Introduction
The clinical use of tacrolimus (Tac), a part of most
immunosuppressive protocols following renal transplantation, is
complicated by its narrow therapeutic range and large
inter-individual variability in pharmacokinetics. This variability
may lead to under-immunosuppression and acute rejection episodes or
over-immunosuppression, which can be potentially severe due to
adverse effects and toxicity. Hence, therapeutic drug monitoring
(TDM) of Tac is a required tool for reducing its toxicity and
improving efficacy (1,2). However, there are patients who will
experience adverse effects or a lack in efficacy with a Tac
concentration within the optimal range, which suggests that
additional investigation of the factors that may contribute these
effects is required (3). Previous
studies have shown that genetics are one of the main determinants,
along with demographic factors and drug-drug interactions that
contribute to patient variability in Tac pharmacokinetics (3,4).
Gene polymorphism (6986A>G) in cytochrome P450
3A5 (CYP 3A5) is assumed to be the major factor that contributes to
the pharmacokinetic variability of Tac. The presence of an A allele
at the polymorphic site in the CYP 3A5 gene suggests that an
individual has a functionally active enzyme (expresser) and carries
one of the two genotypes (CYP 3A5*1/*1 or CYP 3A5*1/*3). By
contrast, non-expressers do not have a functionally active enzyme
and carry the CYP 3A5*3/*3 genotype (3,5). The
significance of this gene polymorphism on Tac pharmacokinetics is
well documented, while its role in the pharmacodynamics of Tac has
not been elucidated completely (6–8). Tac is
also a substrate of P-glycoprotein (PGP), the product of the
ATP-binding cassette transporter (ABCB1) gene, which acts as an
efflux transporter that limits the oral absorption of drugs.
Genetic variability in the ABCB1 gene results in inter-individual
differences in its expression and activity. The most studied ABCB1
gene polymorphism is 3435C>T, but its role in Tac
pharmacokinetics remains controversial (9,10).
Considering CYP 3A5 and ABCB1 polymorphisms, the majority of
pharmacogenetic studies have investigated their influence on Tac
pharmacokinetics and pharmacodynamics in the early period following
renal transplantation up to 1 year post-transplant, while data from
subsequent periods are insufficient (5,11).
In addition to variability in pharmacokinetics that
may complicate post-transplantation immunosuppressive treatment,
chronic nephrotoxicity may also lead to undesirable outcomes,
foremost chronic allograft nephropathy (CAN). This is defined as a
gradual deterioration in graft function and represents the main
cause of late kidney graft loss (12,13).
The main goal of this study was to evaluate the
potential influence of CYP 3A5 and ABCB1 gene polymorphisms on Tac
dose requirements and dose-adjusted concentrations in different
long-term periods following renal transplantation. A further aim
was to investigate whether these polymorphisms affect renal
function in the late post-transplant period.
Materials and methods
Subjects
The pharmacokinetic-pharmacogenetic retrospective
study was conducted at the Clinic of Nephrology, Clinical Center
Nis (Nis, Serbia) and at the Research Centre for Biomedicine,
Faculty of Medicine, University of Nis (Nis, Serbia) during 2013.
The genotyping analysis included 9 renal transplant recipients
(including patients on Tac, cyclosporine and sirolimus), who were
monitored at the Clinic of Nephrology at the time of study
initiation. Of all patients enrolled in the genotyping study, only
53 patients underwent pharmacokinetic examination, due to exclusion
criteria that limited the number of the patients. Patients were
excluded if they were on cyclosporine and sirolimus, had any sign
of chronic graft rejection or concomitant disease or state that can
interfere with this type of study, or were taking medications known
to interfere with Tac metabolism (such as ketoconazole,
fluconazole, diltiazem, erythromycin or rifampicin). The study
involved a period from 6–24 months after transplantation (follow-up
period, 18 months). Of patients enrolled into the pharmacokinetic
study, 35 were men and 18 were women, mean age 39.38±10.48 years at
the beginning of the study. Regarding the type of transplantation,
41 of 53 patients received transplanted kidney from a living (L)
donor, and 12 of 53 received their organ from deceased (D) donors.
In addition to standard immunosuppressive therapy, patients also
received antihypertensive drugs (β-blocker bisoprolol or
metoprolol, and the calcium channel blocker amlodipine in
monotherapy as well as in combination) and omeprazole as a
gastroprotective agent. The study was approved by Ethics Committee
of Medical Faculty of the University of Nis and fully informed
written consent was obtained from each patient (No.
01-10204-13).
Immunosuppressive protocol
All patients started with a quaternary
immunosuppressive protocol that included Tac and intravenous
methylprednisolone (MP), with an initial dose of 0.5 g/day and 2 or
3 days later it was switched to prednisone (Pre) at an initial dose
of 1 mg/kg/day, mycophenolate mofetil (MMF) at a dose of 1.5 g/day
or mycophenolic acid (MPA) at a dose of 1,080 mg/day orally and 20
mg monoclonal antibody basiliximab (Bas) which was administered at
the first and the fourth day after transplantation. The first oral
Tac dose was administered on day 5 post-transplant at 8.00 h before
breakfast (0.05 mg/kg). Furthermore, Tac was administered twice
daily (08:00 h and 20:00 h), and the dose was adjusted according to
the trough concentration of the drug in the blood, in order to
maintain the drug trough concentration (C0) in the appropriate
range (5–15 ng/ml). Tac blood trough concentration was measured by
immunoassay method according to the manufacturer's instructions
(Architect immunoassay analyzer; Abbott, Abbott Park, IL, USA). For
the purpose of the study and to facilitate interpretation, the MMF
dose has been expressed in a dose of MPA and therefore this
abbreviation is used for both drugs. During absorption, the prodrug
MMF is hydrolyzed completely into the active metabolite MPA. A
dose-adjusted concentration (C0/D) of Tac was also calculated as
the trough concentration divided by the corresponding dose of Tac
(mg/kg/day); it represents a surrogate index of Tac
bioavailability. For the purposes of the analysis, the dose, C0 and
C0/D of Tac at 6, 12 and 24 months after transplantation were used.
