Introduction
Intestinal ischemia-reperfusion (IR) is a common
symptom of various diseases, including acute mesenteric ischemia,
small bowel transplantation, abdominal aortic aneurysm,
hemorrhagic, traumatic or septic shock and severe burn wounds
(1). Numerous mediators and
processes are involved in the pathogenesis of IR-induced intestinal
injury, including reactive oxygen/nitrogen species,
pro-inflammatory cytokines and leukocyte adhesion/infiltration
(2–3). It is established that apoptosis and
inflammation are increased significantly during IR in the gut and
may serve key functions in the pathogenesis of IR-induced
intestinal injury. Intestinal IR models have been used frequently
in the study of apoptosis (4). There
is a requirement for the identification of experimental agents that
may be administered as adjunctive therapy to surgery in order to
mitigate intestinal IR injury (5–7).
However, these experimental agents may not be administrable during
surgery or in the intensive care unit (ICU) (8). Therefore, the effects of anesthetic and
sedative agents on IR injury may be significant factors in patient
outcomes and thus require further study.
Dexmedetomidine (DEX) is a potent and selective α2
adrenergic receptor agonist. Clinically, DEX has been used as an
adjunct to anesthesia, analgesia and ICU sedation (9). In addition, DEX offers good
perioperative hemodynamic stability and reduces intraoperative
anesthetic requirements; a number of prior studies have
demonstrated that DEX reduces intestinal IR injury (10,11).
However, the effects of DEX on IR-associated reduced contractility
of intestinal smooth muscle remains unclear, and there are a
limited number of studies addressing the effects of DEX on
apoptosis and inflammation in IR-induced intestinal injury.
The aim of the present study was to investigate the
effects of DEX on intestinal contractile activity, inflammation and
apoptosis in a rat model of IR-induced intestinal injury. These
effects were examined via the evaluation of acetylcholine (Ach) and
potassium chloride (KCl)-induced contractile responses. In
addition, the protein levels of nitric oxide (NO), tumor necrosis
factor (TNF)-α, interleukin (IL)-6, Bax and Bcl-2, and the mRNA
expression levels of telomerase and caspase-3 were determined.
Materials and methods
TNF-α and IL-6 enzyme-linked
immunosorbent assay (ELISA)
ELISA kits were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China) and all other reagents
were purchased from commercial sources.
Animals
Male Sprague-Dawley rats (age range, 2–2.5 months)
were purchased from the animal facility of Qingdao Medical
University, Qingdao, China. The present study was approved by the
local Medical Ethics Committee of Qingdao Women and Children's
Hospital, Qingdao, China. Rats were reared under standard
laboratory conditions (22±2°C, 60±10% relative humidity and a 12-h
light-dark cycle) and had free access to food and water, but fasted
overnight prior to the experiments.
Induction of IR injury
Rats were anesthetized intraperitoneally (i.p.) with
ketamine (100 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and
chlorpromazine (0.75 mg/kg; Sigma-Aldrich). Following induction of
anesthesia, the abdomen was opened with a midline abdominal
incision. Intestinal IR injury was produced by complete occlusion
of the superior mesenteric artery followed by a period of
reperfusion. The superior mesenteric artery was clamped for 60 min.
Following 60 min of ischemia, the vascular clamp at the superior
mesenteric artery was removed and three drops of 2% lidocaine
(Sigma-Aldrich) were applied directly to the superior mesenteric
artery to facilitate reperfusion. Blood circulation was restarted
for a 60-min reperfusion period. Following the reperfusion period,
the rats were euthanized with an overdose of ketamine (200 mg/kg)
and chlorpromazine (1 mg/kg). A 20-cm incision was made 1 cm distal
to the ileocecal junction. The internal cavity was exposed and
feces were cleaned, then washed with phosphate-buffered saline
(PBS; Sigma-Aldrich) and dried. A mucosal smear of the small
intestine was collected with a glass slide.
Experimental protocol
Rats were selected at random and divided into three
groups (n=10 in each group) as follows: Sham group rats underwent
an abdominal incision and their organs were exposed for 120 min,
but without clamping of the mesenteric artery, in order to
distinguish the differences between the effects of intestinal IR
and those of non-specific surgical stress; IR group rats received
an i.p. injection of normal saline (10 ml/kg) 30 min prior to the
intestinal IR; and IR + DEX group rats received an i.p. injection
of 25 µg/kg DEX dissolved in normal saline 30 min prior to the
intestinal IR. The dose of DEX administered was based on a previous
study (10).
