Introduction
Gangliosides are molecules composed of a
glycosphingolipid, consisting of ceramide and oligosaccharides,
with one or more sialic acids linked to the sugar chain. The
molecules are components of the cell plasma membrane that modulate
cell signal transduction events, and have been shown to be
concentrated in lipid rafts. Gangliosides have recently been
identified as crucial molecules in neuronal apoptosis (1) and endoplasmic reticulum stress response
(2). Natural and semisynthetic
gangliosides are considered to be possible therapeutics for
neurodegenerative disorders (3).
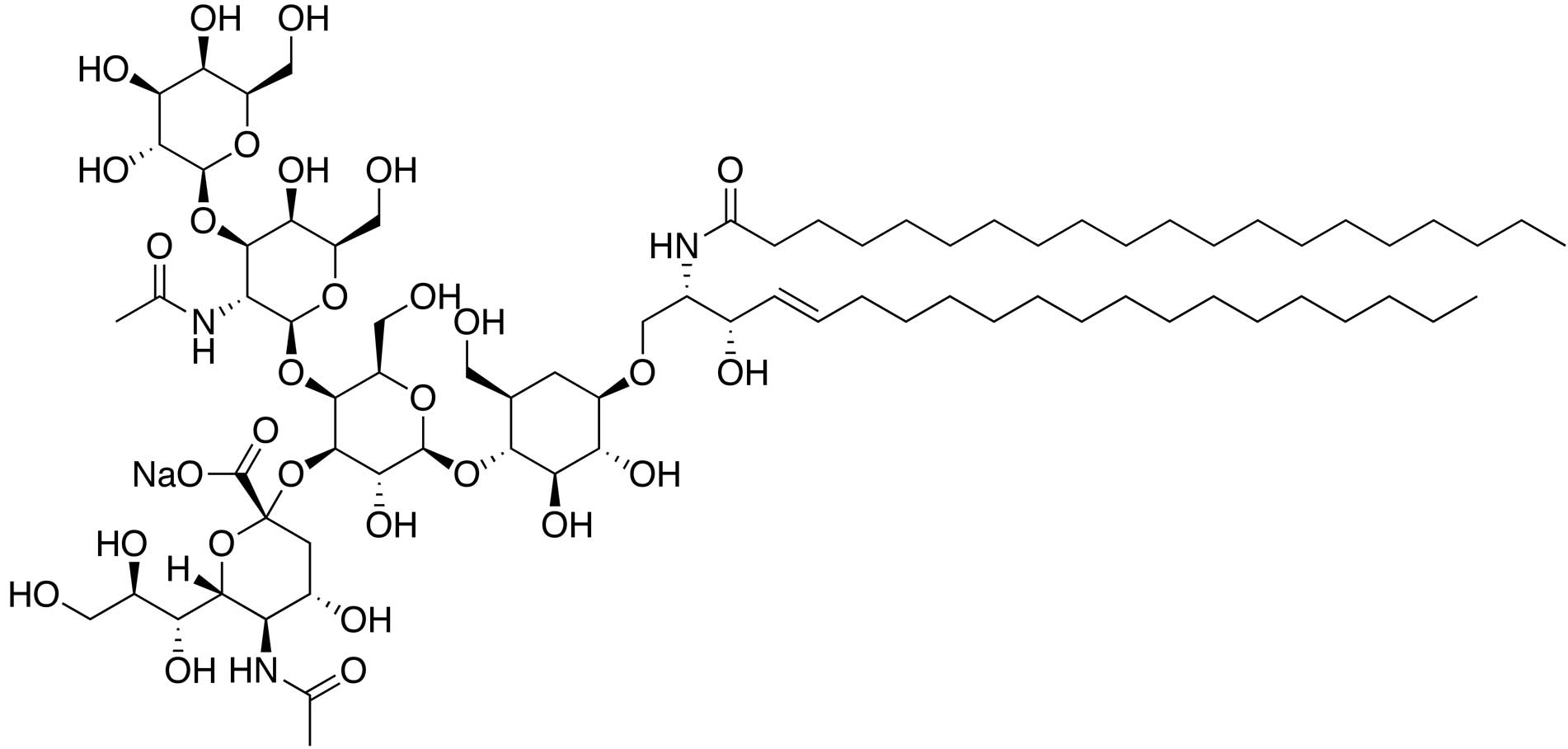
The natural structure of
monosialotetrahexosylganglioside (GM1) is galactose-N-acetyl
galactosamine-galactose-(glucose-ceramide)-sialic acid (Fig. 1). Semisynthetic gangliosides are
sodium salts of GM1, with a molecular formula of
C73H130N3NaO31 or
C75H134N3NaO31 and a
molecular weight of 1,568.84 or 1,597.18 Da, respectively (4). GM1 has been widely used to treat
neonatal hypoxic-ischemic brain injury, Parkinson's disease, acute
cerebral infarction, retinal ischemia and spinal cord injury
(5–7). In addition to neuroprotective effects
(8), GM1 has been shown to exert
protective effects on brain microvascular endothelial cells
(8), although the underlying
mechanisms remain unclear.