The patients' data for dose and concentration of Tac from two
successive routine controls for each analyzed period were
included.
Biochemical data
A fasting blood sample was taken from each patient
during routine control at the clinic. Of the whole blood sample,
200 µl was taken for DNA isolation. DNA was extracted from the
whole blood with EDTA as an anticoagulant using a Genomic DNA
Purification kit (Fermentas, Thermo Scientific, Vilnius, Lithuania)
according to the manufacturer's instructions. Serum urea (Ure) and
creatinine (Cre) concentration were measured by standard methods in
the Biochemical laboratory at the Clinic of Nephrology. Analyses
were performed with an automated random access clinical chemistry
analyzer (ERBA XL-600; ERBA Diagnostics Mannheim GmbH, Mannheim,
Germany). Glomerular filtration rate (GFR) was estimated by
Modification of Diet in Renal Disease (MDRD) formula (14).
Genotyping CYP 3A5 and ABCB1
polymorphism
In order to determine polymorphisms of CYP 3A5 and
ABCB1 genes, a modification of the allele-specific polymerase chain
reaction (PCR) method of Ashavaid et al (15) was used. Each reaction mixture (for a
single patient) was prepared in duplicate, one for determination of
wild-type allele (CYP 3A5*1 and ABCB1 3435C) and the second for
determination of mutant-type allele (CYP 3A5*3 and ABCB1 3435T) of
the two polymorphisms. To identify each of the tested
polymorphisms, one forward and two reverse primers (wild-type and
mutant-type reverse primer) were used for a single polymorphism.
The forward primer is mutual for both reaction mixtures while the
reverse primer is different. Sequences of the forward primers: CYP
3A5: 5′-CAC TTG ATG ATT TAC CTG CCT TC-3′, ABCB1: 5′-ACT ATA GGC
CAG AGA GGCvTGC-3′. Sequences of the reverse primers: CYP 3A5
(wild-type): 5′-GGT CCA AAC AGG GAA GAG ATAT-3′, CYP 3A5
(mutant-type): 5′-GGT CCA AAC AGG GAA GAG ATAC-3′, ABCB1
(wild-type): 5′-GTG GTG TCA CAG GAA GAGCTC-3′, ABCB1 (mutant-type):
5′-GTG GTG TCA CAG GAA GAGCTT-3′. In total volume of 25 µl, each
reaction mixture contained 12.5 µl KAPA2G Readymix (KAPA2G ReadyMix
FastHotStart; Kapa Biosystems, Boston, MA, USA), which already
contains Hot Start DNA polymerase, dNTPs, MgCl2 and
stabilizers. In addition to the commercial mix, 0.5 µl of both
primers (forward and reverse, concentration of 10 pmol/µl), 10.5 µl
deionized water and 1 µl isolated DNA (average concentration 50
ng/µl) were added. For the amplification of PCR products, the
following program was used: Initial denaturation for 2 min at 95°C,
followed by 35 cycles of denaturation for 15 sec at 95°C, annealing
for 15 sec at 60°C and elongation for 15 sec at 72°C with a final
elongation for 30 sec at 72°C. Amplification products were detected
on 3% agarose gel. The length of an amplified product in the
determination of CYP 3A5 is 218 base pairs (bp) and for ABCB1, 134
bp.
Statistical analysis
The distribution of genotypes for each polymorphism
was assessed for deviation from Hardy-Weinberg equilibrium (HWE),
and differences in genotype frequency and in allele frequency
between the groups were assessed using the χ2 test.
Characteristics of the study group were expressed as median and
interquartile range or number. Student's t-test was used for
normally distributed data and the Mann Whitney U test was used for
data that were not normally distributed to compare pharmacokinetic
data (dose, C0 and C0/D) and renal function parameters (GFR and
Cre) between the groups of patients based on the CYP 3A5 and ABCB1
genotypes within the same period following transplantation. The
multivariate linear regression model considered Tac C0/D as the
dependent variable, whereas gender, age, corticoid dosage and CYP
3A5 and ABCB1 polymorphisms were used as independent variables.