The animals in the IR and IR + DEX groups, to which
normal saline or DEX were applied, were euthanized 2.5 h after the
injections. The sham group rats were euthanized 2 h after the sham
operation in order to imitate the conditions of the rats in the IR
group. Subsequent to the experiments, samples from the jejunum were
collected from the animals for examination. Immediately following
euthanasia, the rat intestinal tissues were rapidly removed and
cleaned with PBS and frozen in liquid nitrogen (Tiangen Biotech Co.
Ltd., Beijing, China). Tissues were homogenized (10% wt/vol) in
ice-cold 10 mM phosphate buffer (pH 7.4), sonicated (Ultrasonic
Instruments, Misonix, MI, USA) on ice for 15 sec and centrifuged at
3,000 × g for 20 min. The resulting supernatants were collected for
biochemical assays.
Preparation of terminal ileum
Ileal longitudinal muscle contractile activity was
evaluated in isolated ileal segments following 1-h reperfusion in
an organ bath (12). Strips of
longitudinal muscle were removed 1 cm cephalad of the ileocecal
junction. Strips were longitudinally suspended under a 2-g load in
an organ bath containing 20 ml Kreb's solution (in mM: NaCl, 118.5;
KCl, 4.8; KH2PO4, 1.2;
MgSO4.7H2O, 1.2; CaCl2, 1.9;
NaHCO3, 25; and glucose, 10.1). The solution was
continually gassed with a mixture of 5% CO2 and 95%
O2 and maintained at 3°C. After 60-min equilibration
with a 2-g load, Ach was added to the organ bath fluid at a final
concentration of 10−6 M. Following 60-min equilibration
with a 2-g load, KCl was added to the organ bath separately at a
final concentration of 30 mM, in order to measure changes in the
contractile responses of the samples. In the preparation of high
K+ solutions, NaCl was exchanged for equimolar amounts
of KCl, in order to maintain the physiological osmolarity of the
Krebs solution. Drugs were prepared daily in distilled water and
stored in ice during the course of the experiments. Isometric force
was monitored with an external force displacement transducer using
a BL-420F Data Acquisition & Analysis System (Chengdu Taimeng
Science and Technology Co., Ltd., Chengdu, China).
Measurement of NO levels
The tissue levels of nitrite
(NO2−) and nitrate
(NO3−) were measured in order to estimate NO
production, as NO2− and
NO3− are stable NO oxidative metabolites.
Quantification of NO2− and
NO3− was based on the Griess reaction, in
which a chromophore with a strong absorbance at 550 nm is formed by
the reaction of NO2− with a mixture of 0.01%
naphthyl ethylenediamine, 1% sulfanilamide and 5%
H3PO4 (5,7,8). The results are expressed as µmol/g
protein.
ELISA analysis of IL-6, TNF-α, Bcl-2
and Bax levels
IL-6, TNF-α, Bcl-2 and Bax expression levels in the
intestinal tissues were detected using commercial ELISA kits
(Tiangen Biotech). The association between optical density (OD) and
cytokine concentration was defined using the standard curve
according to the manufacturers instructions. These expression
levels are expressed as pg/ml.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of telomerase and
caspase-3
Total RNA from the jejunum was isolated using TRIzol
reagent (Gibco Life Technologies, Carlsbad, CA, USA) according to
the manufacturers instructions (13). RNA was quantified by measuring the OD
at 260 nm and reverse transcribed into single-stranded cDNA using a
RevertAid H Minus First Strand cDNA Synthesis kit (#K1632; Thermo
Fisher Scientific, Pittsburgh, PA, USA). The cDNA was amplified for
35 cycles in order to maintain the PCR product in the linear range.
The PCRs were performed at 94°C for 5 min, followed by 35 cycles at
94°C for 30 sec, annealing at the corresponding temperature
(Table I) for 30 sec and 72°C for 30
sec, and a final extension at 72°C for 8 min. PCR was performed
using a Golden Easy PCR System (#KT221; Tiangen Biotech) with
gene-specific primers for β-actin, telomerase and caspase-3
(Table I). β-Actin was used as an
internal control to confirm mRNA integrity. The identities of all
PCR products were confirmed by size, based on the known length of
the DNA sequence on 1% agarose gel (Tiangen Biotech) stained by
ethidium bromide (Tiangen Biotech). The OD was analyzed using a
GeneSnap system (version 2.0; Syngene, Frederick, MD, USA).
 | Table I.Primer sequences for mRNA fragments
amplified by quantitative polymerase chain reaction. |
Table I.
