Introduction
Retinal neovascularization diseases, such as
proliferative diabetic retinopathy (DR), retinopathy of prematurity
(ROP) and secondary neovascular glaucoma, may be caused by a
variety of angiogenic factors in the retinal tissue under ischemic
and hypoxic conditions, leading to the generation of new blood
vessels. Retinal neovascularization is a complex process and
involves the participation of a variety of angiogenic cytokines,
including basic fibroblast growth factor, platelet-derived growth
factor (1), epidermal growth factor
(2), hepatocyte growth factor
(3), tumor necrosis factor and
vascular endothelial growth factor (VEGF). Among these, VEGF has
been considered to be the most important factor (4). Previous studies have indicated that
VEGF expression levels are significantly increased in tissues with
DR (5), ROP (6) and other retinal neovascularization
diseases, indicating that VEGF is a key factor in the promotion of
angiogenesis.
Since VEGF is able to significantly promote
angiogenesis, the growth factor may provide a target for the
prevention and treatment of angiogenesis-associated diseases.
Inhibitors for VEGF include anti-VEGF antibodies (such as
bevacizumab, a recombinant human VEGF antibody) (7), soluble VEGF receptors (such as
pegaptanib, a VEGF-165 RNA antagonist) (8), VEGF receptor antagonists, antisense
VEGF (9) and VEGF-associated signal
pathway inhibitors. Although these drugs may be useful for the
inhibition of retinal neovascularization, and a number of these
drugs have been used in a clinical context, one issue with their
application is the requirement for frequent administration in order
to maintain a suppressive effect against VEGF. Thus, the
identification of VEGF inhibitors with longer lasting effects, as
compared with the aforementioned drugs, is necessary.
RNA interference (RNAi) is a post-transcriptional
gene-silencing mechanism that can be initiated by a double-stranded
RNA (dsRNA) homologous in sequence to the targeted gene, which
induces cells to present a specific gene deletion phenotype
(10). RNAi has previously been used
for the functional analysis of genes in invertebrates, plants and
mammals (11). In the present study,
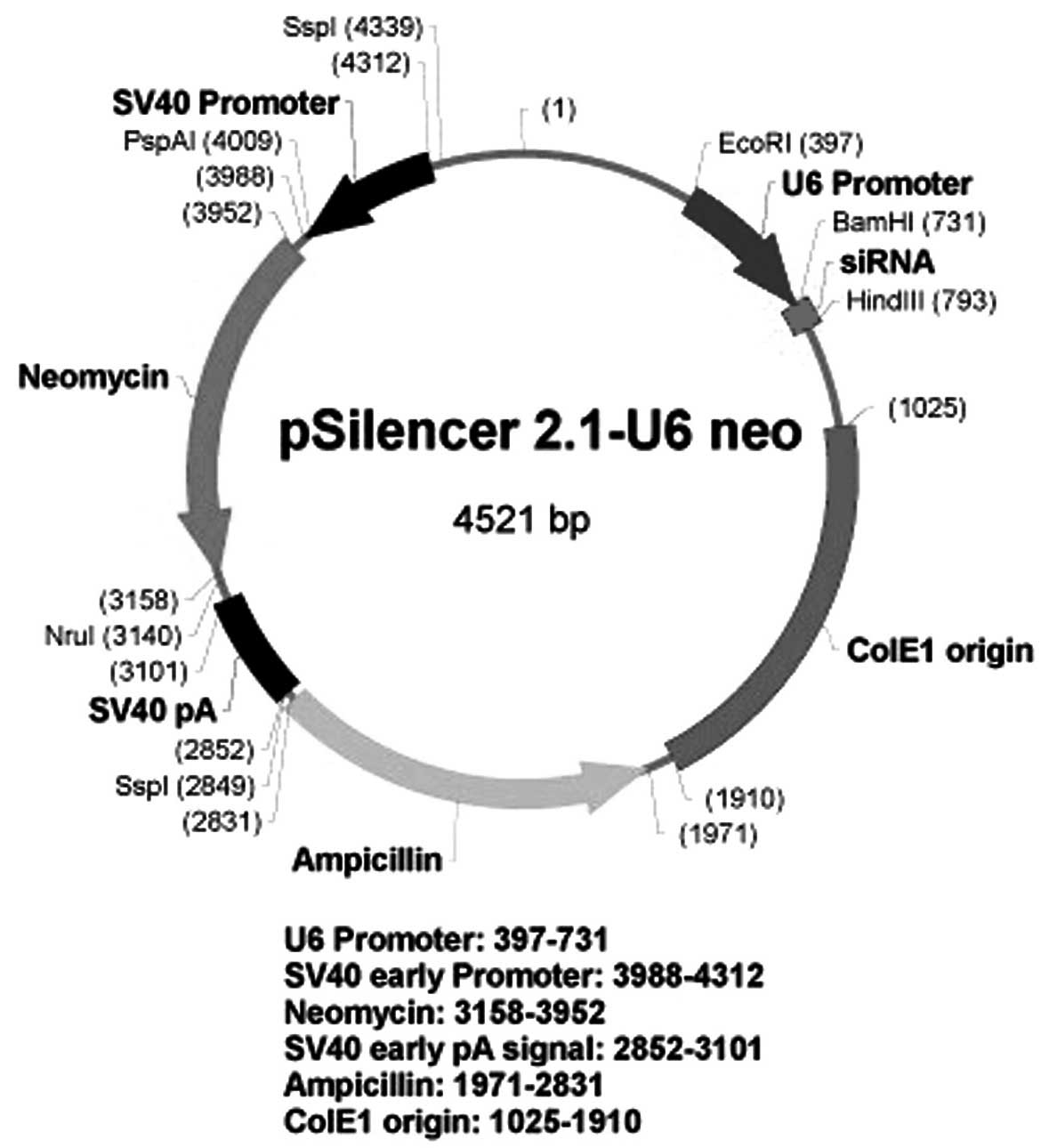
a recombinant pSilencer 2.1-U6 neo plasmid was constructed
containing short hairpin RNA (shRNA) targeted against the
eucaryotic VEGF gene. The recombinant plasmid was subsequently
transfected into a monkey choroid-retinal endothelial cell line
(RF/6A). The effects of the plasmid on the mRNA and protein
expression levels of VEGF and on the proliferation of the RF/6A
cell line were evaluated.