Also, bivariate linear regression analysis was performed to
estimate the effect of CYP 3A5 and ABCB1 polymorphism on Cre
clearance. All analyses were performed with SPSS statistical
analysis software, version 16.0 (SPSS, Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Genotype and allelic frequencies of
the CYP 3A5 and ABCB1 polymorphisms
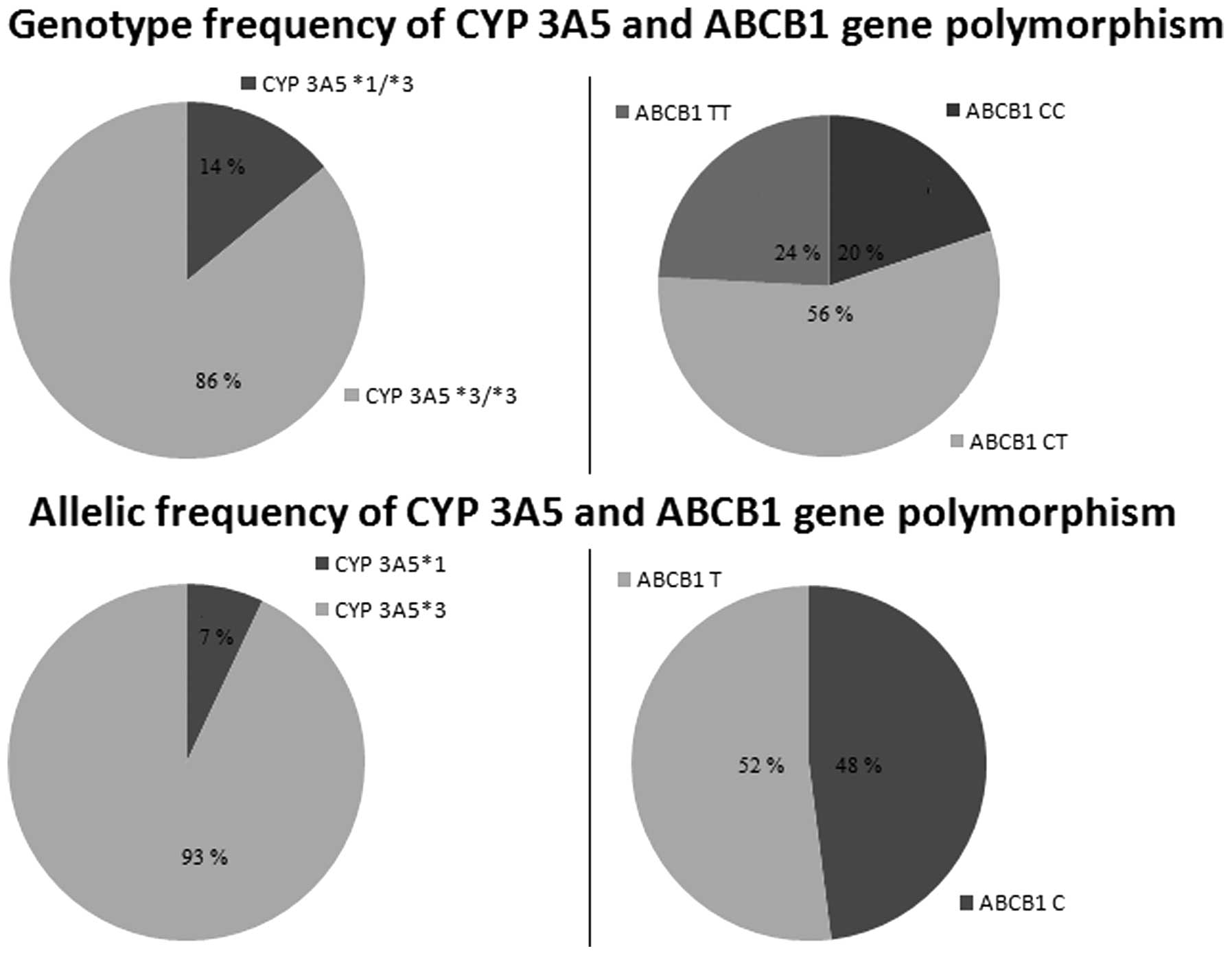
Fig. 1 shows the
genotype and allelic frequencies of the CYP 3A5 and ABCB1
polymorphisms. Genotyping analysis showed that 13 of 91 patients
had the CYP 3A5*1/*3 genotype and 78 of 91 had CYP 3A5*3/*3. There
were no patients with the CYP 3A5 *1/*1 genotype. Also, 18 of the
91 patients had ABCB1 CC, 51 of 91 had CT and 22 of 91 had TT
genotypes. Genotype and allele frequencies did not deviate from
Hardy-Weinberg equilibrium for the two tested polymorphisms.
The clinical and demographic characteristics of the
study population are presented in Table
I.
 | Table I.Clinical and demographic
characteristics of the renal transplant recipients on
tacrolimus-based immunosuppression in the different periods after
transplantation. |
Table I.
Clinical and demographic
characteristics of the renal transplant recipients on
tacrolimus-based immunosuppression in the different periods after
transplantation.
