Introduction
Chorioamnionitis is an important clinical factor
that leads to premature rupture of the fetal membrane (PROM), and
can be classified as subclinical or histological chorioamnionitis.
During pregnancy, immune function is relatively low; thus, various
pathogens from the vulva and cervix invade the uterus, which
commonly results in subclinical chorioamnionitis (1). Subclinical chorioamnionitis may lead to
inflammatory cell exudation, leukocyte infiltration edema, fibrous
tissue proliferation and reduced elasticity/increased brittleness
of fetal membrane, ultimately leading to premature rupture
(2). Following the PROM, the
environment of the uterus and vagina is altered in response,
promoting bacterial proliferation and exacerbating the subclinical
chorioamnionitis (3).
Chorioamnionitis is an easily overlooked condition,
as the early clinical symptoms are not evident in the majority of
pregnant patients and it is difficult to establish an accurate
prenatal diagnosis (4). To date,
there is no accurate method or index for predicting PROM in cases
of subclinical chorioamnionitis, although there are a number of
studies concerning the disease (5–9).
Matrix metalloproteinases (MMPs), including MMP-2,
serve key functions in the development of various diseases by
contributing to the degradation of type IV collagen, which is a
major component of the extracellular matrix (10–12).
MMP-2 is secreted as an inactive proenzyme and activated by other
factors or signals. It has been reported that MMP-2 expression
levels are reduced in maternal blood serum, and it is possible that
MMP-2 concentrations are associated with preterm labor and fetal
inflammatory responses (13).
However, other studies demonstrated no association between MMP-2
concentration and preterm labor or fetal inflammatory responses
(14,15). Therefore, it is important to
determine whether alterations in MMP-2 levels are associated with
the PROM in patients with subclinical chorioamnionitis.
In the present study, the effects of PROM on
pregnancy outcomes were investigated in cases of subclinical
chorioamnionitis. PROM, combined with subclinical chorioamnionitis,
was observed to be associated with reduced levels of MMP-2.
Materials and methods
Patients
In total, 80 patients (age range, 24–32 years) that
exhibited PROM were recruited and divided evenly into the control
and experimental groups, according to their final placental
pathology results. The 40 patients in the experimental group
suffered from subclinical chorioamnionitis, while the 40 patients
in the control group exhibited no lesions of the placenta or fetal
membrane. All procedures were approved by the Ethics Committee of
Yan'an University (Yan'an, China). Informed consent was obtained
from all the patients or their families.
Patients included in the study agreed to undergo the
following protocol: Newborns were immediately transferred into the
intensive care unit for treatment after birth; the mother accepted
ultrasound examination to monitor fetal and amniotic fluid
conditions following admission to hospital; the mother underwent
laboratory tests following admission to measure levels of
C-reactive protein (CRP) and white blood cell count; and the
neonatal outcomes were effectively followed-up. Patients were
excluded if they exhibited any gestational diabetes, gestational
hypertension, heart disease or if they refused to participate in
the research or effective follow-up.
Parameters
Maternal clinical indicators, including age,
gravidity, gestational age, body temperature, white blood cell
count and CRP level were summarized and compared between the two
groups. In addition, the rates of preterm incidence, cesarean
section, puerperal infection, postpartum hemorrhage, placenta
accreta, retained placenta and stillbirth were summarized and
compared between the two groups.
Finally, the birth weights, Apgar scores, infection
rates, incidence of respiratory distress syndrome, jaundice and
neonatal mortality rates of the two groups of newborns were
compared. The health conditions of the newborns were evaluated
using Apgar scoring as described by Dai, Zuo and Li (16), which includes skin color, breathing,
heart rate, reflection and muscle tension. The newborns with total
scores ≥7 were classed as healthy, those with scores <7 were
considered to suffer from asphyxia.
Biopsy
Placenta samples were extracted from the center of
the placental vertical section where the umbilical cord was
attached during cesarean section. The size of the tissue samples
was ~1×1×1 cm. Frozen 10-µm sections were prepared according to
normal procedures (17), followed by
fixation with cold acetone.