Materials and methods
Construction of the pSilencer 2.1-U6
neo-shRNA plasmid
A 21-mer short hairpin RNA (shRNA) against VEGF mRNA
(GenBank accession no. NM_001089925) was designed. According to the
targeting sequences, two pairs of 66-mer oligonucleotides coding
the shRNA were designed. The shRNAs were manufactured by Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Each shRNA sequence
contained a 7-bp loop sequence that separated the two complementary
domains. The 3′ end of the shRNA template was a 5-nucleotide poly
(T) tract recognized as an RNA Pol III termination signal, while
the 5′ ends of the two oligonucleotides contained BamHI and
HindIII restriction site overhangs. The sequence for the
complete VEGF shRNA insert template was as follows (lower case
indicates an inverted repeat sequence): 5′-GAT CCC gaa gag aag gaa
gaa gag agg TCA AGA GCC TCT CTT CTT CCT TCT CTT CTT TTT TGG AAA-3′
(sense) and 5′-AGC TTT TCC AAA AAA gaa gag aag gaa gaa gag agg CTC
TTG ACC TCT CTT CTT CCT TCT CTT CGG-3′ (antisense). These
oligonucleotides were synthesized, annealed and ligated into the
BamHI and HindIII sites of the pSilencer 2.1-U6 neo
shRNA vector (Ambion Life Technologies, Carlsbad, CA, USA) using T4
DNA ligase (Takara Bio, Inc., Otsu, Japan) (Fig. 1), as described in a previous study
(12). The recombinant vectors were
transformed into Escherichia coli XL1-Blue, and positive
clones were selected using ampicillin (Sigma-Aldrich, St. Louis,
MO, USA) and identified using BamHI and HindIII
restriction and DNA sequencing (Invitrogen Life Technologies, Grand
Island, NY, USA). A negative control plasmid, pSilencer 2.1-U6
neo-NC (p-NC), was supplied by Ambion Life Technologies.
Cell culture and transfection
RF/6A cells were cultured in RPMI 1640 medium
(Invitrogen Life Technologies, Grand Island, NY, USA), supplemented
with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), at 37°C in
5% CO2 and 95% humidified air. The RF/6A cells were
divided into four groups, which included the normal group,
CoCl2 group, CoCl2 plus negative control
vector transfection (CoCl2+ p-NC) group, and the
CoCl2 plus VEGF-targeting shRNA vector transfection
(CoCl2 + p-shRNA) group. The cells treated with
CoCl2 were exposed to 100 µmol/l CoCl2
(Sigma-Aldrich) for 24 h in order to induce a model hypoxic
response. Subsequently, the RF/6A cells were seeded onto 24-well
plates at a density of 2.0×105 cells/well and cultured
for 24 h. The recombinant pSilencer 2.1-U6 neo-shRNA vector and the
negative control pSilencer 2.1-U6 neo-NC vector were transfected
into the RF/6A cells using Lipofectamine™ 2000 (Invitrogen Life
Technologies).
