Introduction
Lung cancer is the leading cause of cancer-related
mortality (1). The majority of
patients with lung cancer present with advanced-stage disease.
According to the Surveillance, Epidemiology and End Results
database (2), 65% of non-small-cell
lung cancer (NSCLC) cases are diagnosed at stage IIIB or IV.
Curative and palliative treatments are provided for NSCLC patients
at stage I–IIIA and IV, respectively. With stage IIIB NSCLC, some
eligible patients receive curative-intent treatments, which combine
radiotherapy (RT) and chemotherapy; however, the overall 5-year
survival rate remains at 3–8% (3,4).
With regard to locally advanced NSCLC, sequential
chemoradiotherapy (CRT) was first compared with prior RT alone.
Following the identification of the superior outcomes of sequential
CRT, concurrent CRT was compared with sequential CRT (5). A meta-analysis demonstrated a
significant advantage of concurrent CRT in overall survival, due to
increased locoregional disease control (6); however, a proportion of patients with
locally advanced NSCLC still receive RT alone, as they are
unsuitable for combined CRT due to advanced age, underlying
diseases, poor reserve of critical organs or low-grade performance.
In addition, certain patients are reluctant to receive systemic
chemotherapy due to excessive concern over its toxicity.
Hyperthermia is an old form of cancer therapy that
involves attacking the malignant disease by administering heat in
various ways. It has been usually applied as an adjunct to an
already established treatment modality (7); however, medical and technical problems
have prevented its wide acceptance. It is suggested that
oncothermia, a new concept of hyperthermia, can overcome these
shortcomings (8). The present study
records the case of a patient with stage IIIB NSCLC who received
concurrent oncothermia and definitive RT.
Case report
A 75-year-old male visited the Soonchunhyang
University Hospital (Cheonan, Republic of Korea) presenting with
hoarseness. He was an active smoker with a 50-pack-year history.
Simple chest radiography showed a mass-like lesion in the left
upper lobe. Computed tomography (CT) revealed a 2.6-cm irregular
mass with peripheral enhancement in the left upper lobe abutting
the descending thoracic aorta. The left lower paratracheal lymph
node was enlarged and appeared to have extranodal extension and
recurrent laryngeal nerve invasion. The endobronchial lesion was
not detected by flexible bronchoscopy. Pathological examination by
percutaneous needle biopsy revealed a poorly differentiated
adenocarcinoma. Positron emission tomography-CT showed an increased
18F-fluorodeoxyglucose uptake in both the primary mass
and the lymph node. The pretreatment serum level of
carcinoembryonic antigen was 2.65 ng/ml. Pulmonary function testing
showed a forced expiratory volume in 1 sec of 2.48 liters (78%). A
whole-body bone scan, brain magnetic resonance imaging and positron
emission tomography-CT showed no evidence of distant metastasis.
The TNM clinical stage was determined as T4N2M0 (stage IIIB)
(9). The present study was conducted
in accordance with the guidelines of the Institutional Review Board
and informed consent was obtained from the patient.
The patient was deemed unfit for chemotherapy due to
advanced age and an Eastern Cooperative Oncology Group performance
scale score of 2. Definitive RT was scheduled and the patient
agreed to undergo oncothermia concurrently with RT. During the RT
simulation, the patient was immobilized in the supine position,
with the arms above the head, in a vacuum-bag restriction system
(Vac-Lock; CIVCO Medical Solutions, Kalona, IA, USA). CT was
performed using a 16-slice CT scanner (Brilliance CT Big Bore;
Philips Medical Systems, Cleveland, OH, USA) and intravenous
contrast. Only the gross tumor volume, including the primary lesion
and involved lymph node, was included in the RT target; elective
nodal irradiation was not considered. A three-dimensional conformal
plan was created using the Eclipse treatment planning system
(Varian Medical Systems, Inc., Palo Alto, CA, USA) and 15-MV
photons. The RT fractionation scheme was 64.8 Gy in 36 fractions.
RT was performed using a Novalis Tx system (Varian Medical Systems,
Inc./BrainLab, Feldkirchen, Germany).
Oncothermia was carried out using the EHY 2000
device (Oncotherm GmbH, Troisdorf, Germany). A 30-cm-diameter
electrode was applied using the position of the RT simulation
target. Oncothermia was performed for 60 min per session, two
sessions per week, for a total of 12 sessions. The applied power
was gradually and linearly increased from 60 to 140 W, depending on
the tolerance of the patient.