Primer sequences for mRNA fragments
amplified by quantitative polymerase chain reaction.
| Gene | Primer sequences |
|---|
| Human telomerase
reverse transcriptase |
5-GCAGAATTCATGCCAGGGACTCCCCGCAGGTTG-3 |
|
|
5′-CGGGTCGACTTACTCGTAGTTGAGGACGCTGAAC-3 |
| Caspase-3 |
5-TGTCGATGCAGCTAACC-3 |
|
|
5-GGCCTCCACTGGTATCTTCTG-3 |
| β-Actin |
5-GGAAATCGTGCGTGAC-3 |
|
|
5-GGAAGGTGGACAGTGAG-3 |
Statistical analysis
Values are expressed as the mean ± standard error.
One-way analysis of variance was used, followed by Fishers
least-significant difference test for the homogeneity testing of
variance (Levenes test). Data were analyzed using Dunnetts T3 test
for the heteroscedasticity of variance test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were conducted using SPSS software, version
13.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
DEX inhibits IR-induced attenuation of
ileal longitudinal muscle contractility
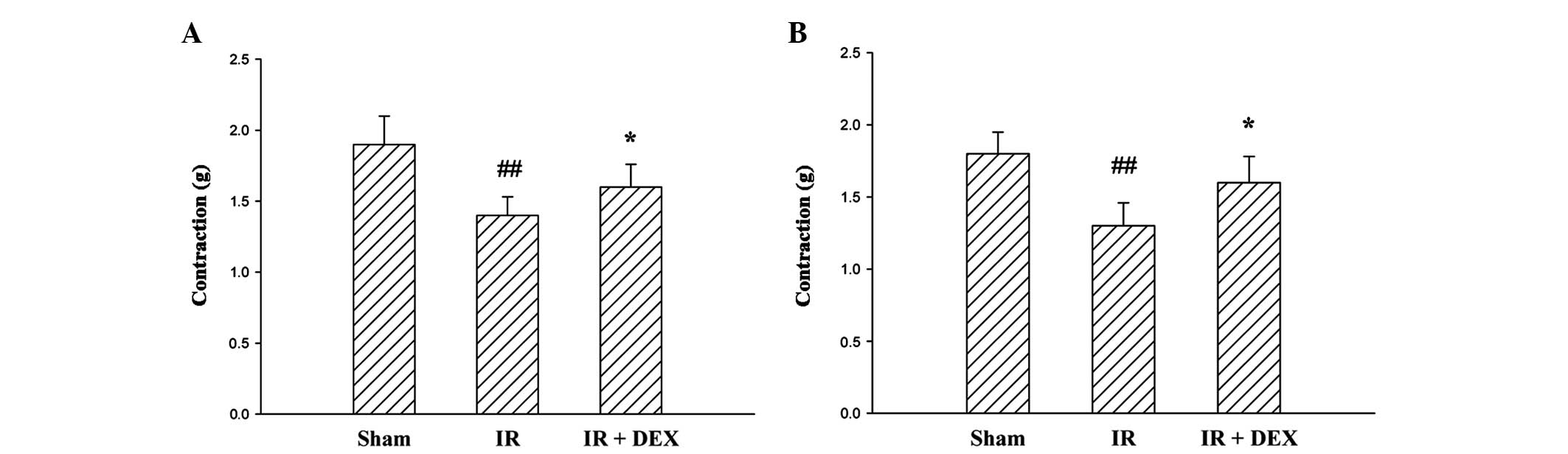
The contractile responses induced by Ach were
significantly inhibited by the induction of IR (P<0.01; Fig. 1A). Administration of DEX at a dose of
25 µg/kg resulted in a significant reduction in the inhibition of
contractility observed due to IR (P<0.05).
The addition of KCl at a final concentration of 30
mM into the organ bath fluid resulted in the contraction of the
terminal ileum segment (Fig. 1B).
The contraction obtained from the tissues of rats in the IR group
was reduced compared with the sham group rats (P<0.01). The
contractile response of the IR + DEX group rats following treatment
with 30 mM KCl was similar to that of the rats in the sham group.
The administration of DEX (25 µg/kg) to the IR + DEX group resulted
in an amelioration of the KCl-induced contractile response in the
ischemic tissue (P<0.05).