|
| Period after
transplantation |
|---|
|
|
|
|---|
| Parameters | 6 months | 12 months | 24 months |
|---|
| Gender
(male/female) | 35/18 | 35/18 | 35/18 |
| Age (years) | 39 (33–48) | 40 (34–49) | 39 (35–50) |
| Donor type
(living/deceased) | 41/12 | 41/12 | 41/12 |
| Body weight (kg) | 72 (65.5–77.5) | 73 (66–80) | 73.5 (66.3–84) |
| Cre (µmol/l) | 136 (115–164) | 122 (108–154) | 141 (114–157) |
| eGFR (ml/min/1.73
m2) | 49.36
(40.54–58.42) | 51.69
(44.46–59.02) | 48.48
(41.70–56.65) |
| Ure (mmol/l) | 8.20 (6.70–9.70) | 7.70
(6.48–10.13) | 8.00
(5.90–11.00) |
| Dose of Tac
(mg/kg/day) | 0.06 (0.05–0.09) | 0.05 (0.04–0.07) | 0.04 (0.03–0.07) |
| C0 of Tac
(ng/ml) | 7.40 (6.20–8.90) | 7.25 (5.68–8.45) | 6.45 (5.50–7.75) |
| C0/D of Tac
[(ng/ml)/(mg/kg/day)] | 110.92
(82.69–164.81) | 129.10
(85.72–207.91) | 146.43
(114.31–204.43) |
| Dose of Pre
(mg/kg/day) | 0.15
(0.14–0.17) | 0.14
(0.13–0.16) | 0.14
(0.12–0.15) |
| Dose of MPA
(mg/day)a | 1080
(720–1440) | 1080
(720–1440) | 1080
(720–1080) |
| CYP 3A5 genotype
(*1*3/*3*3) | 11/42 | 11/42 | 11/42 |
| ABCB1 genotype
(CC/CT/TT) | 11/32/10 | 11/32/10 | 11/32/10 |
Influence of the CYP 3A5 and ABCB1
gene polymorphism on the tacrolimus dosage regimen
Table II shows a
comparison of Tac dose, C0 and C0/D among patients with different
CYP 3A5 genotype in the different periods following renal
transplantation. Individuals carrying the CYP 3A5*1/*3 genotype
(n=11) had lower C0/D than *3/*3 carriers (n=42) at 6, 12 and 24
months post-transplant (P<0.05). Also, CYP 3A5 expressers
required a higher dose of Tac at 6 and 12 months following
transplantation (P<0.05), but not after 24 months. By contrast,
there was no difference in C0 in relation to CYP 3A5 genotype in
the observed period of time.
 | Table II.Dose, trough concentration and
dose-adjusted trough concentration of tacrolimus in relation to CYP
3A5 genotype up to 24 months after transplantation. |
Table II.
Dose, trough concentration and
dose-adjusted trough concentration of tacrolimus in relation to CYP
3A5 genotype up to 24 months after transplantation.
|
| Periods after
transplantation |
|---|
|
|
|
|---|
| Variable | 6 months | 12 months | 24 months |
|---|
| Dose
(mg/kg/day) |
|
|
|
| CYP
3A5*1/*3 | 0.09
(0.08–0.15)a | 0.08
(0.05–0.09)a | 0.07
(0.04–0.10) |
| CYP
3A5*3/*3 | 0.06
(0.04–0.08) | 0.05
(0.04–0.07) | 0.04
(0.03–0.06) |
| C0 (ng/ml) |
|
|
|
| CYP
3A5*1/*3 | 7.60
(6.40–9.10) | 7.10
(5.50–7.30) | 5.10
(4.60–7.50) |
| CYP
3A5*3/*3 | 7.30
(6.20–8.70) | 7.60
(5.70–8.60) | 6.50
(5.70–7.80) |
| C0/D
[(ng/ml)/(mg/kg/day)] |
|
|
|
| CYP
3A5*1/*3 | 52.31
(49.82–103.13)a | 81.59
(64.80–121.50)a | 115.24
(75.38–151.30)a |
| CYP
3A5*3/*3 | 122.19
(88.92–172.36) | 135.88
(93.97–216.45) | 150.75
(115.58–227.52) |
Considering ABCB1 genotype (Table III), the only difference was found
in Tac dose 6 months after transplantation (P<0.05), between
patients with at least one C allele (CC and CT genotype, n=43) and
carriers of the TT genotype (n=10). There was a significant
difference in C0/D between CC+CT and TT at 24 months after renal
transplantation. Also, in order to eliminate the influence of CYP
3A5 polymorphism, carriers of the *1/*3 genotype (n=11) were
excluded and then dose, C0 and C0/D of Tac were compared in
relation to the ABCB1 genotype, but no significant difference was
identified (Table III).
 | Table III.Dose, trough concentration and
dose-adjusted trough concentration of tacrolimus in relation to
ABCB1 genotype up to 24 months after transplantation. |
Table III.
Dose, trough concentration and
dose-adjusted trough concentration of tacrolimus in relation to
ABCB1 genotype up to 24 months after transplantation.
|
| Period after
transplantation |
|---|
|
|
|
|---|
| Variable | 6 months | 12 months | 24 months |
|---|
| Dose
(mg/kg/day) |
|
|
|
| ABCB1
3435 genotype |
|
|
|
|
CC+CT | 0.07
(0.05–0.10)b | 0.06
(0.04–0.07) | 0.05
(0.03–0.07) |
|
TT | 0.05
(0.04–0.06) | 0.05
(0.04–0.06) | 0.04
(0.03–0.04) |
| ABCB1
3435 genotypea |
|
|
|
|
CYP*3/*3,
CC+CT | 0.06
(0.05–0.08) | 0.06
(0.04–0.07) | 0.05
(0.03–0.07) |
|
CYP*3/*3, TT | 0.05
(0.04–0.06) | 0.05
(0.04–0.06) | 0.04
(0.03–0.04) |
| C0 (ng/ml) |
|
|
|
| ABCB1
3435 genotype |
|
|
|
|
CC+CT | 7.65
(6.05–9.18) | 7.25
(5.78–8.35) | 6.30
(5.10–7.80) |
|
TT | 7.20
(7.10–7.60) | 7.50
(5.38–8.60) | 6.30
(5.83–6.68) |
| ABCB1
3435 genotypea |
|
|
|
|
CYP*3/*3,
CC+CT | 7.70
(6.00–9.20) | 7.60
(6.40–8.60) | 6.50
(5.30–7.90) |
|
CYP*3/*3, TT | 7.20
(7.10–7.60) | 7.50
(5.38–8.60) | 6.30
(5.83–6.68) |
| C0/D (ng
ml−1/mg kg−1 day−1) |
|
|
|
| ABCB1
3435 genotype |
|
|
|
|
CC+CT | 110.13
(80.27–156.00) | 129.10
(82.97–177.53) | 128.57
(83.58–182.24)b |
|
TT | 150.11
(107.10–189.93) | 138.00
(92.45–216.61) | 159.57
(149.39–274.36) |
| ABCB1
3435 genotypea |
|
|
|
|
CYP*3/*3,
CC+CT | 121.71
(85.44–158.60) | 135.88
(104.27–214.65) | 136.03
(111.83–207.20) |
|
CYP*3/*3, TT | 150.11
(107.10–189.93) | 138.00
(92.45–216.61) | 159.57
(149.39–274.36) |
Multivariate analysis showed that the CYP 3A5
polymorphism affected C0/D up to 24 months after transplantation,
and this effect remained significant even after adjusting the model
for gender, age and corticoid dose (Table IV). By contrast, ABCB1 polymorphism
did not show a significant impact on Tac exposure.
