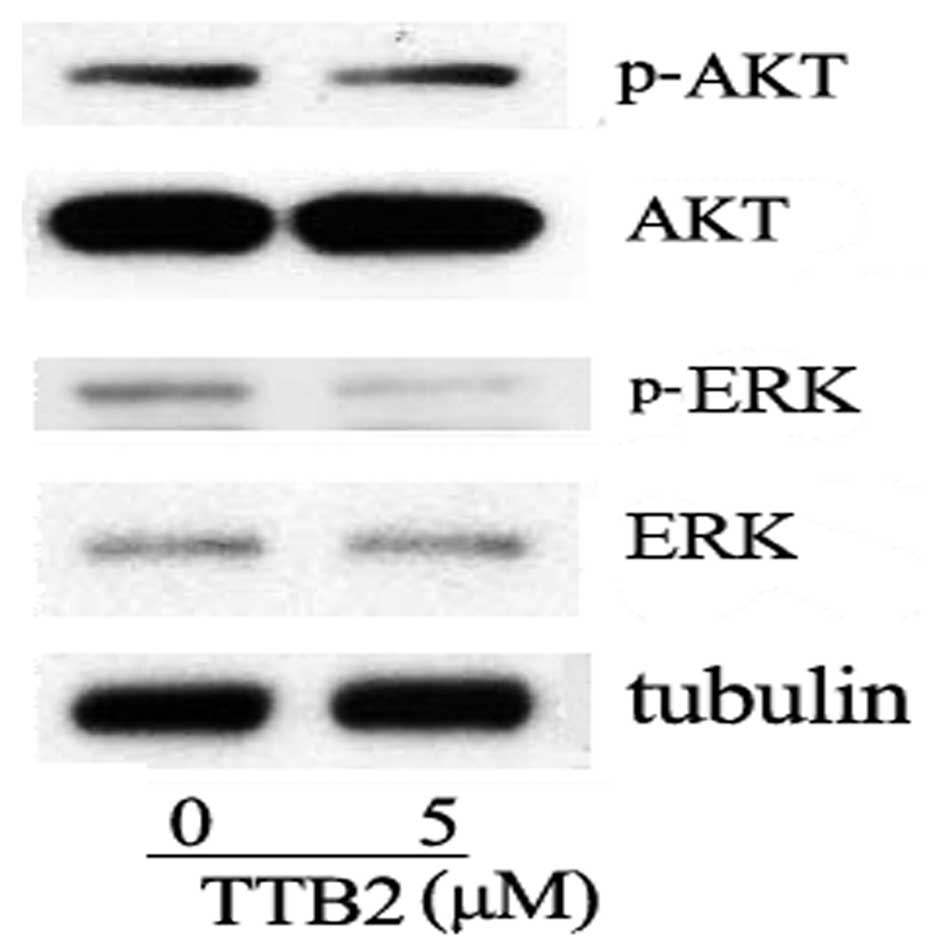
TTB2 inhibits the phosphorylation of
ERK
It has been widely reported that activation of Akt
or ERK exists in cancer cells (14).
In the present results, TTB2 did not inhibit the phosphorylation of
Akt (Ser473). However, TTB2 was found to modulate the activity of
ERK, a member of the mitogen-activated protein kinase family. As
shown in Fig. 3, following TTB2
treatment the phosphorylation of ERK was significantly
decreased.
Discussion
With an increasing cancer rate worldwide, there is
an urgent requirement for improvements in the therapeutic activity
and selectivity of anticancer agents. Steroidal saponins are widely
distributed in plants and exhibit pharmacological functions and
biological activities (12,15). The compound TTB2, pennogenin
3-O-α-L-rhamnopyranosyl-(1→2)
[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside, is a steroidal
saponin that has been isolated from T. kamtschaticum
(8) and Paris polyphylla var.
yunnanensis (16). However,
few studies have focused on its bioactivity and the associated
mechanisms. It has been reported that certain pennogenin steroid
analogues from other plants exhibit diverse bioactivity. For
example, pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)
[α-L-arabinofuranosyl-(1→4)]-β-D-glucopyranoside, which is
extracted from P. polyphylla var. yunnanensis, has
been shown to markedly inhibit gastric lesions induced by ethanol
and indomethacin (17). Furthermore,
certain analogues, such as pennogenin 3-O-α-l-rhamnopyranosyl-(1→2)
[α-l-rhamnopyranosyl-(1→3)]-[6-O-acetyl]-β-D-glucopyranoside from
Dracaena (18),
pennogenin-O-R-L-rhamnopyranosyl-(1→2)-[R-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside
from D. deisteliana (18) and
pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside from
P. vietnamensis (19), have
been found to be cytotoxic to cancer cells. The present results
showed that TTB2, the pennogenin steroid from T. tschonoskii
Maxim, could inhibit the growth of one type of tumor of the
mesenchymal tissue.