Hematoxylin and eosin (HE) staining
procedure
For conventional smear preparations, smear glass
slides were fixed with 95% ethanol for ≥15 min. The slides were
then treated with phosphate-buffered saline (PBS) for 1 min,
hematoxylin for 10 min, running water for 15 min, eosin for 1 min,
95% ethanol for 1 min and 100% ethanol for 2 min. Stained slides
were cover-slipped with Permount. Finally, the HE-stained cells
were examined under an SMT light microscope (1:7; binocular;
Shanghai Milite Precise Instrument Co., Ltd., Shanghai, China) at
x100-400 magnification. Milite Imaging Software was used for
visualization (Shanghai Milite Precise Instrument Co., Ltd.,
Shanghai, China). Acetone was purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Enzyme-linked immunosorbent assay
(ELISA)
MMP-2 concentrations in the sera of the patients
were determined using a commercially available ELISA kit (MMP-2
human ELISA kit; Life Technologies, Grand Island, NY, USA). The
experiment was repeated independently at least six times. The
results are expressed as the mean ± standard deviation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA USA) and mRNA was
reverse transcribed to cDNA using Reverse Transcriptase M-MLV
(Takara Biotechnology Co., Ltd, Dalian, China). PCR reactions were
conducted using a One-Step SYBR PrimeScript RT-PCR kit II (Takara
Biotechnology Co., Ltd). Relative expression levels of MMP-2 mRNA
were normalized against GAPDH. The primer sequences for MMP-2 and
GAPDH detection were as follows: MMP-2, F 5-AGG CTT AAC TGA TTA AGG
CAC-3 and R 5-GAT GGC TAC GAA TTC GAT AGC-3; GAPDH, F 5-CAT GCG CCT
CAC TAG TCA GCT-3′ and R 5-TAC GCT GAG GAT ACA GGA TAC-3. The qPCR
was performed using a 7500 Real-Time PCR system (Applied Biosystems
Life Technologies, Foster City, CA, USA). Each experiment was
repeated three times.
Western blot analysis
Total protein was prepared from the tissue samples
scraped from the uterus. The total proteins were separated on 10%
SDS-PAGE gels (Beyotime Institute of Biotechnology, Shanghai,
China) and transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were incubated with
monoclonal mouse primary antibodies against MMP-2 or GAPDH (1:200;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at
4°C. The membranes were subsequently washed in PBS then incubated
with secondary antibody conjugated to peroxidase (1:5,000; Santa
Cruz Biotechnology, Inc.) for 1 h. Subsequent to rinsing three
times, the membranes were visualized using an ECL chemiluminescence
kit (EMD Millipore). GAPDH was used for normalization. The relative
intensity of the target bands was analyzed by Quantity One 1-D
analysis software, version 4.6 (Bio-Rad Laboratories, Hercules, CA,
USA). Each assay was independently repeated three times.
Statistical analysis
All data were analyzed using SPSS software, version
18.0 (SPSS, Inc., Chicago, IL, USA). Measurement data are presented
as the mean ± standard deviation and the t-test was used for
comparison between groups. The count data are presented using
percentages and the χ2 test was used for comparison
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Subclinical chorioamnionitis
histologically affects the placenta and fetal membrane
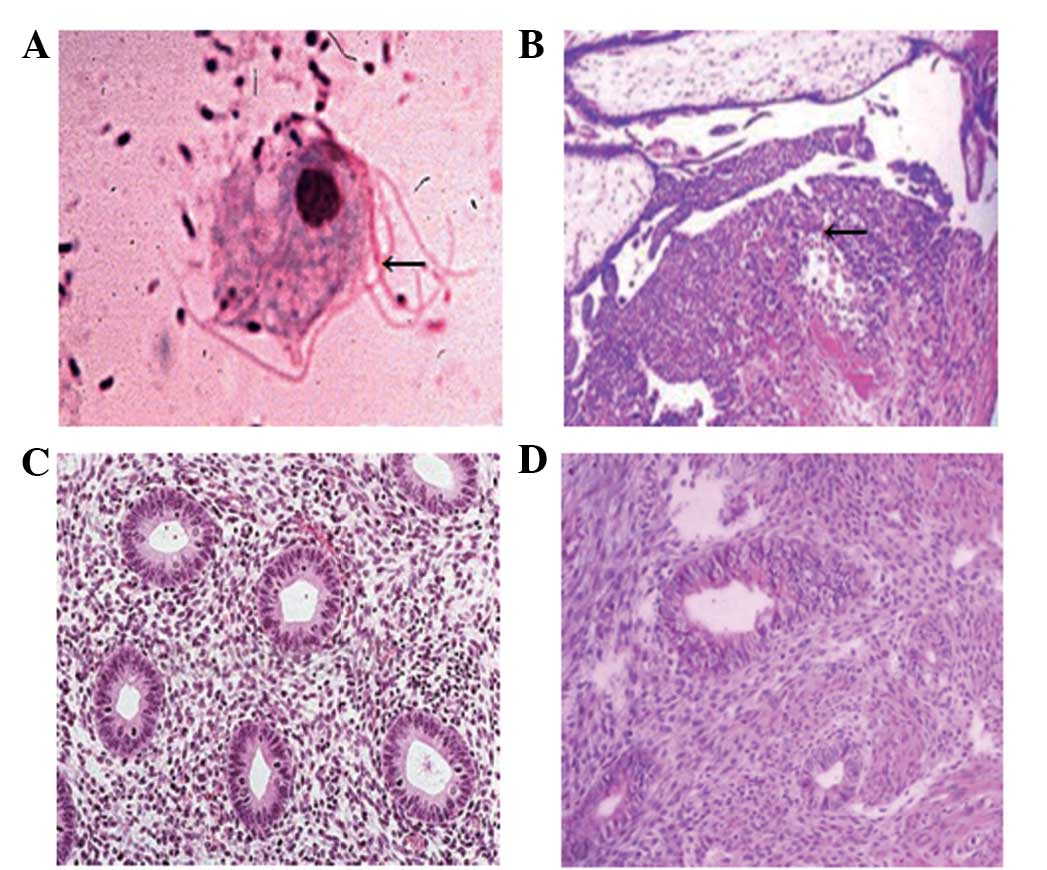