 | Table IV.Effects of different predictors on
tacrolimus dose-adjusted concentration up to 24 months after
transplantation as determined by linear multivariate
regression. |
Table IV.
Effects of different predictors on
tacrolimus dose-adjusted concentration up to 24 months after
transplantation as determined by linear multivariate
regression.
| Predictor | Estimate | P-value | Model |
|---|
| CYP 3A5 (*1) | −0.274 | 0.001 | R=0.35,
R2=0.13, P<0.01 |
| ABCB1 (TT) | −0.048 | 0.595 |
|
| Male gender |
0.084 | 0.345 |
|
| Age (years) | −0.137 | 0.095 |
|
| Corticoid dose
(mg/kg/day) | −0.143 | 0.086 |
|
Influence of the CYP 3A5 and ABCB1
gene polymorphism on creatinine clearance up to 24 months after
renal transplantation
This study showed that CYP 3A5, but not the tested
ABCB1 polymorphism might explain the variability in inter-patient
difference in renal function parameters within two years after
transplantation (Table V). The
results of bivariate analysis suggest that variance in Cre
clearance might be partially explained by CYP 3A5 polymorphism and
that patients carrying the CYP 3A5*1 allele had lower Cre
clearance. Based on the results of bivariate analysis, Cre
clearance and serum Cre level were compared with respect to
patients' CYP 3A5 genotype in the different periods following renal
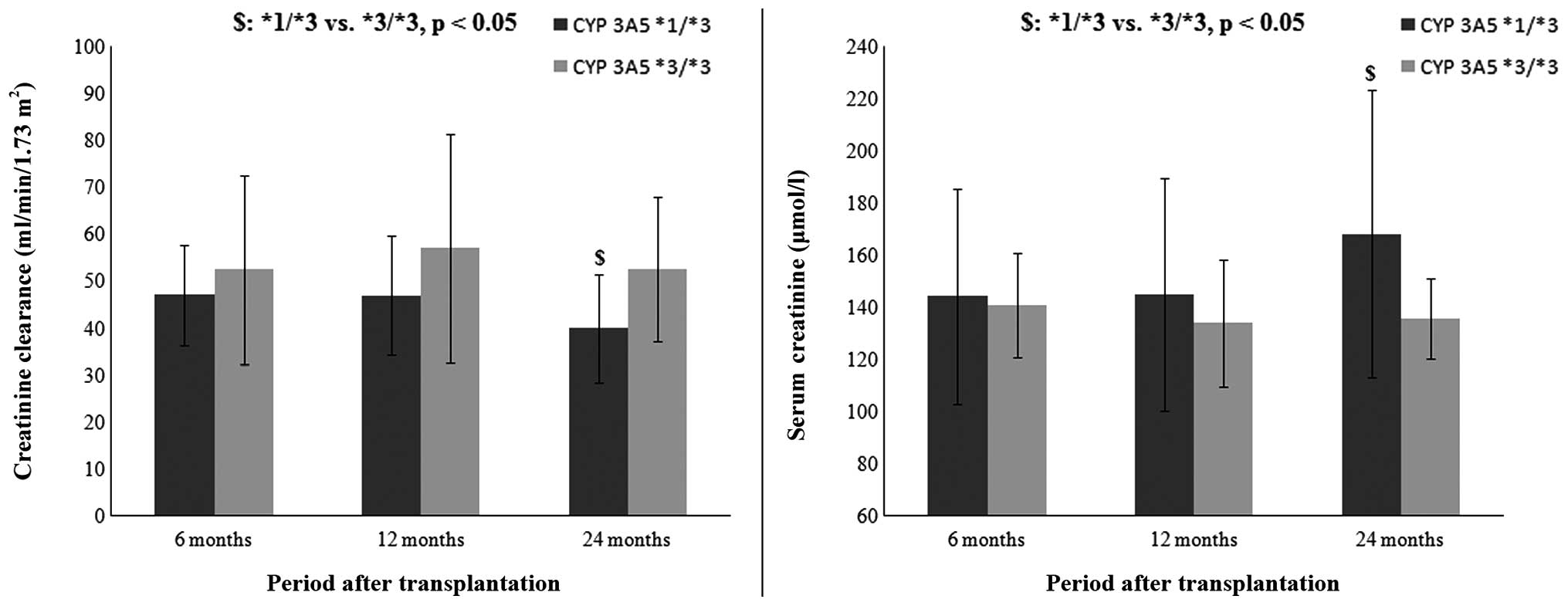
transplantation (Fig. 2). The
results indicate that patients with the CYP 3A5*1/*3 genotype had
lower Cre clearance (39.92±11.46 vs. 52.64±15.32 ml/min/1.73
m2, P<0.05) and higher serum Cre levels (168.11±55.20
vs. 135.79±39.03 µmol/l, P<0.05) when compared with patients
with the CYP 3A5*3/*3 genotype after 24 months, but not after 6
(47.07±10.66 vs. 52.57±20.12 ml/min/1.73 m2, P>0.05;
144.23±41.30 vs. 140.94±43.05 µmol/l, P>0.05) and 12
(47.00±12.67 vs. 57.09±24.45 ml/min/1.73 m2, P>0.05;
144.82±44.62 vs. 134.07±47.74 µmol/l, P>0.05) months
post-transplant.