Reverse transcription quantitative
polymerase chain reaction (qPCR)
Total cellular RNA was extracted from the cells
using TRIzol reagent (Invitrogen Life Technologies) and quantified
using UV absorbance spectroscopy (NanoDrop 2000 spectrophotometer;
Thermo Fisher Scientific, Waltham, MA, USA) and 1.2% agarose
formaldehyde gels. Reverse transcription was performed using an
ExScript™ RT reagent kit (Takara Bio, Inc.). Subsequently, the
real-time experiments were conducted using an ABI PRISM 7300
sequence detection system and SYBR Green Real-time PCR Master Mix
(Applied Biosystems Life Technologies, Beijing, China). The thermal
cycling conditions for the PCR assay consisted of an initial
denaturation step for 30 sec at 95°C, followed by 40 cycles at 95°C
for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The sequences of
the PCR primers were as follows: VEGF, 5′-ACT CTT CCC ACA GGC ATC
AG-3′ (sense) and 5′-CCC TAT CCC ATT CTT GCC TAT-3′ (antisense);
GAPDH, 5′-GTG GTG AAG TCG GCA TCA GA-3′ (sense) and 5′-AGG TGG AAG
AGT GGG TGT CG-3′ (antisense). Relative mRNA expression levels were
calculated using the 2−ΔΔCt method, where GAPDH was used
as a reference gene.
Western blot analysis
RF/6A cells were lysed in radioimmunoprecipitation
assay lysis buffer supplemented with protease inhibitor. The
protein concentration of the lysate was measured using a
bicinchoninic protein assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Equal quantities of protein (20 µg) were
subjected to SDS-PAGE, and transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked by incubation with 1% bovine serum albumin for 1 h at room
temperature. The membranes were incubated with a primary rabbit
polyclonal antibody against human VEGF (1:2,000; sc-507; Santa Cruz
Biotechnology, Inc.) overnight at 4°C, and subsequently with a goat
horseradish peroxidase-conjugated secondary antibody against rabbit
IgG (1:500; #32260; Invitrogen Life Technologies) for 2 h.
Immunoreactive bands were visualized using an enhanced
chemiluminescence kit.
Cell viability
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
Cells in the exponential growth phase were plated in
96-well plates at a density of 1×104 cells/well in 115
µl RPMI 1640 medium. Following shRNA transfection or hypoxia
treatment for 24, 48 or 72 h, the culture medium was replaced with
115 µl FBS-free RPMI 1640 medium containing MTT (Sigma-Aldrich) and
incubated at 37°C for 4 h. The medium was gently aspirated and 150
µl dimethyl sulfoxide (Tianjin Kermel Chemical Reagent Co., Ltd.,
Tianjin, China) was added, after which the plates were gently
shaken for 10 min. Finally, absorbance values were determined at
570 nm using a Bio-Rad 680 microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The inhibitory rate of cell proliferation
was calculated as follows: Inhibition rate (%) = [(absorbance of
CoCl2 + p-NC group - absorbance of CoCl2 +
p-shRNA group)/absorbance of CoCl2 + p-NC group)] ×
100%.
Statistical analysis
Continuous data are expressed as the mean ± standard
deviation. One way analysis of variance and the least significant
difference test were used for statistical analysis. All statistical
tests were two-sided, where P<0.05 was considered to indicate a
statistically significant difference. At least three independent
experiments were performed in duplicate. SPSS software, version
11.5 for Windows (SPSS, Inc., Chicago, IL, USA), was used for
statistical analysis.
Results
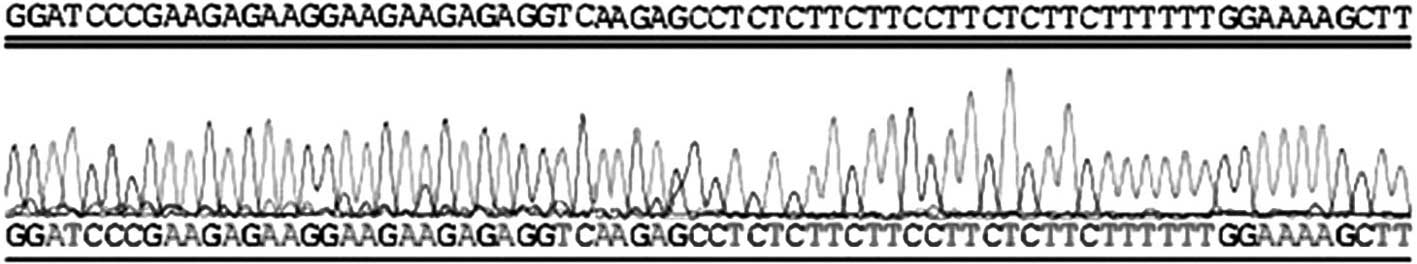
Identification of the recombinant
shRNA plasmid vectors
VEGF-targeted shRNA was designed and synthesized,
and the corresponding pSilencer 2.1-U6 neo-shRNA expressing vector
(p-shRNA) was constructed and identified using enzyme restriction
and DNA sequencing. The results indicated that the p-shRNA plasmid
was successfully constructed, in accordance with the initial design
(Fig. 2).