Histochemical analysis by optical microscopy was
used to investigate the placental pathology of the patients. The
experimental group consisted of 40 patients with subclinical
chorioamnionitis (Fig. 1A and B). By
contrast, no lesions of the placenta or fetal membrane were
observed in the 40 subjects in the control group (Fig. 1C and D). The data indicated that
subclinical chorioamnionitis impacted histologically on the
placenta and fetal membrane.
PROM combined with subclinical
chorioamnionitis significantly influences maternal clinical
indicators, clinical outcomes and neonatal outcomes
Statistical analyses were performed in order to
examine maternal clinical indicators, clinical outcomes and
neonatal outcomes. No statistically significant difference was
observed between the groups in age, gravidity or body temperature
(P>0.05), while the gestational age, white blood cell count and
CRP expression level did exhibit statistically significant
differences (P<0.05; Table I).
The incidence of preterm birth and the rates of cesarean section,
postpartum hemorrhage, puerperal infection, placenta accreta,
retained placenta and stillbirth were all significantly higher in
the experimental group compared with the control group (P<0.05;
Table II). With regard to the
neonatal outcomes, 36/40 newborns survived in the experimental
group, while 40/40 newborns survived in the control group. The
average body weight of newborns in the experimental group was lower
than that of the control group (t = 5.879; P<0.05).
Statistically significant differences were identified between the
two groups, in the Apgar scores, infection rates, incidence of
respiratory distress syndrome, incidence of jaundice and stillbirth
rates of the newborns (P<0.05; Table III). These results suggest that
PROM combined with subclinical chorioamnionitis significant
influenced maternal clinical indicators, clinical outcomes and
neonatal outcomes.
 | Table I.Clinical indicators of the two
maternal groups. |
Table I.
Clinical indicators of the two
maternal groups.
| Group | Age (years) | Gravidity | Body temperature
(°C) | Gestation age
(weeks) | White blood cell
count (x109/l) | CRP level (mg/l) |
|---|
| aExperimental (n=40) | 28.0±2.0 | 3.5±1.0 | 37.0±0.2 | 32.0±1.0 | 14.0±3.0 | 12.9±5.5 |
| aControl (n=40) | 27.0±2.5 | 3.0±1.0 | 37.1±0.2 | 36.0±1.0 | 9.0±1.0 | 7.0±3.5 |
| t-test | 1.975 | 2.236 | 2.237 | 17.888 | 10.000 | 5.724 |
| P-value | >0.05 | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 |
 | Table II.Comparison of the clinical outcomes of
the two maternal groups [n (%)]. |
Table II.
Comparison of the clinical outcomes of
the two maternal groups [n (%)].
| Group | Incidence of
preterm | Cesarian section | Postpartum
hemorrhage | Puerperal
infection | Placenta accreta | Retained
placenta | Stillbirth |
|---|
| Experimental
(n=40) | 25 (62.5) | 30 (75.0) | 15 (37.5) | 18 (45.0) | 8 (20.0) | 9 (22.5) | 4 (10.0) |
| Control (n=40) | 8 (20.0) | 10 (25.0) | 4 (10.0) | 7 (17.5) | 4 (10.0) | 3 (7.5) | 0 (0) |
| χ2 | 37.267 | 50.000 | 20.881 | 17.600 | 3.922 | 8.823 | 10.526 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
 | Table III.Comparison of the neonatal outcomes of
the two groups of newborns [n (%)]. |
Table III.