The phosphatidylinositol 3-kinase (PI3K)/glycogen
synthase kinase (GSK)-3 signaling pathway functions primarily as an
inhibitory pathway in cells, exerting effects on cell
proliferation. Nuclear factor (NF)-κB is a ubiquitous nuclear
factor in cells that induces numerous pathological processes,
including inflammation, immune cell proliferation and apoptosis.
The aim of the present study was to clarify the protective effects
and the mechanism of action of GM1 on human umbilical vein
endothelial cells (HUVECs).
Materials and methods
Reagents and antibodies
GM1 was supplied by Jilin Yinglian Biopharmaceutical
Co., Ltd. (Panshi, China). Dulbecco's modified Eagle's medium
(DMEM; SH30021.01) and newborn calf serum (SH30401.01) were
obtained from GE Healthcare Life Sciences (HyClone; Logan, UT,
USA). A Cell Counting Kit (CCK)-8 was purchased from Guangzhou
Yiyuan Biotechnology Co., Ltd. (Guangzhou, China). Mouse monoclonal
anti-PI3K p85 (sc-377482), anti-NF-κB p65 (sc-8008) and
anti-p-GSK-3 (sc-81496) antibodies were acquired from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). In addition, a mouse
anti-GAPDH monoclonal antibody (TA-08), horseradish peroxidase
(HRP)-labeled goat anti-mouse IgG (ZB-2305) and
tetramethylrhodamine (TRITC)-conjugated AffiniPure IgG (ZF-0313)
were obtained from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. (Beijing, China). An enhanced chemiluminescence (ECL)
detection kit (NCI5079) was purchased from EMD Millipore
(Billerica, MA, USA). Furthermore, a Bradford protein assay kit
(P0006), radioimmunoprecipitation assay (RIPA) lysis buffer
(P0013B) and a nuclear and cytosolic protein extraction kit (P0027)
were acquired from the Beyotime Institute of Biotechnology
(Guangzhou, China).
Cell culture and grouping
HUVECs were purchased from Shanghai Bogoo
Biotechnology Co., Ltd. (Shanghai, China). The HUVEC strain was
cultured and passaged routinely in DMEM culture medium containing
10% fetal bovine serum in 5% CO2 at 37°C. Subsequently,
the cells were divided into five groups. Only serum-free DMEM was
used in the control group. H2O2 was used to
induce lesions in the HUVECS. The
H2O2-treated cells
(H2O2 group) were cultured with 500 mmol/l
H2O2 in serum-free DMEM, while the cells in
the high-dose GM1 group (10-mg/l GM1; GM1 H group) were cultured in
serum-free DMEM with 500 mmol/l H2O2
containing 10 mg/l GM1. In addition, the medium-dose GM1 group
cells (5-mg/l GM1; GM1 M group) were cultured in serum-free DMEM
with 500 mmol/l H2O2 containing 5 mg/l GM1,
and the low-dose GM1 group cells (1-mg/l GM1; GM1 L group) were
cultured in serum-free DMEM with 500 mmol/l
H2O2 containing 1 mg/l GM1. All the cells
were cultured in serum-free DMEM for 12 h, which was exchanged for
the conditional medium, as aforementioned, in order to synchronize
the cells.
Detection of cell proliferation
Cell proliferation was detected using a CCK-8 assay.
HUVECs were plated in 96-well plates at a density of
~1×103 cells per well. Following treatment under the
different culture medium conditions for 24 h, 10 µl CCK-8 was added
and the plates were cultured for an additional 2 h, and the optical
density value at 490 nm was determined using a DNM-9606 plate
reader (Beijing Perlong Medical Equipment Co., Ltd., Beijing,
China). The cell viability was calculated according to the
following formula: Cell viability (%) = (treated group - control
group) × 100%. The experiment was repeated three times, using a
minimum of six wells for each group each time.