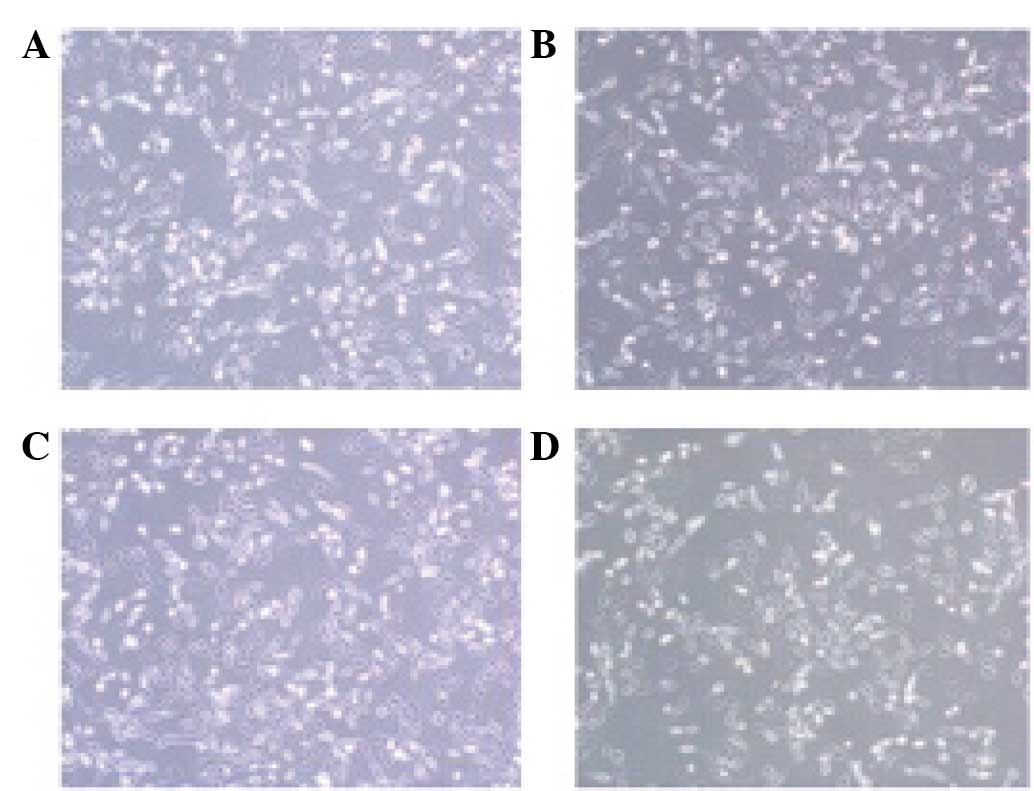
Effect of VEGF shRNA on the morphology
of RF/6A cells treated with CoCl2
Morphological differences were identified between
the cells exposed to CoCl2 or the vehicle for 24 h. The
results revealed that the cells in the CoCl2 and
CoCl2 + p-NC groups exhibited reduced cell connectivity,
irregular morphology and reduced thickness when compared with the
cells in the normal group. However, the cells in the
CoCl2 + p-shRNA group exhibited improvements with regard
to the morphological alterations when compared with the cells in
the CoCl2 + p-NC group (Fig.
3).
Effect of VEGF shRNA on the
proliferation of RF/6A cells treated with CoCl2
Proliferation of the RF/6A cells was evaluated
following exposure to CoCl2 or normal saline for 24, 48
and 72 h. The results indicated that cell proliferation in the
CoCl2 and CoCl2 + p-NC groups was enhanced
compared with the normal group, and this effect was time-dependent.
No statistically significant difference in the rate of cell
proliferation was detected between the CoCl2 and
CoCl2 + p-NC groups. However, the rate of cell
proliferation in the CoCl2 + p-shRNA group was
significantly attenuated when compared with the CoCl2 +
p-NC group (Fig. 4A), with
inhibition rates of 16, 32 and 38% at 24, 48 and 72 h, respectively
(Fig. 4B).