Planned treatments of RT combined with oncothermia
were completed without interruption. Acute toxicity was limited to
mild odynophagia, which subsided with conservative management. No
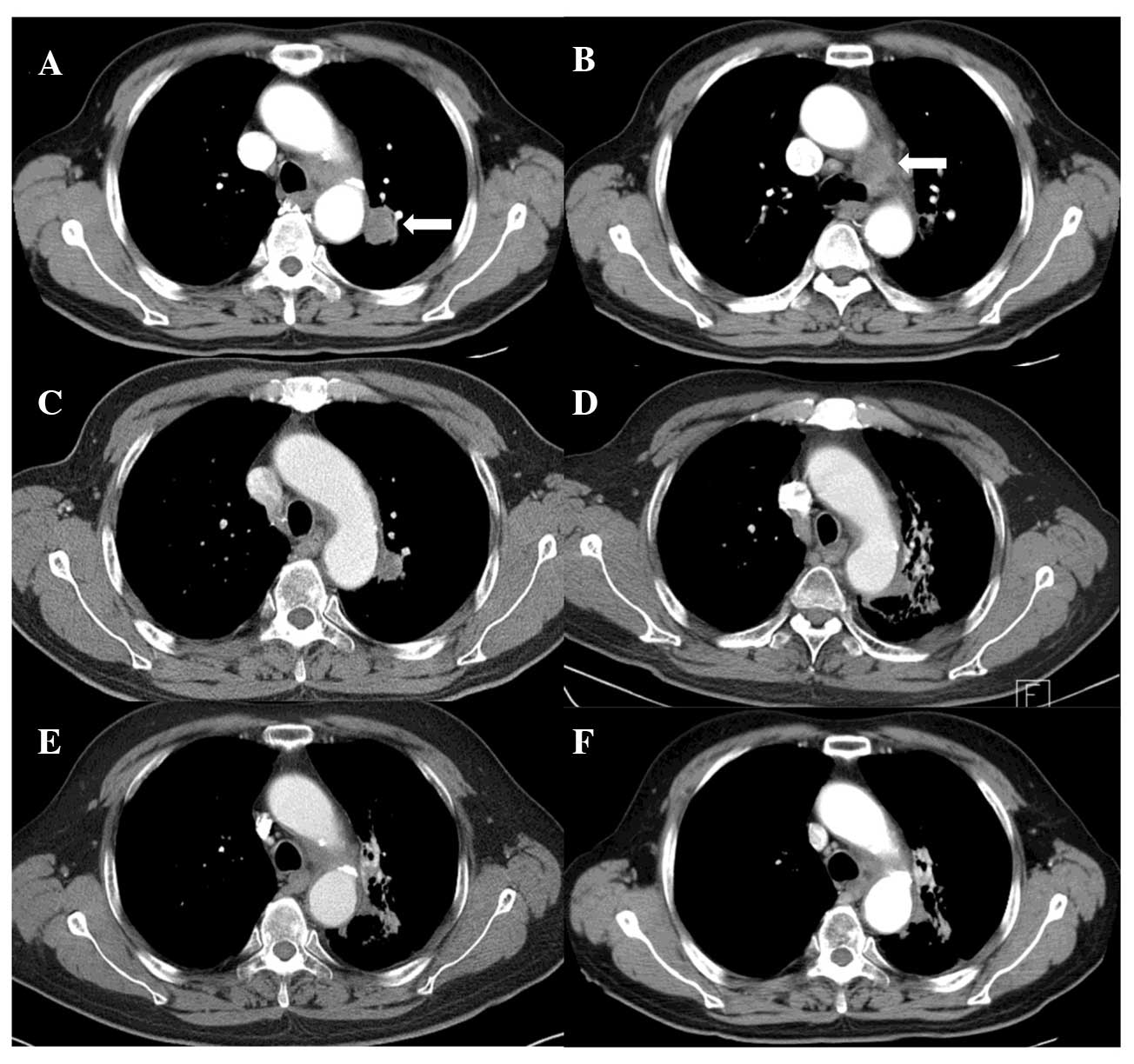
skin reaction developed. Follow-up CT (Fig. 1) showed a complete tumor response
along with signs of radiation pneumonitis and fibrosis around the
treated region; however, the patient developed no specific
associated symptoms. There was an improvement in the presenting
symptom of hoarseness and the patient was alive without any
evidence of the disease at 18 months after treatment.