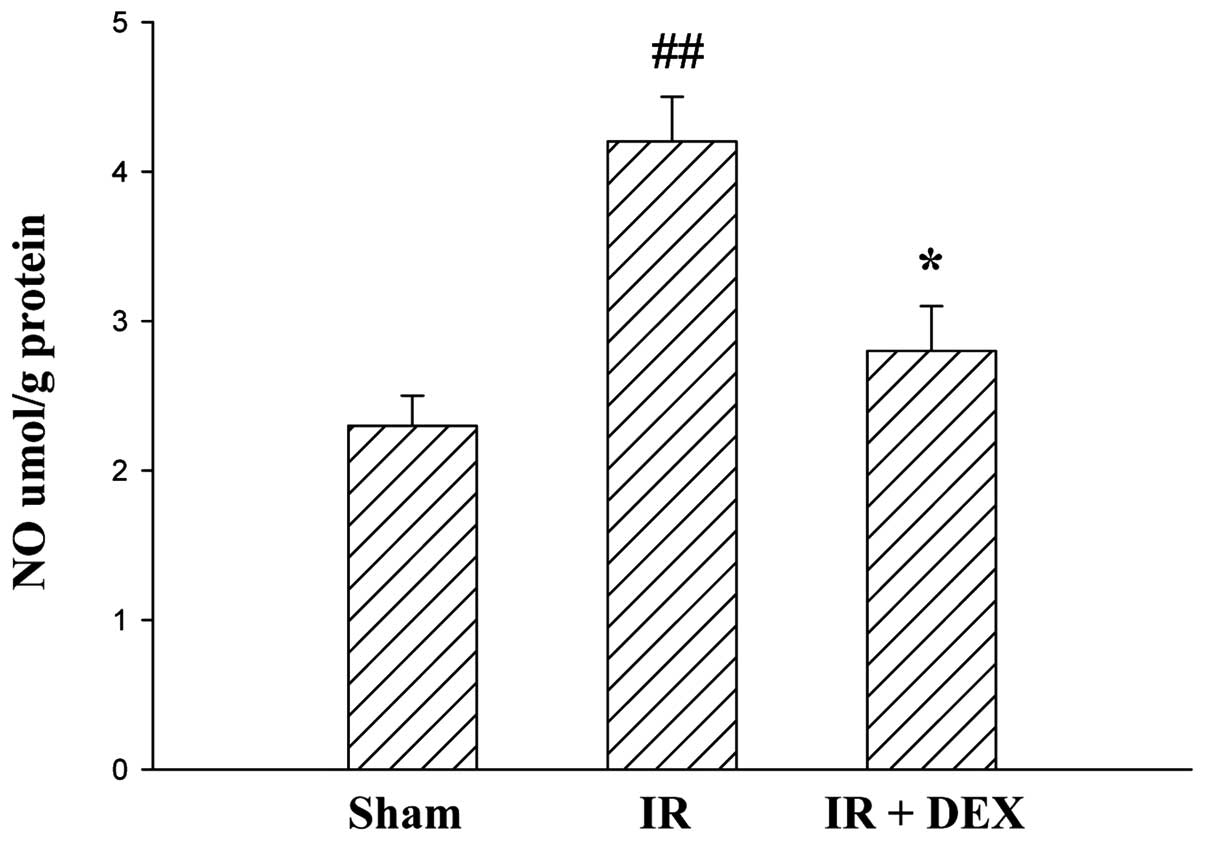
DEX reduces IR-induced increase in
intestinal NO levels
The concentrations of NO in intestinal tissues of
the groups are presented in Fig. 2.
A significant increase in NO levels was observed in the IR group
compared with the sham group (P<0.01). By contrast, the IR + DEX
group exhibited a notable reduction in NO levels compared with the
IR group (P<0.05).