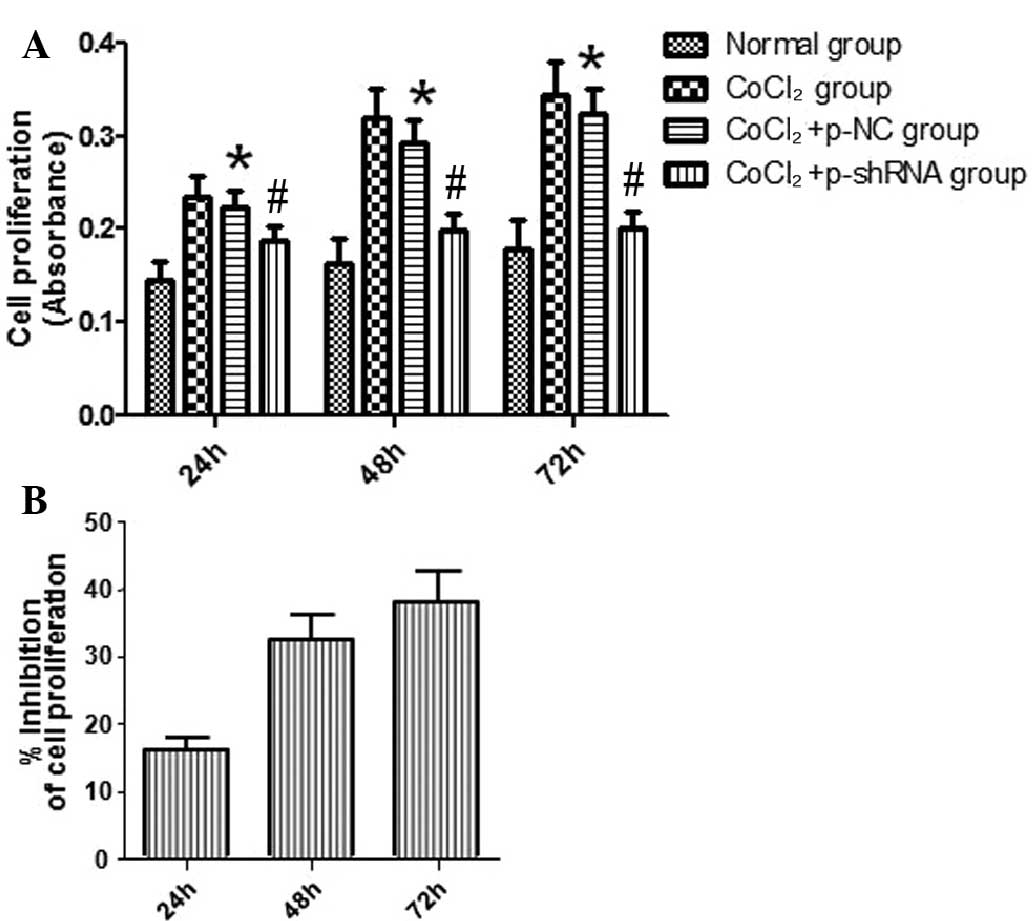 | Figure 4.Effect of vascular endothelial growth
factor short hairpin RNA (shRNA) on the proliferation of RF/6A
cells. The proliferation of RF/6A cells was determined following
exposure to CoCl2 for 24, 48 and 72 h. (A) Results
revealed that RF/6A cell proliferation in the CoCl2 and
CoCl2 + p-NC groups was enhanced in a time-dependent
manner, as compared with the normal group. However, the cell
proliferation in the CoCl2 + p-shRNA group was
significantly inhibited compared with the CoCl2 + p-NC
group. (B) Inhibition rates of the CoCl2 + p-shRNA group
were determined to be 16, 32 and 38% at 24, 48 and 72 h,
respectively. *P<0.05, vs. normal group; #P<0.05,
vs. CoCl2 + p-NC group. p-NC, pSilencer 2.1-U6
neo-normal control plasmid; p-shRNA, pSilencer 2.1-U6 neo-shRNA
plasmid. |
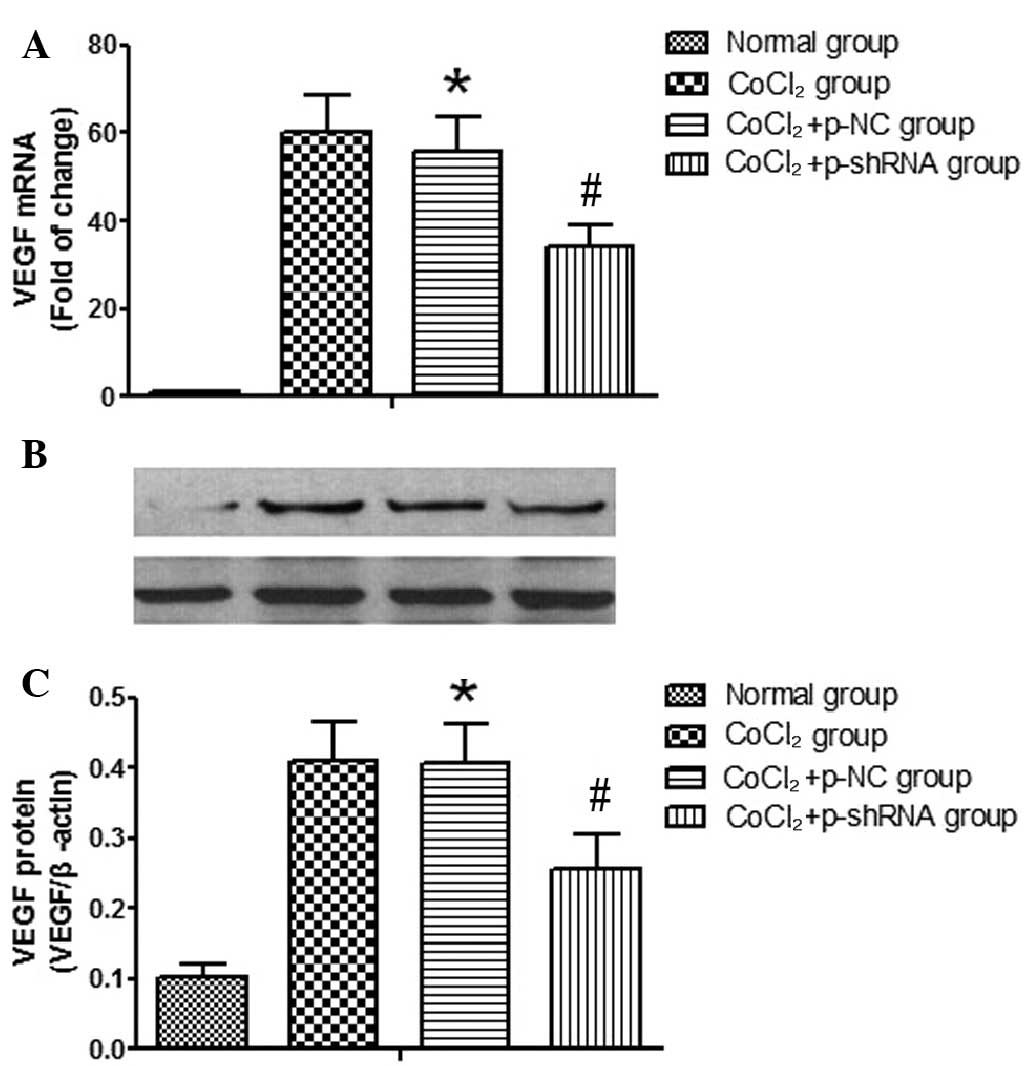
Effect of VEGF shRNA on the mRNA and
protein expression levels of VEGF in RF/6A cells treated with
CoCl2
The VEGF knockdown efficiency of the shRNA in RF/6A
cells was confirmed by determining the mRNA and protein expression
levels of VEGF following the transfection of the p-NC or p-shRNA
vectors into the cells. The results indicated that the transfection
of p-NC had no effect on the mRNA and protein expression levels of
VEGF (data not shown). However, in the CoCl2 and
CoCl2 + p-NC groups, the expression levels of VEGF mRNA
(Fig. 5A) and protein (Fig. 5B) were shown to increase when
compared with the normal group. No statistically significant
differences were detected in the VEGF mRNA and protein expression
levels between the CoCl2 and CoCl2 + p-NC
groups. However, the CoCl2-induced increases in the VEGF
mRNA and protein expression levels were reduced in the
CoCl2 + p-shRNA group, as compared with the
CoCl2 + p-NC group. Cells in the CoCl2 +
p-shRNA group exhibited reduced mRNA and protein expression levels
of VEGF (48 and 52% reductions, respectively) when compared with
the CoCl2 + p-NC group.
Discussion
Vascular endothelial cells are able to proliferate
