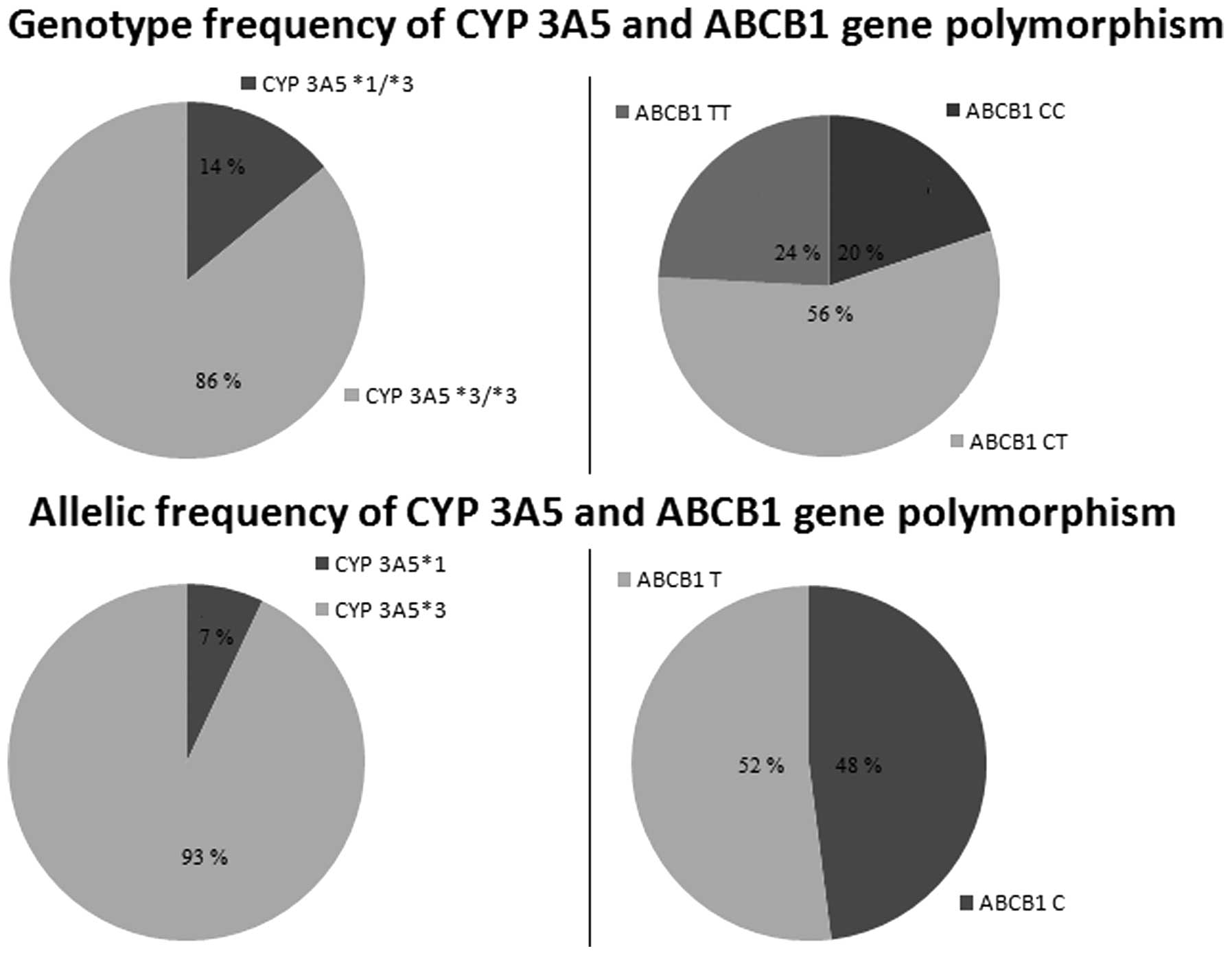
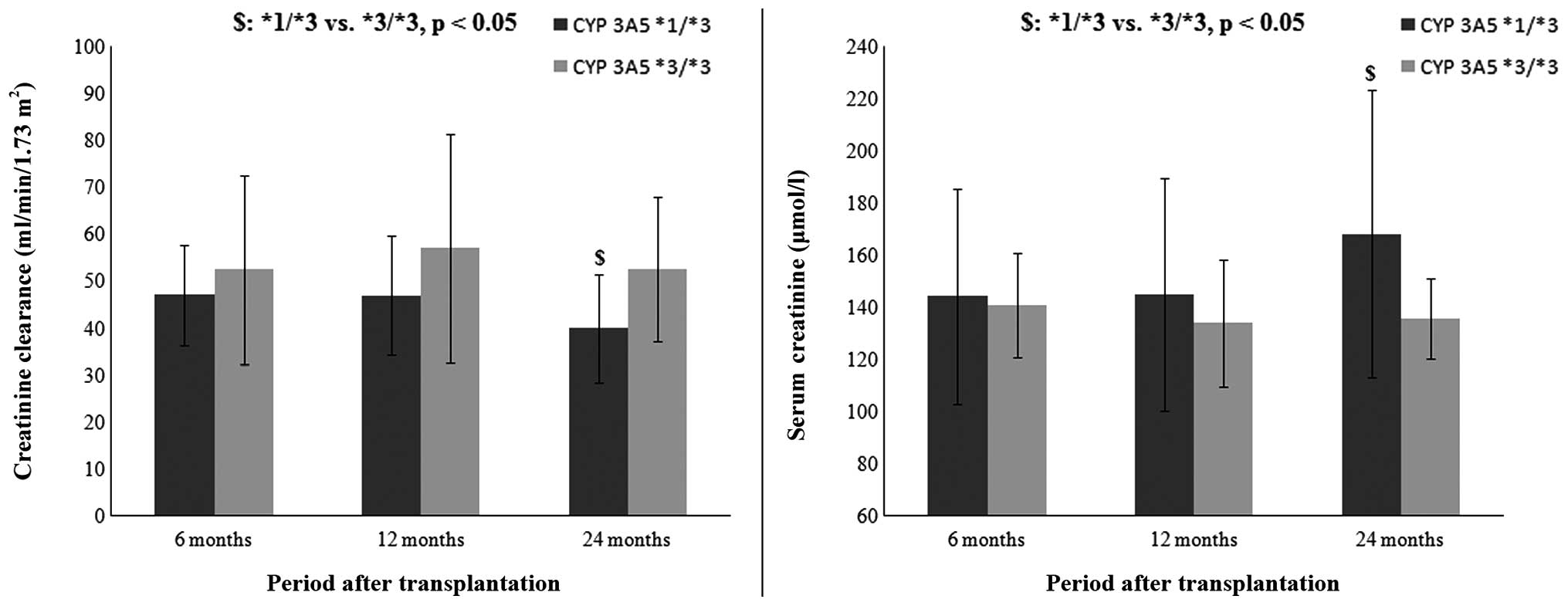
|
1
|
Press RR, de Fijter JW and Guchelaar HJ:
Individualizing calcineurin inhibitor therapy in renal
transplantation - current limitations and perspectives. Curr Pharm
Des. 16:176–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masuda S and Inui K: An up-date review on
individualized dosage adjustment of calcineurin inhibitors in organ
transplant patients. Pharmacol Ther. 112:184–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacPhee IA: Pharmacogenetic biomarkers:
Cytochrome P450 3A5. Clin Chim Acta. 413:1312–1317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velickovic-Radovanovic R, Mikov M,
Catic-Djordjevic A, et al: Tacrolimus as a part of
immunosuppressive treatment in kidney transplantation patients: Sex
differences. Gend Med. 9:471–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staatz CE, Goodman LK and Tett SE: Effect
of CYP3A and ABCB1 single nucleotide polymorphisms on the
pharmacokinetics and pharmacodynamics of calcineurin inhibitors:
Part I. Clin Pharmacokinet. 49:141–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Staatz CE, Goodman LK and Tett SE: Effect
of CYP3A and ABCB1 single nucleotide polymorphisms on the
pharmacokinetics and pharmacodynamics of calcineurin inhibitors:
Part II. Clin Pharmacokinet. 49:207–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuypers DR, de Jonge H, Naesens M, Lerut
E, Verbeke K and Vanrenterghem Y: CYP3A5 and CYP3A4 but not MDR1
single-nucleotide polymorphisms determine long-term tacrolimus
disposition and drug-related nephrotoxicity in renal recipients.