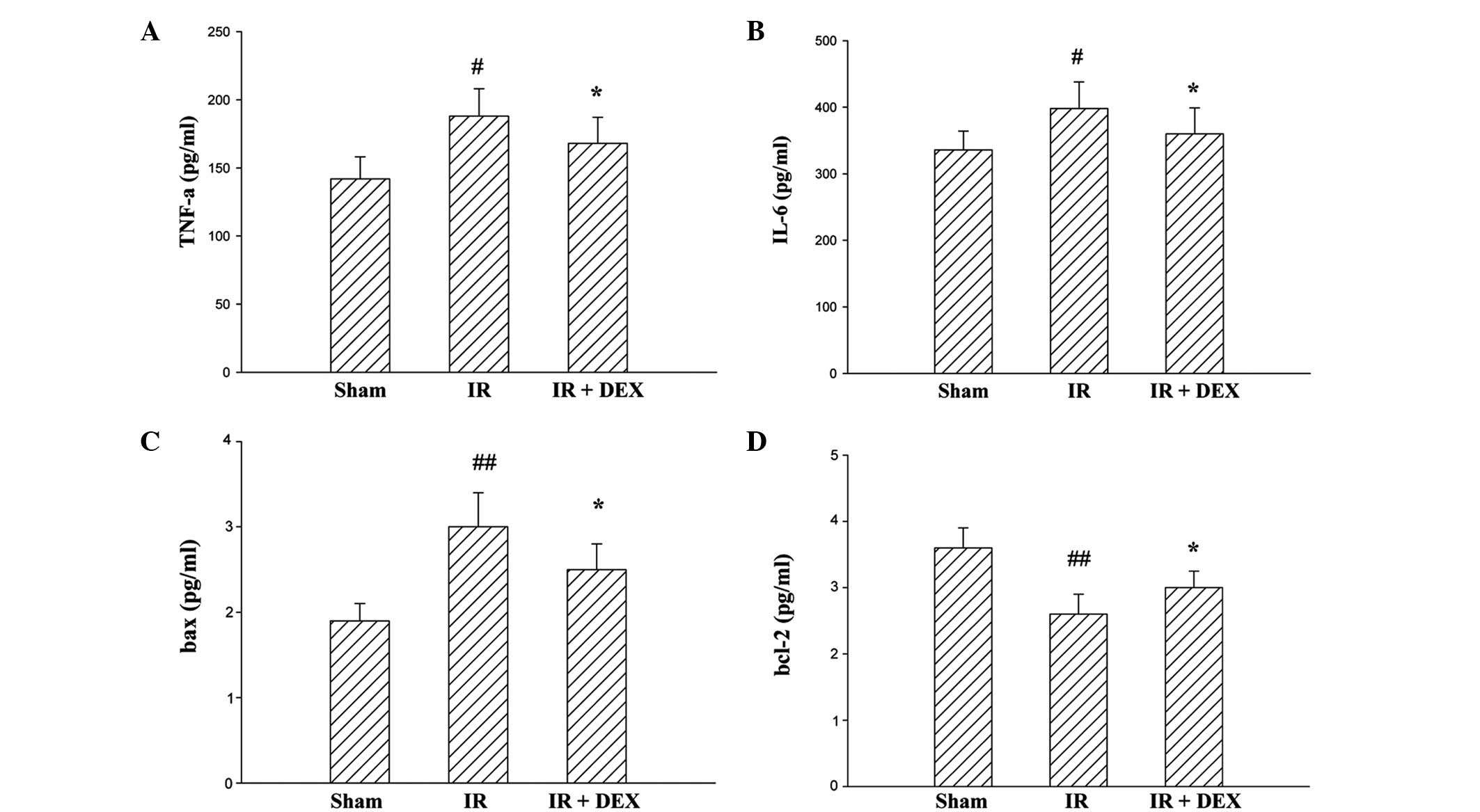
Levels of TNF-α, IL-6 and protein
expressions of Bax and Bcl-2 on intestinal IR injury
To further assess intestinal IR injury, levels of
TNF-α, IL-6, Bax and Bcl-2 were also analyzed (Fig. 3). Compared with the sham group, the
expression levels of TNF-α, IL-6 (P<0.05) and Bax (P<0.01)
were significantly increased in the IR group rats; and this
increase was significantly inhibited in the IR + DEX group rats
(P<0.05). Furthermore, a reduction in tissue Bcl-2 level was
observed following intestinal IR injury. The Bcl-2 content was
significantly higher in the IR + DEX treated group compared with
the IR group (P<0.05). These results indicate that the DEX
pretreatment markedly attenuated intestinal IR injury.