Discussion
Hyperthermia has the ability to kill cancer cells as
a direct response to heat and, more importantly, render cancer
cells more susceptible to RT and certain chemotherapeutic drugs
(10). The synergistic mechanisms of
hyperthermia and RT include inhibition of lethal or sublethal
damage repair, cell cycle sensitivity and tumor oxygenation
(11). The clinical response rates
have been approximately doubled from 25–35%, with RT alone, to
50–70% with combined RT and hyperthermia (12). Seventeen randomized trials of
hyperthermia have been performed, primarily in patients with
sarcoma and cervical, breast and bladder cancer. Following the
addition of hyperthermia to the standard therapy, improvement in
local control and/or patient survival was achieved (12,13);
however, several challenges must be overcome before hyperthermia
can be established as a standard practice in oncology (14,15).
The physical objective of hyperthermia is to achieve
tumor temperatures of 40–45°C and to maintain that temperature for
1 h. This is accomplished using non-ionizing electromagnetic or
ultrasound radiation; however, heating tumor volumes selectively,
precisely and uniformly is technically challenging (7,12).
Common toxicities include superficial tissue burns, subcutaneous
fat necrosis or infection from the use of a catheter to monitor the
intratumoral temperature (12).
The recent development of oncothermia, also known as
electro-hyperthermia, has led to a renewed interest in the
oncologic treatment modality that is hyperthermia. Oncothermia is a
precise impedance-matched system based on capacitive coupling
(7). Due to the high ion
concentration near malignant cells and the low impedance of tumors
(increased metabolism), the modulated radiofrequency current flow
(13.56 MHz) and absorption of energy are concentrated in tumor
tissues, particularly in the extracellular matrix and the membranes
of malignant cells (7). Not only the
heating but also the electric field itself is efficacious
(non-thermal effects); therefore, the tumor temperature required by
oncothermia is lower than that required by conventional
hyperthermia. Traditional hyperthermia relies on temperature, while
oncothermia is energy dose-dependent (7). The complications reported have been
restricted to a small erythema (<8%) (8), and the contraindications are few, with
the exception of the existence of a pacemaker or metallic
implants/replacements in the treated area. Lung and liver tumors
are known to be unsuitable candidates for conventional
hyperthermia, due to the fact that they have their own cooling
systems, such as air ventilation and high blood flow, respectively.
On the contrary, tumors located in the aforementioned organs have
been shown to be good therapeutic targets for oncothermia (8,16). The
present case report supports this suggestion by demonstrating the
favorable outcome in an NSCLC patient treated with combined RT and
oncothermia.
The results of RT alone in early-stage NSCLC have
been considerably improved following the introduction of
stereotactic body RT (17,18), but the survival time of patients with
locally advanced NSCLC remains poor (median survival, ≤1 year)
following conventional RT alone (5).
By contrast, a randomized trial comparing concurrent CRT with
sequential CRT for locally advanced NSCLC patients showed that the
median survival time associated with concurrent CRT was 17 months
(19). Locoregional progression has
been shown to significantly decrease and distant progression to
remain the same (6); however,
numerous patients with locally advanced NSCLC receive RT alone for
various reasons, such as advanced age, comorbidities or poor
performance status. Historically, older cancer patients have been
undertreated, which has made elderly patients with stage III NSCLC
less likely to be selected for concurrent CRT (20). Furthermore, integration of concurrent
CRT has been accompanied by increased toxicities, particularly
esophageal complications. In a previous study, the incidence rate
of severe (grade III or IV) esophageal toxicity was shown to have
increased to 18% (6). Hematological
and pulmonary toxicities are also increased by combined
chemotherapy. Despite the fact that hyperthermia is also
synergistic with chemotherapy and triple-modality treatment is
feasible (21), the present case
report suggests that combined oncothermia with RT, with the former
having radiosensitizing potential and no additional toxicities, may
be a promising alternative for advanced-age and/or frail patients
with locally advanced NSCLC.
Acknowledgements
The present study was supported by the Soonchunhyang
University Research Fund.