 | Table V.Effects of CYP 3A5 and ABCB1 gene
polymorphisms on creatinine clearance up to 24 months after
transplantation as determined by bivariate linear regression. |
Table V.
Effects of CYP 3A5 and ABCB1 gene
polymorphisms on creatinine clearance up to 24 months after
transplantation as determined by bivariate linear regression.
| Predictor | Estimate | P-value | Model |
|---|
| CYP 3A5 (*1) | −0.205 | 0.029 | R=0.26,
R2=0.07, P<0.05 |
| ABCB1 (TT) | −0.131 | 0.129 |
|
Discussion
Hepatic and intestinal CYP 3A5 as well as intestinal
PGP may act synergistically and limit Tac bioavailability and
exposure. Although, the CYP 3A5 gene has 11 different polymorphisms
that have been identified to date, the CYP 3A5 (6986A>G)
polymorphism is the most extensively studied and indicated to be
the major contributor to the inter-patient variability of Tac
pharmacokinetics. It is characterized by an adenine (A) to guanine
(G) transition at position 6986 within intron 3 of the CYP 3A5
gene. Expressers (carriers of the CYP 3A5*1 allele) produce high
levels of full-length messenger RNA (mRNA), which consequently
leads to high levels of CYP 3A5 functional protein in the body. The
ABCB1 gene is also polymorphically expressed with ≥50 different
polymorphisms known to date. In contrast to CYP 3A5, neither of the
ABCB1 polymorphisms leads to complete loss of the PGP function
(5,8). The most studied ABCB1 gene polymorphism
is 3435C>T, which includes a C to T transition at position 3435
within exon 26. Previous studies suggest that the lower activity of
PGP is associated with TT genotype for this particular polymorphism
(5,16).
Previous studies did not show a difference in allele
frequency between renal transplant patients and healthy volunteers,
but revealed that the CYP 3A5*1 allele frequency is largely
dependent on ethnic origin (5,17). The
results of the present study showed that the CYP 3A5*1 allele was
present in 7% of the tested population (which comprised only
Caucasian individuals), which was consistent with previous findings
in Caucasians of ~5–15% (17). The
frequency of this allele varies among other ethnic groups, and has
been reported to be 45–73% in African-Americans, 15% in Japanese,
27–35% in Chinese, 30% in Koreans, 25% in Mexicans, 27% in
Moroccans and 26% in Brazilians (9,
18–20).
Considering ABCB1 3435C>T polymorphism, the C
allele was present in 48% and T allele in 52%, which was in
accordance with previous studies conducted in Caucasian individuals
(21). Additionally, the ABCB1 TT
genotype was identified in ~26% of Caucasians, but it was present
in 20% of Japanese, 16% of Chinese, 5% of African-Americans, and
1.7% of West Africans (22,23).
Previous studies conducted suggest that variability
in Tac pharmacokinetics might be partially explained by CYP 3A5 and
ABCB1 gene polymorphisms. According to this, the findings of the
present study suggest that carriers of the CYP 3A5*1/*3 genotype in
comparison with CYP 3A5*3/*3 carriers had higher Tac dose
requirements to maintain the drug concentration in the optimal
range in different long-term periods after transplantation
(Table II). Although, the majority
of previous studies emphasized the role of CYP 3A5 polymorphism in
the early period after renal transplantation (5,9,24,25), the
present study showed that CYP 3A5 polymorphism may be a significant
predictor of Tac exposure within the 2 years after
transplantation.
By contrast, results for ABCB1 3435 polymorphisms
showed that the only significant difference between patient
genotype for Tac dose requirements were 6 months after renal
transplantation. However, this effect was not independent from the
influence of CYP 3A5, due to the fact that this significance was
lost when carriers of the CYP 3A5*1 allele were excluded (Table III). Although, certain previous
studies did not find a difference in Tac doses between ABCB1 3435
genotypes (26,27), there have been studies confirming the
significance of ABCB1 polymorphism in the early phase as well as at
6 months post-transplant (28–30).
Additionally, linear multivariate regression analysis confirmed the
influence of the CYP 3A5 polymorphism, but not ABCB1 polymorphism
on the C0/D of Tac up to 24 months after renal transplantation. The
model used in the present study was adjusted for age, gender and
corticosteroid dose (Table IV). The
low R2 was probably the result of the small number of patients with
the CYP 3A5*1/*3 genotype.