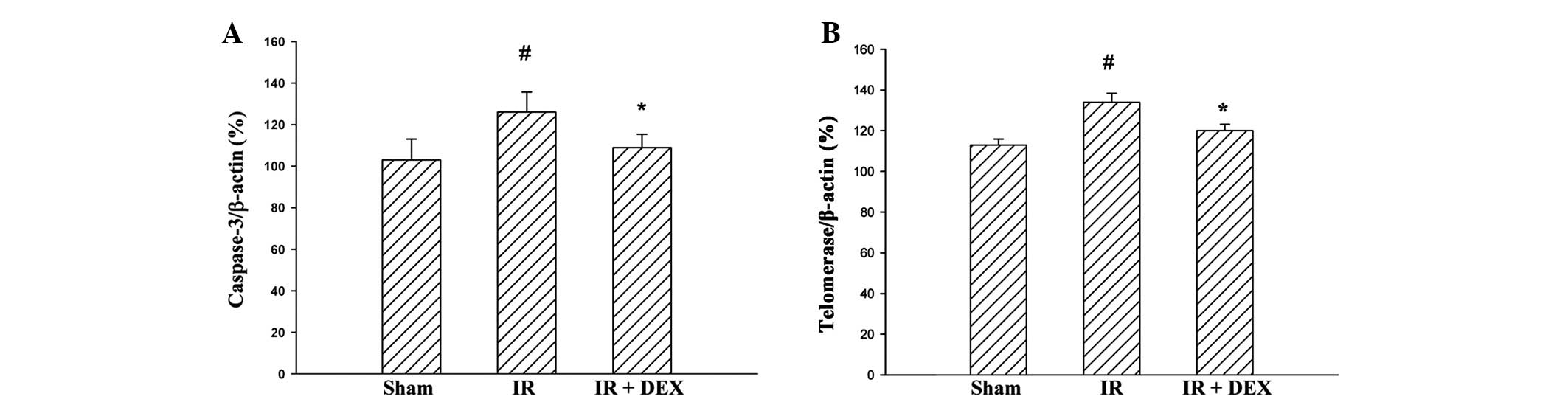
DEX suppresses the IR injury-induced
increase in levels of telomerase and caspase-3 in the jejunum
The effects of DEX on intestinal cell survival
following IR injury were investigated by analyzing the
transcriptional levels of associated genes (telomerase and
caspase-3) following DEX pretreatment. The mRNA expression levels
of telomerase and caspase-3 were enhanced in the IR group compared
with the sham group (P<0.05; Fig.
4). Pretreatment with DEX resulted in a significant reduction
in the mRNA expression levels of telomerase and caspase-3 compared
with the IR group (P<0.05).
Discussion
The present study evaluated the effects of DEX
pretreatment in small intestinal tissue with IR-induced injury. The
results demonstrated that intestinal IR damage led to a reduction
in ileal contractility in response to Ach (receptor-mediated) and
KCl (non-receptor-mediated) induction. Administration of 25 µg/kg
DEX appeared to mitigate this reduction in contractile response.
Intestinal IR induces an inflammatory response within the area of
muscle cells that results in the recruitment and extravasation of
leukocytes into the smooth muscle syncytium (14). Reactive oxygen species and
inflammatory leukocytes are reportedly involved in the progression
of intestinal IR-induced remote organ injury (15). NO, as a toxic metabolite, may serve a
key function in the initiation of intestinal mucosal injury
(15). NO is beneficial as a
messenger or modulator; however, under conditions such as oxidative
stress, NO is potentially toxic. High levels of exogenous NO exert
a cytopathic effect on the intestine that increases the extent of
mucosal injury (15–17). Potoka et al (15) suggested that peroxynitrite may be
able to induce enterocyte apoptosis via a number of mechanisms,
including the inhibition of mitochondrial function, adenosine
triphosphate depletion, activation of caspases via cytochrome
c, mitochondrial release of apoptosis-activating factor-1
and the activation of poly(ADP-ribose) synthetase. Additionally,
peroxynitrate may influence the inhibition of enterocyte
proliferation and differentiation in the intestinal crypts by
interfering with tyrosine kinase signaling cascades. NO, through
its toxic metabolite peroxynitrite, serves a major role in the
initiation of intestinal mucosal injury in clinical conditions
associated with sustained inducible NO synthase upregulation in the
gut (16,17). In the present study, NO levels of the
IR group rats were significantly higher compared with the sham
group rats. In addition, the NO levels of the IR + DEX group were
significantly reduced compared with the IR group. Thus, the results
indicated that NO is a critical mediator of the inflammatory
response during the development of intestinal injury, and that DEX
significantly reduces intestinal tissue levels of NO.
Previous studies have demonstrated that DEX inhibits
the expression of a number of inflammatory mediators, including NO,
prostaglandin E2, TNF-α and IL-6 (18). In previous studies, treatment with
DEX led to a reduction in the expression levels of TNF-α in
ischemic hippocampal tissue, and a reduction of the TNF-α and IL-6
concentrations in endotoxin-exposed rats (19,20).