Comparison of the neonatal outcomes of
the two groups of newborns [n (%)].
| Groups | Apgar score
<7 | Infection | Respiratory distress
syndrome | Jaundice | Neonatal
mortality | Birth weight
(kg) |
|---|
| Experimental group
(n=36) | 18 (50.0) | 8 (22.2) | 14 (38.9) | 7 (19.4) | 4 (10.0) | 1.70±0.35 |
| Control group
(n=40) | 5 (12.5) | 2 (5.0) | 5 (12.5) | 3 (7.5) | 0 (0) | 2.23±0.45 |
| χ2 | 32.727 | 12.588 | 18.250 | 6.080 | 10.526 | 5.879 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
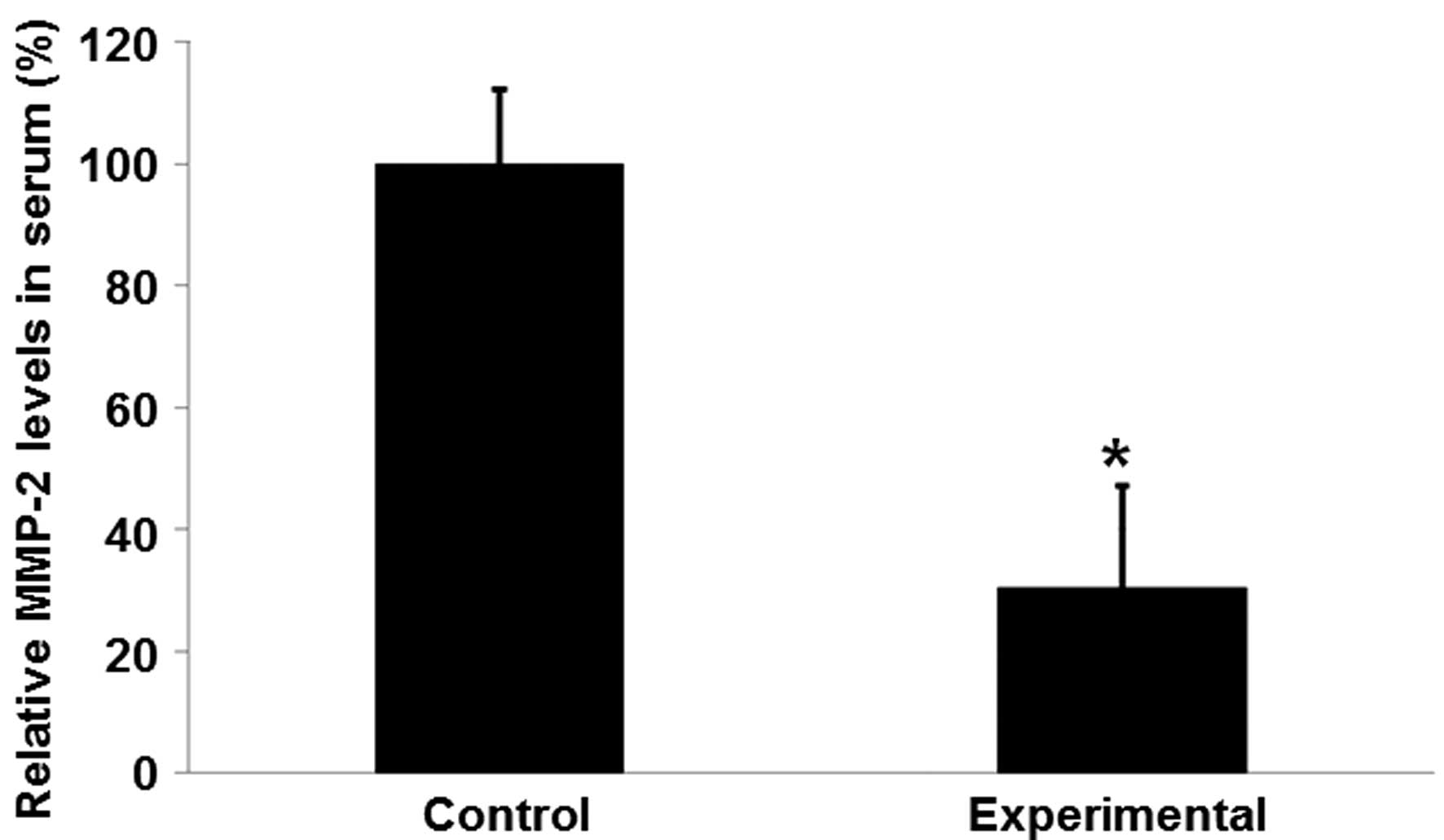
MMP-2 concentrations in the serum of
the experimental group are reduced, compared with those in the
control group
Serum samples were collected and subjected to ELISA
in order to investigate the effects of PROM combined with
subclinical chorioamnionitis on MMP-2 serum concentrations. As
presented in Fig. 2, the mean MMP-2
concentration in the experimental group was significantly reduced
compared with that in the control group (P<0.05). These results
suggest that reduced serum MMP-2 levels may be associated with
rupture of the fetal membrane combined with subclinical
chorioamnionitis.