The controversy in the results concerning the
long-term adverse effects of Tac prompted an assessment of the
contribution of CYP 3A5 and ABCB1 polymorphisms on renal function
parameters in transplant recipients in the present study. The
majority of the previous studies found no correlation between
investigated polymorphisms and the clinical features of Tac
toxicity or renal function attenuation (6,8,31). The bivariate linear regression showed
that CYP 3A5 polymorphism, but not ABCB1 polymorphism,
independently affects renal function from 6 to 24 months after
renal transplantation (Table V).
Furthermore, the CYP 3A5*1 allele led to renal function
deterioration following transplantation. Although, carriers of the
CYP 3A5*1 allele continuously require a higher dose of Tac, it is
not clear how exactly higher Tac dose requirements may lead to
renal function impairment without any marked difference in Tac
exposure. A potential theoretical explanation is that Tac
metabolites are formed in greater amounts in the liver and
therefore may exert adverse affects on renal function. Increased
hepatic and gastrointestinal CYP 3A5 enzymatic activity leads to
higher systemic clearance of the drug and hence higher dose
requirements, but could also result in higher (renal) tissue
concentrations of Tac metabolites (32). These metabolites may enter the
transplanted organ, causing calcineurin inhibitor-related
arteriolar vasoconstriction and therefore reduction in renal
function (32,33).
Based on the results from regression analysis, Cre
clearance was compared in relation to CYP 3A5 genotype at different
periods after renal transplantation (Fig. 2). The results showed that patients
with the CYP 3A5*1/*3 genotype had lower GFR and higher serum Cre
level in comparison with patients with the CYP 3A5*3/*3 genotype.
This is in accordance with the previous assumption that a
constantly high dose of Tac may lead to a worsening of renal
function. This finding may be contradictory to the majority of
previously published studies, but the majority of them limited
their observations at the first year after transplantation
(6,31). However, certain authors found an
association between Tac toxicity and patient genotype. They
suggested that CYP 3A5*1 allele as well as higher Tac dose
requirements led to Tac-associated nephrotoxicity in the long-term
following renal transplantation (32).
In conclusion, this study demonstrates that the CYP
3A5 polymorphism contributes to the inter-individual variability in
Tac dose requirements and exposure not only in the early period
after renal transplantation, but also in the long-term
post-transplant periods. Also, the findings do not show that ABCB1
3435C>T polymorphism independently affects Tac pharmacokinetics.
Furthermore, the obtained results indicate that renal function
decline may be more pronounced in patients with CYP 3A5*1 in the
long-term after renal transplantation and this may suggest that CYP
3A5 polymorphism underlies renal function decline.
Acknowledgements
This study was supported by a grant from the
Ministry of Science and Technological Development of Serbia
(project no. 41018).
References
|
1
|
Press RR, de Fijter JW and Guchelaar HJ:
Individualizing calcineurin inhibitor therapy in renal
transplantation - current limitations and perspectives. Curr Pharm
Des. 16:176–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masuda S and Inui K: An up-date review on
individualized dosage adjustment of calcineurin inhibitors in organ
transplant patients. Pharmacol Ther. 112:184–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacPhee IA: Pharmacogenetic biomarkers:
Cytochrome P450 3A5. Clin Chim Acta. 413:1312–1317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velickovic-Radovanovic R, Mikov M,
Catic-Djordjevic A, et al: Tacrolimus as a part of
immunosuppressive treatment in kidney transplantation patients: Sex
differences. Gend Med. 9:471–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staatz CE, Goodman LK and Tett SE: Effect
of CYP3A and ABCB1 single nucleotide polymorphisms on the
pharmacokinetics and pharmacodynamics of calcineurin inhibitors:
Part I. Clin Pharmacokinet. 49:141–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Staatz CE, Goodman LK and Tett SE: Effect
of CYP3A and ABCB1 single nucleotide polymorphisms on the
pharmacokinetics and pharmacodynamics of calcineurin inhibitors:
Part II. Clin Pharmacokinet. 49:207–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuypers DR, de Jonge H, Naesens M, Lerut
E, Verbeke K and Vanrenterghem Y: CYP3A5 and CYP3A4 but not MDR1
single-nucleotide polymorphisms determine long-term tacrolimus
disposition and drug-related nephrotoxicity in renal recipients.
Clin Pharmacol Ther. 82:711–725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hesselink DA, Bouamar R, Elens L, van
Schaik RH and van Gelder T: The role of pharmacogenetics in the
disposition of and response to tacrolimus in solid organ
transplantation. Clin Pharmacokinet. 53:123–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cusinato DA, Lacchini R, Romao EA,
Moysés-Neto M and Coelho EB: Relationship of CYP3A5 genotype and
ABCB1 diplotype to tacrolimus disposition in Brazilian kidney
transplant patients. Br J Clin Pharmacol. 78:364–372. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurzawski M, Dąbrowska J, Dziewanowski K,
Domański L, Perużyńska M and Droździk M: CYP3A5 and CYP3A4, but not
ABCB1 polymorphisms affect tacrolimus dose-adjusted trough
concentrations in kidney transplant recipients. Pharmacogenomics.