and generate new blood vessels in a variety of conditions,
including wound healing, inflammation, cancer and retinal or
choroidal neovascularization (13).
Vascular endothelial cells are the primary targets of VEGF, which
is a factor involved in angiogenesis. VEGF binds to receptors on
endothelial cells and performs a number of endothelial-associated
functions, including the regulation of endothelial proliferation,
angiogenesis, vascular permeability and thrombosis. Furthermore,
VEGF is able to facilitate the movement of tumor cells into the
blood or adjacent tissues, subsequently promoting tumor invasion
and metastasis.
Previous studies have indicated that hypoxia is a
key regulator and promotor of VEGF expression (14,15).
Elevated expression levels of VEGF have been observed in
endothelial cells, thereby affecting angiogenesis and development.
Firstly, VEGF is a specific mitogen of vascular endothelial cells,
which may cause vascular endothelial cells to deform, migrate,
divide and proliferate (16).
Secondly, VEGF may induce vascular endothelial cells to increase
their vascular permeability, causing vasoconstriction factors,
clotting factors, plasma proteins and fibrins to extravasate into
the extracellular space. Exosmic fibrins and other proteins, such
as fibronectin, are condensed into a fibronectin gel, which
provides a matrix component for endothelial cells and other cells,
facilitating migration and intrusion, and ultimately converting the
cells into vascularized connective tissue (17). In addition, VEGF is a selective
mitogen of endothelial cells that is able to stimulate the growth
of new blood vessels. Finally, VEGF receptors (VEGFRs) are
primarily expressed on endothelial cells, and possess a high
affinity for VEGF. The VEGF/VEGFR system is the control center of
angiogenesis regulation and may serve a key function in the early
stages of angiogenesis. Previous in vitro studies have
indicated that VEGF is secreted by retinal microvascular
endothelial cells, pericytes and retinal pigment epithelial (RPE)
cells (18,19).