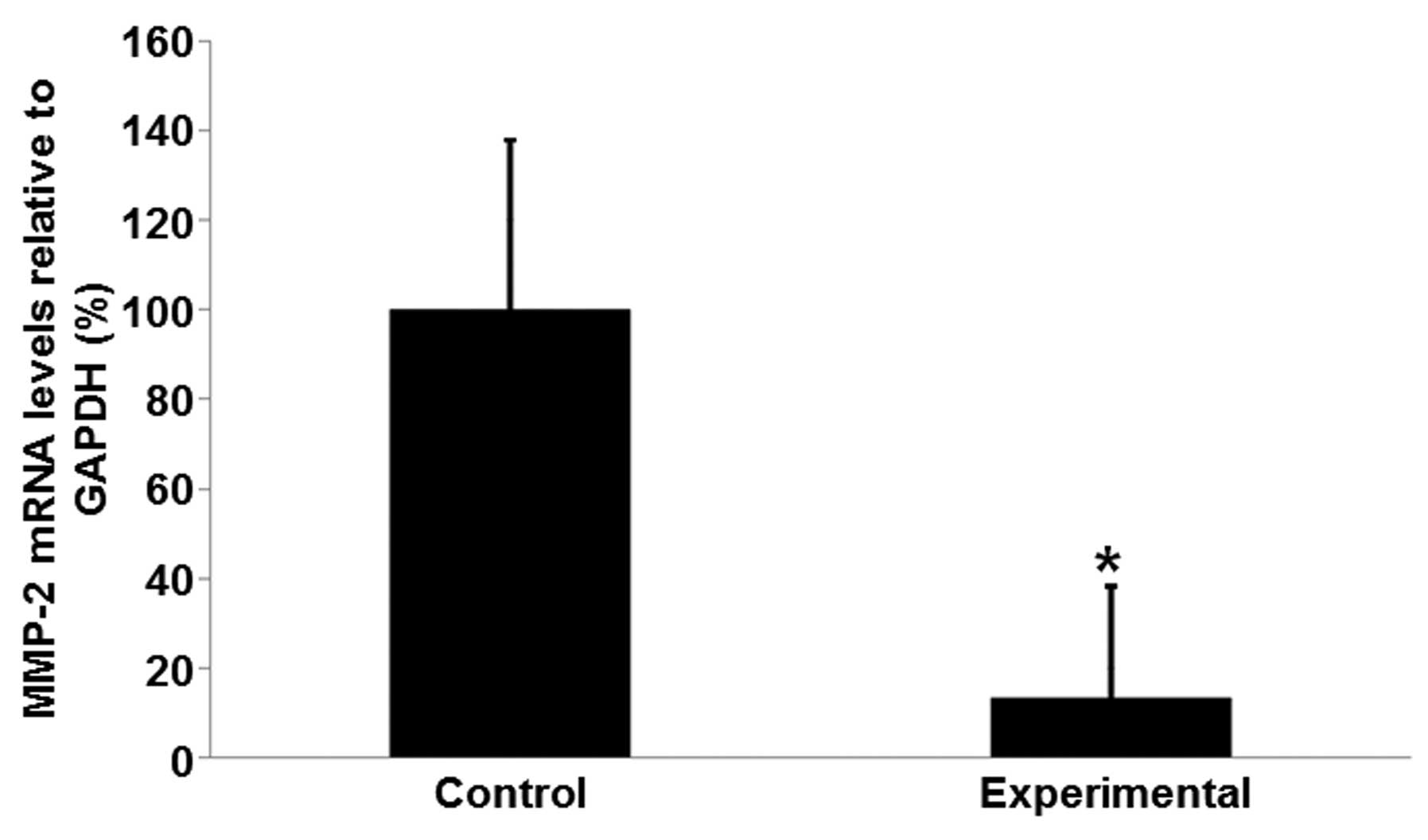
MMP-2 mRNA expression levels in the
experimental group are reduced, compared with those in the control
group
Total RNA was isolated from tissue samples from the
patients in order to further determine if the MMP-2 levels are
associated with rupture of the fetal membrane combined with
subclinical chorioamnionitis. Analysis with qPCR indicated that the
mRNA expression levels of MMP-2 in the experimental group were
significantly lower than those in the control group (Fig. 3). The mean mRNA expression level of
MMP-2 was 13.74% of the mean level in the control group (Fig. 3; P<0.05). These results suggest
that MMP-2 mRNA expression levels are downregulated in patients
with rupture of the fetal membrane combined with subclinical
chorioamnionitis.