The G2/M and S phases are important
checkpoints for DNA damage and are critical to cell cycle
progression (20). The present
results clearly indicated that TTB2 caused an increase in the
percentage of cells in G2/M (low concentration) and/or S
(high concentration) phase, which is one mechanism by which TTB2
exerts its anti-proliferative effect. Since Akt and ERK activation
contributes to anti-apoptosis effects and cell growth, the
activation of Akt or ERK plays an important role in the pathology
of cancer (21,22). In the present study, Akt and ERK
phosphorylation was observed. Although the inhibition of Akt did
not occur in TTB2-treated Rh1 cells, the inhibition of ERK could be
induced by TTB2. The results indicate that the ERK pathway is
involved in TTB2-induced Rh1 apoptosis.
In conclusion, the present data revealed that Rh1
cells are sensitive to growth inhibition by TTB2, which is
associated with cell cycle arrest and the inhibition of ERK.
Therefore, the results of this study indicate that TTB2 isolated
from T. tschonoskii Maxim may be a potential candidate for
the development of anticancer drugs for use in the treatment of
cancer.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (nos. 31070313 and 21272136),
the Yichang Science and Technology Research and Development Project
(no. A201230234) and the CTGU Talent Scientific Research Initial
Foundation (no. KJ2012B063).
References
|
1
|
Mansilla S, Llovera L and Portugal J:
Chemotherapeutic targeting of cell death pathways. Anticancer
Agents Med Chem. 12:226–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rasul A, Song R, Wei W, et al:
Tubeimoside-1 inhibits growth via the induction of cell cycle
arrest and apoptosis in human melanoma A375 cells. Bangladesh J
Pharmacol. 7:150–156. 2012. View Article : Google Scholar
|
|
3
|
Fu L: China Plant Red Data Book: Rare and
Endangered Plants. 1. Science Press; Beijing: 1992
|
|
4
|
Li Q, Xiao M, Guo L, et al: Genetic
diversity and genetic structure of an endangered species,
Trillium tschonoskii. Biochem Genet. 43:445–458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayes PY, Lehmann R, Penman K, Kitching W
and De Voss JJ: Steroidal saponins from the roots of Trillium
erectum (Beth root). Phytochemistry. 70:105–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokosuka A and Mimaki Y: Steroidal
glycosides from the underground parts of Trillium erectum
and their cytotoxic activity. Phytochemistry. 69:2724–2730. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono M, Sugita F, Shigematsu S, et al:
Three new steroid glycosides from the underground parts of
Trillium kamtschaticum. Chem Pharm Bull (Tokyo).
55:1093–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nohara T, Miyahara K and Kawasaki T:
Steroid saponins and sapogenins of underground parts of Trillium
kamtschaticum Pall. II. Pennogenin- and kryptogenin
3-O-glycosides and related compounds. Chem Pharm Bull (Tokyo).
23:872–885. 1975. View Article : Google Scholar
|
|
9
|
Nohara T, Kumamoto F, Miyahara K and
Kawasaki T: Steroid saponins of aerial parts of Paris
tetraphylla A. Gray and of underground parts of Trillium
tschonoskii Maxim. Chem Pharm Bull (Tokyo). 23:1158–1160. 1975.
View Article : Google Scholar
|
|
10
|
Osbourn A, Goss RJ and Field RA: The
saponins: Polar isoprenoids with important and diverse biological
activities. Nat Prod Rep. 28:1261–1268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuchs H, Bachran D, Panjideh H, Schellmann
N, Weng A, Melzig MF, Sutherland M and Bachran C: Saponins as tool
for improved targeted tumor therapies. Curr Drug Targets.
10:140–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang SL, Liu XK, Wu H, Wang HB and Qing C:
Steroidal saponins and cytoxicity of the wild edible
vegetable-Smilacina atropurpurea. Steroids. 74:7–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tyagi A, Bhatia N, Condon MS, Bosland MC,
Agarwal C and Agarwal R: Antiproliferative and apoptotic effects of
silibinin in rat prostate cancer cells. Prostate. 53:211–217. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 Suppl
2:S17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Güçlü-Ustündaǧ O and Mazza G: Saponins:
properties, applications and processing. Crit Rev Food Sci Nutr.
47:231–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CX, Zhou J, Zhang YT and Zhao YY:
Steroid saponins of aerial parts of Paris polyphylla var.
yunnanensis. Acta Bot Yunnan. 12:323–329. 1990.(In
Chinese).
|
|
17
|
Matsuda H, Pongpiriyadacha Y, Morikawa T,
Kishi A, Kataoka S and Yoshikawa M: Protective effects of steroid
saponins from Paris polyphylla var. yunnanensis on
ethanol- or indomethacin-induced gastric mucosal lesions in rats:
structural requirement for activity and mode of action. Bioorg Med
Chem Lett. 13:1101–1106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kougan GB, Miyamoto T, Tanaka C, et al:
Steroidal saponins from two species of Dracaena. J Nat Prod.
73:1266–1270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Cui LJ, Wang Q and Ye WC:
Separation and identification of active constituents of Paris
vietnamensis. Yao Xue Xue Bao. 41:361–364. 2006.(In Chinese).
PubMed/NCBI
|
|
20
|
Zhou BB and Elledge SJ: The DNA damage
response: putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moelling K, Schad K, Bosse M, Zimmermann S
and Schweneker M: Regulation of Raf-Akt Cross-talk. J Biol Chem.
277:31099–31106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|