Thus, monkey retinal microvascular endothelial cells
were employed in the present study to observe the effect of VEGF
shRNA on retinal microvascular endothelial cell growth and VEGF
mRNA and protein expression levels. The results indicated that the
mRNA and protein expression levels of VEGF were significantly
enhanced in the cells treated with CoCl2 when compared
with those cultured under normoxic conditions, confirming that VEGF
expression was oxygen-dependent.
In the present study, a pSilencer 2.1-U6 neo-shRNA
recombinant plasmid was constructed, and a hypoxia model was
established in cultured RF/6A cells via treatment with
CoCl2. The morphological differences in the transfected
cells were observed and an MTT colorimetric assay was used to
detect the effects of the recombinant material on cell survival and
growth.
Previous studies have observed that the cell number
increases significantly and the cellular morphology becomes
irregular under hypoxic conditions. In addition, following VEGF
shRNA transfection, the cells appear irregular, with polymerization
between the cells reduced and the intercellular gap junctions
enlarged (20,21). The present results were consistent
with these observations. Due to the compensatory mechanism in
response to hypoxic conditions, the cell number is increased and
morphological abnormalities become evident. For the VEGF
shRNA-transfected cells, the decreased expression of VEGF affects
angiogenesis, resulting in cell nutrition disorders and a slowed
cell cycle. Therefore, the results of the present study indicate
that the hypoxia-induced growth of in vitro-cultured monkey
retinal endothelial cells may be inhibited by RNAi.
The results of the present study indicated that cell
proliferation was enhanced in the CoCl2 and
CoCl2 + p-NC groups, while the rate of cell
proliferation was decreased in the CoCl2 + p-shRNA
group. Furthermore, the growth rate of the cells transfected with
VEGF shRNA was significantly reduced compared with the other
groups; the growth inhibition rates of the cells were 31.56, 41.22
and 44.68% following transfection for 24, 48 and 72 h,
respectively. The present results are consistent with those of
previous studies, and indicate that VEGF shRNA is able to suppress
the stimulating effects of VEGF on cell proliferation via a
post-transcriptional gene silencing mechanism (22,23). In
addition, the present study demonstrated that the p-shRNA VEGF
interference plasmid inhibited vascular endothelial cell
proliferation, reduced cell growth and impeded angiogenesis.
To date, siRNA for VEGF have been used in the
treatment of ocular neovascularization. Murata et al
(24) reported that the mRNA
expression levels of VEGF in human RPE cells were significantly
reduced following transfection with VEGF-targeting siRNA. Specific
sequences were designed to bind to the VEGF promoter, and siRNA
targeting the intended gene was transcribed and synthesized by RNA
polymerase in vitro, and appeared to efficiently and
specifically inhibit VEGF expression in human RPE cells (25). Xia et al (26) transfected human umbilical vein
endothelial cells with VEGF-165 siRNA, and observed that VEGF mRNA
and protein expression levels were decreased in the VEGF-165
siRNA-transfected cells, as compared with the control cells.
The effects of RNAi at a molecular level may be
determined by evaluating the mRNA and protein expression levels. In
the present study, the mRNA expression level of VEGF was reduced in
the normoxia cells, while expression was significantly upregulated
in the CoCl2 and CoCl2 + p-NC groups when
compared with the normal group. No statistically significant
difference in VEGF mRNA expression was detected between the
CoCl2 and CoCl2 + p-NC groups. Although VEGF
expression n the CoCl2 + p-shRNA group remained
significantly increased compared with the normoxia group
(P<0.05), the level of VEGF mRNA expression was reduced compared
with the CoCl2 group, indicating that VEGF shRNA was
able to suppress the expression of VEGF. Furthermore, the level of
VEGF protein expression was detected using western blot analysis,
and the results indicated that the expression levels of VEGF
protein were in accordance with the levels of mRNA expression.
In conclusion, the results of the present study
demonstrated that the targeted knockdown of VEGF inhibited the
proliferation of vascular endothelial cells under hypoxic
conditions, which may be effective for the treatment of retinal
neovascularization diseases.
Acknowledgements
The study was supported by a grant from the Tianjin
Science and Technology Committee Foundation (no.
06YFJMJC07200).
References
|
1
|
Heldin CH: Development possible clinical
use of antagonists for PDGF and TGF-beta. Ups J Med Sci.