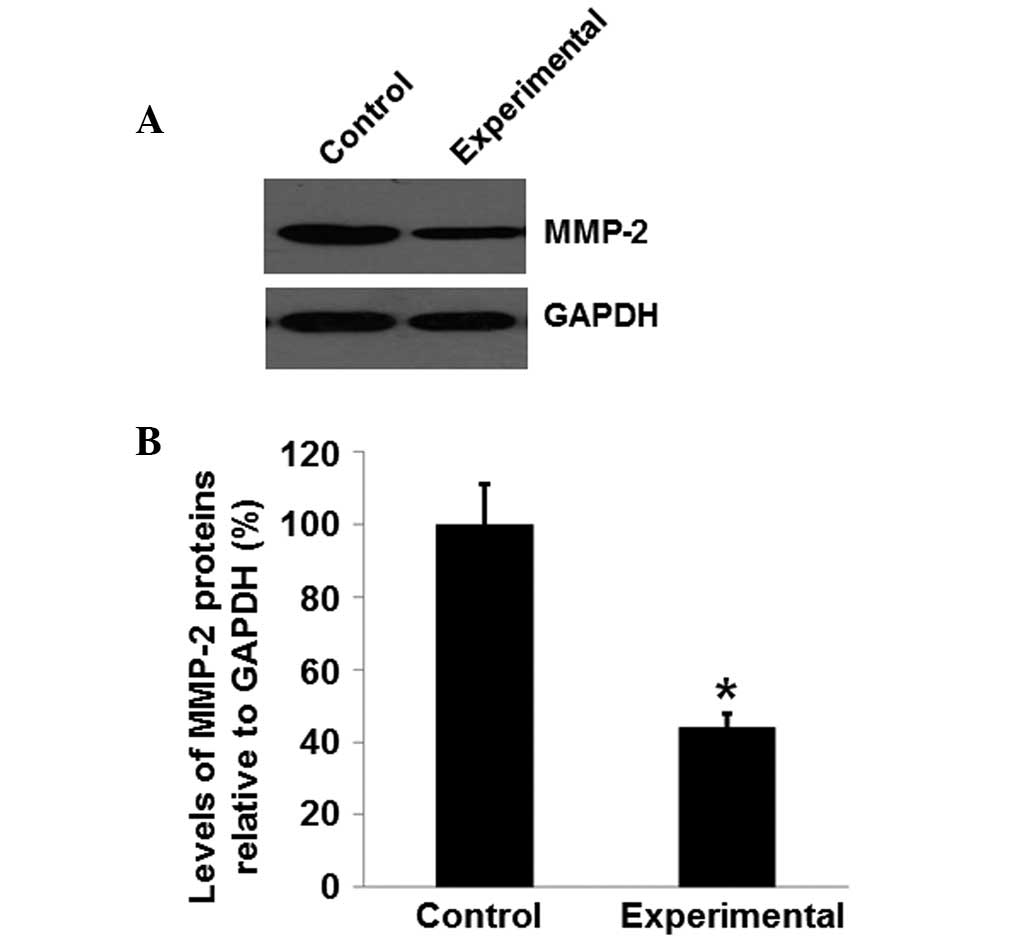
MMP-2 protein expression levels in the
experimental group are reduced, compared with those in the control
group
Total proteins were isolated from tissue samples
from the patients in order to further determine if the MMP-2
protein expression levels may be associated with rupture of the
fetal membrane combined with subclinical chorioamnionitis. As
presented in Fig. 4A, protein levels
of MMP-2 in the experimental group were significantly reduced
compared with those in the control group (P<0.05). The mean
MMP-2 protein level in the experimental group was 42.23% of the
level in the control group (Fig.