15:179–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Provenzani A, Notarbartolo M, Labbozzetta
M, et al: Influence of CYP3A5 and ABCB1 gene polymorphisms and
other factors on tacrolimus dosing in Caucasian liver and kidney
transplant patients. Int J Mol Med. 28:1093–1102. 2011.PubMed/NCBI
|
|
12
|
Ganji MR and Harririan A: Chronic
allograft dysfunction: Major contributing factors. Iran J Kidney
Dis. 6:88–93. 2012.PubMed/NCBI
|
|
13
|
Câmara NO, Williams WW Jr and
Pacheco-Silva A: Proximal tubular dysfunction as an indicator of
chronic graft dysfunction. Braz J Med Biol Res. 42:229–236. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levey AS, Bosch JP, Lewis JB, Greene T,
Rogers N and Roth D: A more accurate method to estimate glomerular
filtration rate from serum creatinine: A new prediction equation.
Modification of Diet in Renal Disease Study Group. Ann Intern Med.
130:461–470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashavaid TF, Raje HS, Shah BV and Shah SA:
Design of allele specific PCR for rapid detection of CYP3A5
(A6986G) and Mdr-1 (C3435T) polymorphisms. Indian J Clin Biochem.
26:18–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anglicheau D, Verstuyft C, Laurent-Puig P,
et al: Association of the multidrug resistance-1 gene
single-nucleotide polymorphisms with the tacrolimus dose
requirements in renal transplant recipients. J Am Soc Nephrol.
14:1889–1896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Larriba J, Imperiali N, Groppa R, Giordani
C, Algranatti S and Redal MA: Pharmacogenetics of immunosuppressant
polymorphism of CYP3A5 in renal transplant recipients. Transplant
Proc. 42:257–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamba JK, Lin YS, Schuetz EG and Thummel
KE: Genetic contribution to variable human CYP3A-mediated
metabolism. Adv Drug Deliv Rev. 54:1271–1294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gervasini G, Vizcaino S, Gasiba C,
Carrillo JA and Benitez J: Differences in CYP3A5* 3 genotype
distribution and combinations with other polymorphisms between
Spaniards and other Caucasian populations. Ther Drug Monit.
27:819–821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elmachad M, Elkabbaj D, Elkerch F, et al:
Frequencies of CYP3A5* 1/* 3 variants in a Moroccan population and
effect on tacrolimus daily dose requirements in renal transplant
patients. Genet Test Mol Biomarkers. 16:644–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ameyaw MM, Regateiro F, Li T, et al: MDR1
pharmacogenetics: Frequency of the C3435T mutation in exon 26 is
significantly influenced by ethnicity. Pharmacogenetics.
11:217–221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosso Felipe C, de Sandes TV, Sampaio EL,
Park SI, Silva HT Jr and Medina Pestana JO: Clinical impact of
polymorphisms of transport proteins and enzymes involved in the
metabolism of immunosuppressive drugs. Transplant Proc.
41:1441–1455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao LY, Huang CR, Hou JQ and Qian MY:
Association study of ABCB1 and CYP3A5 gene polymorphisms with
sirolimus trough concentration and dose requirements in Chinese
renal transplant recipients. Biopharm Drug Dispos. 29:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tada H, Tsuchiya N, Satoh S, et al: Impact
of CYP3A5 and MDR1 (ABCB1) C3435T polymorphisms on the
pharmacokinetics of tacrolimus in renal transplant recipients.
Transplant Proc. 37:1730–1732. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nie XM, Gui R, Zhao HS, et al: Influence
of CYP3A5 polymorphism on tacrolimus blood concentrations in renal
transplant patients. J Cent South Univ Technol. 12:(Sul 1).
S310–S313. 2005. View Article : Google Scholar
|
|
26
|
Zheng HX, Zeevi A, McCurry K, et al: The
impact of pharmacogenomic factors on acute persistent rejection in
adult lung transplant patients. Transpl Immunol. 14:37–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haufroid V, Mourad M, Van Kerckhove V, et
al: The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on
cyclosporine and tacrolimus dose requirements and trough blood
levels in stable renal transplant patients. Pharmacogenetics.
14:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López-Montenegro Soria MA, Kanter Berga J,
Beltrán Catalán S, Milara Payá J, Pallardó Mateu LM and Jiménez
Torres NV: Genetic polymorphisms and individualized tacrolimus
dosing. Transplant Proc. 42:3031–3033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hesselink DA, van Schaik RH, van der
Heiden IP, et al: Genetic polymorphisms of the CYP3A4, CYP3A5 and
MDR-1 genes and pharmacokinetics of the calcineurin inhibitors
cyclosporine and tacrolimus. Clin Pharmacol Ther. 74:245–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Hu X, Cai B, et al: Meta-analysis of
the effect of MDR1 C3435 polymorphism on tacrolimus
pharmacokinetics in renal transplant recipients. Transpl Immunol.
27:12–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glowacki F, Lionet A, Buob D, et al:
CYP3A5 and ABCB1 polymorphisms in donor and recipient: Impact on
tacrolimus dose requirements and clinical outcome after renal
transplantation. Nephrol Dial Transplant. 26:3046–3050. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuypers DR, Naesens M, de Jonge H, Lerut
E, Verbeke K and Vanrenterghem Y: Tacrolimus dose requirements and
CYP3A5 genotype and the development of calcineurin
inhibitor-associated nephrotoxicity in renal allograft recipients.
Ther Drug Monit. 32:394–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naesens M, Kuypers DR and Sarwal M:
Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol.
4:481–508. 2009.PubMed/NCBI
|