Furthermore, a prior study demonstrated a similar reduction in
TNF-α and IL-6 levels in an experimental spinal cord injury
(21). These experimental data were
supported by clinical studies demonstrating reduced TNF-α and IL-6
levels in critically ill patients with sepsis or postoperative
major surgery (22,23). Therefore, the anti-inflammatory
effects of DEX may be responsible for the prevention of the
mesenteric artery occlusion induced by IR injury. The results of
the current study indicated that intestinal IR injury triggers the
production of pro-inflammatory cytokines and that DEX pretreatment
prevents this production. Intestinal IR injury has been
demonstrated to induce a significant increase in IL-6 levels in
intestine samples, which is consistent with the results of the
present study. Furthermore, the results of the present study are
consistent with prior studies (19),
suggesting that DEX exhibits anti-inflammatory effects.
In addition, the present study suggests that DEX
exerts a protective and anti-apoptotic effect against intestinal
ischemic injury. To the best of our knowledge, there are no
previous studies regarding the effects of DEX on apoptosis in
IR-induced intestinal injury. Proteins of the Bcl-2 family, as
anti-apoptotic proteins, induce and integrate cell survival and
death signals, associated with apoptosis in cells. By contrast, the
increased expression of the pro-apoptotic protein Bax mediates an
enhanced rate of apoptosis. In the present study, the expression
levels of Bax and the labeling index of caspase-3, a key caspase in
the apoptotic pathway, were markedly reduced in the intestinal
tissues of the IR + DEX group rats. Upregulation of Bcl-2 and DEX,
however, downregulated the expression of Bax.
The expression levels of telomerase reverse
transcriptase (TERT) significantly increase following stimulation
of the cerebellar fastigial nucleus (FN). TERT may bind to Bax and
inhibit Bax-mediated apoptosis by suppressing the mitochondrial
relocalization of Bax from the cytosol (24). Telomerase is an enzyme that adds a
six-base DNA repeat sequence (TTA GGG) to chromosome ends and
thereby prevents their shortening during successive rounds of
mitosis (25). In the present study,
IR induced telomerase activity in rat intestine samples. RT-qPCR
analyses demonstrated that the mRNA expression levels of telomerase
and caspase-3 were significantly reduced in the DEX group compared
with the IR group. These results indicate that DEX treatment may
inhibit IR-induced damage by modulating the expression of
telomerase and caspase-3.
In conclusion, the protective effects of DEX in the
intestine may be due to its anti-inflammatory and anti-apoptotic
properties. The results of the present study indicated that the
downregulation of telomerase and caspase-3 mRNA may be involved in
the protective effect of DEX against IR-induced damage. Further
studies are required to clarify the possible mechanisms underlying
this protective effect.
References
|
1
|
Shen J and Fu G, Jiang L, Xu J, Li L and
Fu G: Effect of dexmedetomidine pretreatment on lung injury
following intestinal ischemia-reperfusion. Exp Ther Med.
6:1359–1364. 2013.PubMed/NCBI
|
|
2
|
Xia G, Martin AE, Michalsky MP and Besner
GE: Heparin-binding EGF-like growth factor preserves crypt cell
proliferation and decreases bacterial translocation after
intestinal ischemia/reperfusion injury. J Pediatr Surg.
37:1081–1087. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El Assal ON and Besner GE: Heparin-binding
epidermal growth factor-like growth factor and intestinal
ischemia-reperfusion injury. Semin Pediatr Surg. 13:2–10. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujise T, Iwakiri R, Wu B, Amemori S,
Kakimoto T, Yokoyama F, Sakata Y, Tsunada S and Fujimoto K:
Apoptotic pathway in the rat small intestinal mucosa is different
between fasting and ischemia-reperfusion. Am J Physiol Gastrointest
Liver Physiol. 291:G110–G116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taha MO, Miranda-Ferreira R, Fagundes AL,
Fagundes DJ, Simões RS, Santos JM, Souza PD, Oliveira-Júnior IS,
Marchini A, Gomes IT, et al: Effects of L-nitro-arginine methyl
ester, an inhibitor of nitric oxide biosynthesis, on intestinal
ischemia/reperfusion injury in rabbits. Transplant Proc.
42:457–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang S, Chou WP and Pei L: Effects of
propofol on renal ischemia/reperfusion injury in rats. Exp Ther
Med. 6:1177–1183. 2013.PubMed/NCBI
|
|
7
|
Petrat F and de Groot H: Protection
against severe intestinal ischemia/reperfusion injury in rats by
intravenous resveratrol. J Surg Res. 167:e145–e155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanci V, Erol B, Bektaş S, Mungan G,
Yurtlu S, Tokgöz H, Can M and Ozkoçak Turan I: Effect of
dexmedetomidine on testicular torsion/detorsion damage in rats.