Clin Pharmacol Ther. 82:711–725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hesselink DA, Bouamar R, Elens L, van
Schaik RH and van Gelder T: The role of pharmacogenetics in the
disposition of and response to tacrolimus in solid organ
transplantation. Clin Pharmacokinet. 53:123–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cusinato DA, Lacchini R, Romao EA,
Moysés-Neto M and Coelho EB: Relationship of CYP3A5 genotype and
ABCB1 diplotype to tacrolimus disposition in Brazilian kidney
transplant patients. Br J Clin Pharmacol. 78:364–372. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurzawski M, Dąbrowska J, Dziewanowski K,
Domański L, Perużyńska M and Droździk M: CYP3A5 and CYP3A4, but not
ABCB1 polymorphisms affect tacrolimus dose-adjusted trough
concentrations in kidney transplant recipients. Pharmacogenomics.
15:179–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Provenzani A, Notarbartolo M, Labbozzetta
M, et al: Influence of CYP3A5 and ABCB1 gene polymorphisms and
other factors on tacrolimus dosing in Caucasian liver and kidney
transplant patients. Int J Mol Med. 28:1093–1102. 2011.PubMed/NCBI
|
|
12
|
Ganji MR and Harririan A: Chronic
allograft dysfunction: Major contributing factors. Iran J Kidney
Dis. 6:88–93. 2012.PubMed/NCBI
|
|
13
|
Câmara NO, Williams WW Jr and
Pacheco-Silva A: Proximal tubular dysfunction as an indicator of
chronic graft dysfunction. Braz J Med Biol Res. 42:229–236. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levey AS, Bosch JP, Lewis JB, Greene T,
Rogers N and Roth D: A more accurate method to estimate glomerular
filtration rate from serum creatinine: A new prediction equation.