4B). These results suggest that MMP-2 is downregulated in the
patients with rupture of the fetal membrane combined with
subclinical chorioamnionitis.
Discussion
The clinical symptoms of subclinical
chorioamnionitis may lead to long-term intrauterine infection that
can increase the possibility of fetal distress or stillbirth, in
addition to the rates of maternal placenta accreta, preterm birth
and cesarean section (18).
Furthermore, subclinical chorioamnionitis commonly leads to
intrauterine adhesion, which affects the smooth discharge of
maternal placenta following childbirth. As a result, the rates of
retained placenta, postpartum hemorrhage and puerperal infection
are higher in patients with PROM combined with subclinical
chorioamnionitis (19). The present
study demonstrated that the rates of preterm birth, cesarean
section, puerperal infection, postpartum hemorrhage, placenta
implantation, retained placenta and stillbirth were significantly
higher in the experimental group than those in the control group.
Although subclinical chorioamnionitis led to no evident clinical
symptoms, it remained a threat to maternal health and safety.
Numerous studies have demonstrated that subclinical
chorioamnionitis is closely associated with neonatal jaundice
incidence, infection rate and respiratory distress syndrome
incidence (20,21). The maternal occurrence of subclinical
chorioamnionitis has been demonstrated to enhance the risk of
intrauterine infection. Furthermore, the hypoxic conditions of
these fetuses were more severe than those of healthy fetuses; thus,
the risk of asphyxia in childbirth was notably increased (22). In addition, subclinical
chorioamnionitis has been demonstrated to reduce
glucocorticoid-induced kinase expression in fetuses, affect fetal
pulmonary gas exchange and induce pulmonary hypoplasia, resulting
in poor clinical neonatal outcomes (23). In the present study, the Apgar scores
and body weights of the experimental group of newborns were lower
than those in the control group. Furthermore, the rates of neonatal
mortality, respiratory distress syndrome, jaundice and infection
were higher in the experimental group than in the control group.
These results demonstrate that subclinical chorioamnionitis
produces a negative impact on the clinical outcomes of
newborns.
According to Been and Zimmermann (24), inflammatory responses in the body
rapidly produce CRP, a reactive protein located in the blood
plasma, the concentration of which is positively correlated with
the severity of inflammation and tissue injury. Therefore, the
monitoring of maternal CRP expression levels has important clinical
value in the prevention of chorioamnionitis. The present study
demonstrated that the maternal CRP expression levels in the
experimental group were significantly higher than in the control
group. Therefore, dynamic monitoring of CRP expression levels in
pregnant patients with PROM may aid in the choice of suitable
treatments, and the improvement of the outcomes of pregnancy.
MMP-2 is important in various processes (25–27). A
previous study indicated that MMP-2 may be associated with the PROM
(21). However, other reports
suggest that the relationship is uncertain (14,15). The
present study demonstrated that the MMP-2 mRNA and protein
expression levels were reduced in the experimental group compared
with the control group. In addition, ELISA results indicated that
the serum MMP-2 concentrations were reduced in the patients with
PROM. The limitation of the present study is the limited number of
cases. In the future, we will increase the size of studied
populations and investigate other proteins that are related to
premature rupture of the fetal membrane combined with subclinical
chorioamnionitis. In conclusion, PROM combined with subclinical
chorioamnionitis was indicated to be associated with reduced levels
of MMP-2.
Acknowledgements
The current study was supported by a grant from the
Special Funds for the Construction of High-level Universities in
Shaanxi Province, China (no. 2013SXTS02).
References
|
1
|
Vallejo MC, Kaul B, Adler LJ, et al:
Chorioamnionitis, not epidural analgesia, is associated with
maternal fever during labour. Can J Anaesth. 48:1122–1126. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang H: Correlation between the time of
premature rupture of membranes and chorioamnionitis. Zhong Guo Bao
Jian Ying Yang. 20:4247–4248. 2012.(In Chinese).
|
|
3
|
Liu J, Tang A and Chen M: Research on the
correlation between the time of premature rupture of membranes and
chorioamnionitis. Zhong Hua Zhong Xi Yi Xue Za Zhi. 19:14–15.
2012.(In Chinese).
|
|
4
|
Cui XF and Lou JY: The relationship
between fetal fibronectin and chorionitis of premature rupture of
membranes. Zhong Guo You Sheng Yu Yi Chuan Za Zhi She. 6:79–80.