Urol Int. 84:105–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai YC, Tsai PS and Huang CJ: Effects of
dexmedetomidine on regulating endotoxin-induced up-regulation of
inflammatory molecules in murine macrophages. J Surg Res.
154:212–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin S, Dai N, Cheng Z, Shao W and Fu Z:
Effect of dexmedetomidine-etomidate-fentanyl combined anesthesia on
somatosensory- and motor-evoked potentials in patients undergoing
spinal surgery. Exp Ther Med. 7:1383–1387. 2014.PubMed/NCBI
|
|
11
|
Kiliç K, Hanci V, Selek S, Sözmen M, Kiliç
N, Citil M, Yurtlu DA and Yurtlu BS: The effects of dexmedetomidine
on mesenteric arterial occlusion-associated gut ischemia and
reperfusion-induced gut and kidney injury in rabbits. J Surg Res.
178:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozacmak VH, Sayan H, Arslan SO, Altaner S
and Aktas RG: Protective effect of melatonin on contractile
activity and oxidative injury induced by ischemia and reperfusion
of rat ileum. Life Sci. 76:1575–1588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao Y, Deng XG, Sun QN and Zhong ZQ:
Ganoderma spore lipid inhibits N-methyl-N-nitrosourea-induced
retinal photoreceptor apoptosis in vivo. Exp Eye Res. 90:397–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassoun HT, Weisbrodt NW, Mercer DW, Kozar
RA, Moody FG and Moore FA: Inducible nitric oxide synthase mediates
gut ischemia/reperfusion-induced ileus only after severe insults. J
Surg Res. 97:150–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Potoka DA, Nadler EP, Upperman JS and Ford
HR: Role of nitric oxide and peroxynitrite in gut barrier failure.
World J Surg. 26:806–811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Upperman JS, Potoka D, Grishin A, Hackam
D, Zamora R and Ford HR: Mechanisms of nitric oxide-mediated
intestinal barrier failure in necrotizing enterocolitis. Semin
Pediatr Surg. 14:159–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szadujkis-Szadurska K, Grzesk G,
Szadujkis-Szadurski L, Gajdus M and Matusiak G: Role of nitric
oxide and cGMP in the modulation of vascular contraction induced by
angiotensin II and Bay K8644 during ischemia/reperfusion. Exp Ther
Med. 5:616–620. 2013.PubMed/NCBI
|
|
18
|
Lin CY, Tsai PS, Hung YC and Huang CJ:
L-type calcium channels are involved in mediating the
anti-inflammatory effects of magnesium sulphate. Br J Anaesth.
104:44–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eser O, Fidan H, Sahin O, Cosar M, Yaman
M, Mollaoglu H, Songur A and Buyukbas S: The influence of
dexmedetomidine on ischemic rat hippocampus. Brain Res.
1218:250–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taniguchi T, Kurita A, Kobayashi K,
Yamamoto K and Inaba H: Dose- and time-related effects of
dexmedetomidine on mortality and inflammatory responses to
endotoxin-induced shock in rats. J Anesth. 22:221–228. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Can M, Gul S, Bektas S, Hanci V and
Acikgoz S: Effects of dexmedetomidine or methylprednisolone on
inflammatory responses in spinal cord injury. Acta Anaesthesiol
Scand. 53:1068–1072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Memiş D, Hekimoğlu S, Vatan I, Yandim T,
Yüksel M and Süt N: Effects of midazolam and dexmedetomidine on
inflammatory responses and gastric intramucosal pH to sepsis, in
critically ill patients. Br J Anaesth. 98:550–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venn RM, Bryant A, Hall GM and Grounds RM:
Effects of dexmedetomidine on adrenocortical function, and
cardiovascular, endocrine and inflammatory responses in
post-operative patients needing sedation in the intensive care
unit. Br J Anaesth. 86:650–656. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Liu JL, Qin C, Li JP, Wang XL,
Zhang ZX and Zhang L: Effects of cerebellar fastigial nucleus
electrical stimulation on telomerase reverse transcriptase
expression and mitochondrial apoptotic pathway in rats with focal
cerebral ischemia and reperfusion. Zhonghua Yi Xue Za Zhi.
91:1643–1648. 2011.(In Chinese). PubMed/NCBI
|
|
25
|
Lau BW, Wong AO, Tsao GS, So KF and Yip
HK: Molecular cloning and characterization of the zebra fish (Danio
rerio) telomerase catalytic subunit (telomerase reverse
transcriptase, TERT). J Mol Neurosci. 34:63–75. 2008. View Article : Google Scholar : PubMed/NCBI
|