109:165–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YM, Bae MH, Lee OH, et al: Synergistic
induction of in vivo angiogenesis by the combination of
insulin-like growth factor-II and epidermal growth factor. Oncol
Rep. 12:843–848. 2004.PubMed/NCBI
|
|
3
|
Cantón A, Burgos R, Hernández C, et al:
Hepatocyte growth factor in vitreous and serum from patients with
proliferative diabetic retinopathy. Br J Ophthalmol. 84:732–735.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du S, Wang S, Wu Q, Hu J and Li T: Decorin
inhibits angiogenic potential of choroid-retinal endothelial cells
by downregulating hypoxia-induced Met, Rac1, HIF-1α and VEGF
expression in cocultured retinal pigment epithelial cells. Exp Eye
Res. 116:151–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank RN: Diabetic retinopathy. N Engl J
Med. 350:48–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozaki H, Yu AY, Della N, et al: Hypoxia
inducible factor-1 alpha is increased in ischemic retina: Temporal
and spatial correlation with VEGF expression. Invest Ophthalmol Vis
Sci. 40:182–189. 1999.PubMed/NCBI
|
|
7
|
Willett CG, Boucher Y, di Tomaso E, et al:
Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gragoudas ES, Adamis AP, Cunningham et Jr,
et al: Pegaptanib for neovascular age-related macular degeneration.
N Engl J Med. 351:2805–2816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mongerard-Coulanges M, Migianu-Griffoni E,
Lecouvey M and Jolles B: Impact of alendronate and VEGF-antisense
combined treatment on highly VEGF-expressing A431 cells. Biochem
Pharmacol. 77:1580–1585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shuey DJ, MeCallus DE and Giordano T:
RNAi: Gene-silencing in therapeutic intervention. Drug Discov
Today. 7:1040–1046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aravin AA, Klenov MS, Vagin VV, Rozovskiĭ
IaM and Gvozdev VA: Role of double-stranded RNA in eukaryotic gene
silencing. Mol Biol (Mosk). 36:240–251. 2002.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stewart SA, Dykxhoorn DM, Palliser D,
Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hanh WC, Sharp PA, et
al: Lentivirus-delivered stable gene silencing by RNAi in primary
cells. RNA. 9:493–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Itakura J, Ishiwata T, Shen B, Kornmann M
and Korc M: Concomitant over-expression of vascular endothelial
growth factor and its receptors in pancreatic cancer. Int J Cancer.
85:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharkey AM, Day K, Mcpherson A, Malik S,
Licence D, Smith SK and Charnock-Jones DS: Vascular endothelial
growth factor expression in human endometrium is regulated by
hypoxia. J Clin Endocrinol Metab. 85:402–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shweiki D, Itin A, Soffer D and Keshet E:
Vascular endothelial growth factor induced by hypoxia may mediate
hypoxia-initiated angiogenesis. Nature. 359:843–845. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klagsburn M and D'Amore PA: Vascular
endothelial growth factor and its receptors. Cytokine Growth Factor
Rev. 7:259–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elahy M, Baindur-Hudson S, Newsholme P and
Dass C: Mechanisms of PEDF mediated protection against ROS damage
in diabetic retinopathy and neuropathy. J Endocrinol.
222:R129–R139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aiello LP, Northrup JM, Keyt BA, Takagi H
and Iwamoto MA: Hypoxic regulation of vascular endothelial growth
factor in retinal cells. Arch Ophthalmol. 113:1538–1544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thieme H, Aiello LP, Takagi H, Ferrara N
and King GL: Comparative analysis of vascular endothelial growth
factor receptors on retinal and aortic vascular endothelial cells.
Diabetes. 44:98–103. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song E, Zhu P, Lee SK, et al: Antibody
mediated in vivo delivery of small interfering RNAs via
cell-surface receptors. Nat Biotechnol. 23:709–717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia RB, Fan XQ, Wang XL, Zhang XQ, Zhang P
and Lu J: Inhibition of VEGF expression by plasmid-based RNA
interference in the retinoblastoma cells. Zhonghua Yan Ke Za Zhi.
43:493–498. 2007.(In Chinese). PubMed/NCBI
|
|
22
|
Wang J, Shi YQ, Yi J, et al: Suppression
of growth of pancreatic cancer cell and expression of vascular
endothelial growth factor by gene silencing with RNA interference.
J Dig Dis. 9:228–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen J, Yang X, Xiao WH, Hackett SF, Sato
Y and Campochiaro PA: Vasohibin is up-regulated by VEGF in the
retina and suppresses VEGF receptor 2 and retinal
neovascularization. FASEB J. 20:723–725. 2006.PubMed/NCBI
|
|
24
|
Murata M, Takanmi T, Shimizu S, et al:
Inhibition of ocular angiogenesis by diced small interfering RNAs
(siRNAs) specific to vascular endothelial growth factor (VEGF).
Curr Eye Res. 31:171–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai CM, Sun BC and Liu XY: Short hairpin
RNA targeting vascular endothelial growth factor effectively
inhibits expression of vascular endothelial growth factor in human
retinal pigment epithelium. Zhonghua Yan Ke Za Zhi. 42:334–337.
2006.(In Chinese). PubMed/NCBI
|
|
26
|
Xia XB, Xiong SQ, Song WT, Luo J, Wang YK
and Zhou RR: Inhibition of retinal neovascularization by siRNA
targeting VEGF (165). Mol Vis. 14:1965–1973. 2008.PubMed/NCBI
|