2007.(In Chinese).
|
|
5
|
Aguin E, Van De Ven C, Cordoba M, Albayrak
S and Bahado-Singh R: Cerclage retention versus removal following
preterm premature rupture of membranes and association with
amniotic fluid markers. Int J Gynaecol Obstet. 125:37–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fortner KB, Grotegut CA, Ransom CE, et al:
Bacteria localization and chorion thinning among preterm premature
rupture of membranes. PLoS One. 9:e833382014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Magee B and Smith G: Histological
chorioamnionitis associated with preterm prelabour rupture of
membranes at Kingston General Hospital: a practice audit. J Obstet
Gynaecol Can. 35:1083–1089. 2013.PubMed/NCBI
|
|
8
|
Chauhan SP and Ananth CV: Periviable
births: epidemiology and obstetrical antecedents. Semin Perinatol.
37:382–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stanek J and Biesiada J: Relation of
placental diagnosis in stillbirth to fetal maceration and
gestational age at delivery. J Perinat Med. 42:457–471.
2014.PubMed/NCBI
|
|
10
|
Liotta LA, Tryggvason K, Garbisa A, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
12
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koucký M, Germanová A, Kalousová M, et al:
Low maternal serum matrix metalloproteinase (MMP)-2 concentrations
are associated with preterm labor and fetal inflammatory response.
J Perinat Med. 38:589–596. 2010.PubMed/NCBI
|
|
14
|
Maymon E, Romero R, Pacora P, et al: A
role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2)
in human parturition, premature rupture of membranes and
intraamniotic infection. J Perinat Med. 29:308–316. 2001.PubMed/NCBI
|
|
15
|
La Sala GB, Ardizzoni A, Capodanno F, et
al: Protein microarrays on midtrimester amniotic fluids: a novel
approach for the diagnosis of early intrauterine inflammation
related to preterm delivery. Int J Immunopathol Pharmacol.
25:1029–1040. 2012.PubMed/NCBI
|
|
16
|
Dai B, Zuo F and Li Q: Correlation
analysis of time of premature rupture of membranes with
chorioamnionitis. Shong Guo Fu You Bao Jian. 23:3227–3228. 2008.(In
Chinese).
|
|
17
|
Zhang W, Zhou L and Dang Y: Analysis of
related factors of occult chorioamnionitis in mature pregnancy.
Zhong Hua Yi Xue Za Zhi. 9:618–620. 2010.(In Chinese).
|
|
18
|
Chen X: Clinical analysis of 102 cases of
premature rupture of membranes. Zhong Guo Yi Yao Zhi Nan.
18:193–200. 2012.(In Chinese).
|
|
19
|
He L: Clinical analysis of 117 cases of
fetal distress caused by premature rupture of membranes. Zhong Guo
Yi Yao Zhi Nan. 30:110–111. 2012.(In Chinese).
|
|
20
|
Bian X: Cause of premature rupture of
membranes in early birth and progress in treatments. Zhong Wai Yi
Liao. 33:191–192. 2012.(In Chinese).
|
|
21
|
Xie A, Di X, Chen X, et al: Factors
influencing chorioamnionitis with premature rupture of membranes in
early birth and the outcome of newborn. Zhong Hua Fu Chan Ke Za
Zhi. 47:105–108. 2011.(In Chinese).
|
|
22
|
Dang Y, Ma X and Zhang W: Analysis of
pregnancy outcomes of 185 cases of premature rupture of membranes.
Shan Xi Yi Xue Za Zhi. 38:69–77. 2009.(In Chinese).
|
|
23
|
Wang X, Li L and Cui S: The effect of type
III collagen, CTGF and TNF-α in the pathologic mechanisms of
premature rupture of membranes. Si Chuan Da Xue Due Bao. 40:58–60.
2009.
|
|
24
|
Been JV and Zimmermann LJ: Histological
chorioamnionitis and respiratory outcome in preterm infants. Arch
Dis Child Fetal Neonatal Ed. 94:F218–F225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi H, Liu L, Liu L, Geng J, Zhou Y and
Chen L: β-Elemene inhibits the metastasis of B16F10 melanoma cells
by downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9.
Melanoma Res. 24:99–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malz R, Weithaeuser A, Tschöpe C,
Schultheiss HP and Rauch U: Inhibition of coagulation factor Xa
improves myocardial function during CVB3-induced myocarditis.
Cardiovasc Ther. 32:113–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pandurangan A, Dharmalingam P, Sadagopan S
and Ganapasam S: Luteolin inhibits matrix metalloproteinase 9 and 2
in azoxymethane-induced colon carcinogenesis. Hum Exp Toxicol.
33:1176–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|