References
|
1
|
Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH
and Lee JS: Prediction of cancer incidence and mortality in Korea,
2014. Cancer Res Treat. 46:124–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
William WN Jr, Lin HY, Lee JJ, Lippman SM,
Roth JA and Kim ES: Revisiting stage IIIB and IV non-small cell
lung cancer: Analysis of the surveillance, epidemiology and end
results data. Chest. 136:701–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russell K, Healy B, Pantarotto J, Laurie
SA, MacRae R, Sabri E and Wheatley-Price P: Prognostic factors in
the radical nonsurgical treatment of stage IIIB non-small-cell lung
cancer. Clin Lung Cancer. 15:237–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laine AM, Westover KD and Choy H:
Radiation therapy as a backbone of treatment of locally advanced
non-small cell lung cancer. Semin Oncol. 41:57–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aupérin A, Le Péchoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus
R, Yamanaka T, et al: Meta-analysis of concomitant versus
sequential radiochemotherapy in locally advanced non-small-cell
lung cancer. J Clin Oncol. 28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hegyi G, Szigeti GP and Szász A:
Hyperthermia versus oncothermia: Cellular effects in complementary
cancer therapy. Evid Based Complement Alternat Med.
2013:6728732013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andocs G, Szasz O and Szasz A: Oncothermia
treatment of cancer: From the laboratory to clinic. Electromagn
Biol Med. 28:148–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: 2010
|
|
10
|
Coffey DS, Getzenberg RH and DeWeese TL:
Hyperthermic biology and cancer therapies: A hypothesis for the
‘Lance Armstrong effect’. JAMA. 296:445–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roti Roti JL: Cellular responses to
hyperthermia (40–46 degrees C): Cell killing and molecular events.
Int J Hyperthermia. 24:3–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Halperin EC, Brady LW, Perez CA and Wazer
DE: Perez and Brady's Principles and Practice of Radiation
Oncology. 6th. Lippincott Williams & Wilkins; Baltimore, MD:
2013
|
|
13
|
van der Zee J, González González D, van
Rhoon GC, van Dijk JD, van Putten WL and Hart AA: Dutch Deep
Hyperthermia Group: Comparison of radiotherapy alone with
radiotherapy plus hyperthermia in locally advanced pelvic tumours:
A prospective, randomised, multicentre trial. Lancet.
355:1119–1125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sauer R, Creeze H, Hulshof M, Issels R and
Ott O: Interdisciplinary Working Group for Clinical Hyperthermia
(Atzelsberg Circle) of the German Cancer Society and the German
Society of Radiooncology: Concerning the final report
‘Hyperthermia: A systematic review’ of the Ludwig Boltzmann
Institute for Health Technology Assessment, Vienna, March 2010.
Strahlenther Onkol. 188:209–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wild C: Should hyperthermia be included in
the benefit catalogue for oncologic indications? Commercial
interests are presumed behind the editorial of R. Sauer et al.
Strahlenther Onkol. 189:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szasz A: Current status of oncothermia
therapy for lung cancer. Korean J Thorac Cardiovasc Surg. 47:77–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MJ, Yeo SG, Kim ES, Min CK and Se An
P: Intensity-modulated stereotactic body radiotherapy for stage I
non-small cell lung cancer. Oncol Lett. 5:840–844. 2013.PubMed/NCBI
|
|
18
|
Yeo SG and Kim ES: Efficient approach for
determining four-dimensional computed tomography-based internal
target volume in stereotactic radiotherapy of lung cancer. Radiat
Oncol J. 31:247–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curran WJ Jr, Paulus R, Langer CJ, Komaki
R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E,
Machtay M, Sause W and Cox JD: Sequential vs. concurrent
chemoradiation for stage III non-small cell lung cancer: Randomized
phase III trial RTOG 9410. J Natl Cancer Inst. 103:1452–1460. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bayman N, Blackhall F, McCloskey P, Taylor
P and Faivre-Finn C: How can we optimise concurrent
chemoradiotherapy for inoperable stage III non-small cell lung
cancer? Lung Cancer. 83:117–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Westermann AM, Jones EL, Schem BC, van der
Steen-Banasik EM, Koper P, Mella O, Uitterhoeve AL, de Wit R, van
der Velden J, Burger C, van der Wilt CL, et al: First results of
triple-modality treatment combining radiotherapy, chemotherapy and
hyperthermia for the treatment of patients with stage IIB, III and
IVA cervical carcinoma. Cancer. 104:763–770. 2005. View Article : Google Scholar : PubMed/NCBI
|