Modification of Diet in Renal Disease Study Group. Ann Intern Med.
130:461–470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashavaid TF, Raje HS, Shah BV and Shah SA:
Design of allele specific PCR for rapid detection of CYP3A5
(A6986G) and Mdr-1 (C3435T) polymorphisms. Indian J Clin Biochem.
26:18–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anglicheau D, Verstuyft C, Laurent-Puig P,
et al: Association of the multidrug resistance-1 gene
single-nucleotide polymorphisms with the tacrolimus dose
requirements in renal transplant recipients. J Am Soc Nephrol.
14:1889–1896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Larriba J, Imperiali N, Groppa R, Giordani
C, Algranatti S and Redal MA: Pharmacogenetics of immunosuppressant
polymorphism of CYP3A5 in renal transplant recipients. Transplant
Proc. 42:257–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamba JK, Lin YS, Schuetz EG and Thummel
KE: Genetic contribution to variable human CYP3A-mediated
metabolism. Adv Drug Deliv Rev. 54:1271–1294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gervasini G, Vizcaino S, Gasiba C,
Carrillo JA and Benitez J: Differences in CYP3A5* 3 genotype
distribution and combinations with other polymorphisms between
Spaniards and other Caucasian populations. Ther Drug Monit.
27:819–821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elmachad M, Elkabbaj D, Elkerch F, et al:
Frequencies of CYP3A5* 1/* 3 variants in a Moroccan population and
effect on tacrolimus daily dose requirements in renal transplant
patients. Genet Test Mol Biomarkers. 16:644–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ameyaw MM, Regateiro F, Li T, et al: MDR1
pharmacogenetics: Frequency of the C3435T mutation in exon 26 is
significantly influenced by ethnicity. Pharmacogenetics.
11:217–221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosso Felipe C, de Sandes TV, Sampaio EL,
Park SI, Silva HT Jr and Medina Pestana JO: Clinical impact of
polymorphisms of transport proteins and enzymes involved in the
metabolism of immunosuppressive drugs. Transplant Proc.
41:1441–1455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao LY, Huang CR, Hou JQ and Qian MY:
Association study of ABCB1 and CYP3A5 gene polymorphisms with
sirolimus trough concentration and dose requirements in Chinese
renal transplant recipients. Biopharm Drug Dispos. 29:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tada H, Tsuchiya N, Satoh S, et al: Impact
of CYP3A5 and MDR1 (ABCB1) C3435T polymorphisms on the
pharmacokinetics of tacrolimus in renal transplant recipients.
Transplant Proc. 37:1730–1732. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nie XM, Gui R, Zhao HS, et al: Influence
of CYP3A5 polymorphism on tacrolimus blood concentrations in renal
transplant patients. J Cent South Univ Technol. 12:(Sul 1).
S310–S313. 2005. View Article : Google Scholar
|
|
26
|
Zheng HX, Zeevi A, McCurry K, et al: The
impact of pharmacogenomic factors on acute persistent rejection in
adult lung transplant patients. Transpl Immunol. 14:37–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haufroid V, Mourad M, Van Kerckhove V, et
al: The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on
cyclosporine and tacrolimus dose requirements and trough blood
levels in stable renal transplant patients. Pharmacogenetics.
14:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López-Montenegro Soria MA, Kanter Berga J,
Beltrán Catalán S, Milara Payá J, Pallardó Mateu LM and Jiménez
Torres NV: Genetic polymorphisms and individualized tacrolimus
dosing. Transplant Proc. 42:3031–3033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hesselink DA, van Schaik RH, van der
Heiden IP, et al: Genetic polymorphisms of the CYP3A4, CYP3A5 and
MDR-1 genes and pharmacokinetics of the calcineurin inhibitors
cyclosporine and tacrolimus. Clin Pharmacol Ther. 74:245–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Hu X, Cai B, et al: Meta-analysis of
the effect of MDR1 C3435 polymorphism on tacrolimus
pharmacokinetics in renal transplant recipients. Transpl Immunol.
27:12–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glowacki F, Lionet A, Buob D, et al:
CYP3A5 and ABCB1 polymorphisms in donor and recipient: Impact on
tacrolimus dose requirements and clinical outcome after renal
transplantation. Nephrol Dial Transplant. 26:3046–3050. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuypers DR, Naesens M, de Jonge H, Lerut
E, Verbeke K and Vanrenterghem Y: Tacrolimus dose requirements and
CYP3A5 genotype and the development of calcineurin
inhibitor-associated nephrotoxicity in renal allograft recipients.
Ther Drug Monit. 32:394–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naesens M, Kuypers DR and Sarwal M:
Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol.
4:481–508. 2009.PubMed/NCBI
|