Cell cycle analysis
Flow cytometry (FCM) was applied to analyze the cell
cycle. HUVECs in a logarithmic growth phase were seeded in six-well
plates at a density of 5×105 cells per well. Following
treatment under the different culture medium conditions for 24 h,
the culture medium was discarded and the cells were collected via
routine trypsin digestion. Next, the cells were incubated overnight
with 1 ml alcohol (70%) at 4°C. Following centrifugation at 300 × g
for 5 min, the alcohol was discarded and the pellet was washed
three times with pre-chilled phosphate-buffered saline (PBS)
buffer. Finally, the cells were stained with 50 µm/ml propidium
iodide for 30 min at 4°C and the cell cycle ratio was evaluated
using a FACSCalibur cell analyzer (BD Biosciences, Franklin Lakes,
NJ, USA). At least 3 wells had been used for each group and the all
experiments were repeated 3 times.
Immunofluorescence assay
Briefly, cells in a logarithmic phase were seeded
into 24-well plates at a density of 5×103 cells per
well. After 24 h, following cell adhesion to the sides of the well,
the conditional medium was exchanged as aforementioned. The slides
were treated with 4% paraformaldehyde solution for 30 min, washed
three times in 0.01 mol/l PBS (pH 7.4) and placed in goat
non-immune serum (ZDR-5117; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 20 min at room
temperature. Next, the slides were transferred to an NF-κB p65
monoclonal antibody solution (1:200) and incubated overnight at
4°C. The slides were subsequently washed three times, as
aforementioned, and buffer containing TRITC-conjugated AffiniPure
goat anti-mouse IgG (1:1,000) was added to the slides, followed by
incubation for 1 h at room temperature. Finally, the slides were
sealed with glycerol and photographed using a Nikon 80i
fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Total protein detection
Total protein content in each sample was determined
using the Bradford method. According to the manufacturer's
instructions of the Bradford protein assay kit, 5 µl cell lysis
buffer was diluted to 20 µl in standard dilution buffer, followed
by mixing with 200 µl G-250 solution in a 96-well plate and
incubation for 3–5 min at room temperature. A standard curve was
generated using bovine serum albumin (BSA; A4503; Sigma-Aldrich,
St. Louis, MO, USA) as a reference. The absorbance was measured at
490 nm, and the resulting values were referenced with the standard
curve to calculate the protein concentration in the samples.
Western blot analysis
For the measurement of PI3K and p-GSK3 protein
expression levels, the cells were incubated for 3–5 min at room
temperature, after which the protein was extracted using ice-cold
RIPA buffer containing 2 µg/ml leupeptin, 2 µg/ml aprotinin, from
the previously mentioned RIPA and cytosolic protein extraction
kits, respectively, and 100 µg/ml phenylmethylsulfonyl fluoride
(IPFL00010; EMD Millipore). Protein concentrations were determined
using a Bradford method assay, as aforementioned. Subsequently,
~15-mg samples of whole-cell lysate protein were added to each lane
and resolved using 10% SDS-PAGE, after which the protein was
transferred to a 0.22-µM polyvinylidene fluoride membrane.
Non-specific biding sites on the membrane were blocked with BSA for
1 h at room temperature. Next, the membranes were incubated with
the mouse monoclonal PI3K (1:200) and p-GSK3α/β (1:200) primary
antibodies overnight at 4°C. GAPDH (1:1,000) was used as an
internal control, and the HRP-conjugated goat anti-mouse IgG
(1:2,000; 1 h at room temperature) was used as the secondary
antibody. The resultant signals were detected using ECL analysis,
and ImageJ software, version 1.46 (National Institutes of Health,
Bethesda, MD, USA) was employed to quantify the band densities. The
band densities of PI3K and p-GSK3α/β were normalized against that
of GAPDH throughout the experiment, which was used as the final
measure of expression.
NF-κB p65 expression assay
NF-κB p65 expression levels in the cytoplasm and
nucleus were assessed using western blot analysis. Firstly, total
nuclear and cytoplasmic protein was isolated using a protein
extraction kit. According to the manufacturer's instructions, the
cells were collected via scraping and lysed in cytoplasm extraction
solution. Following centrifugation at 12,000–16,000 × g for 5 min
at 4°C. The supernatant which containing cytoplasm protein was
retained, and nuclear extraction solution was added to the
precipitate in order to extract nuclear protein. Nuclear and the
cytoplasmic protein were analyzed using western blot analysis as
previously described.
Statistical analysis
All the experiments were independently replicated a
minimum of three times. The obtained data are expressed as the mean
± standard deviation, and the results were evaluated via one-way
analysis of variance using GraphPad Prism software, version 5
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Protective proliferation-inducing
effects of GM1 on H2O2-induced HUVEC
lesions
Results of the CCK-8 assay indicated that the cell
survival rate in the H2O2-treated group was
75.97% (79.97±11.77%), which was significantly reduced compared
with the control group (100%; Fig.
2A). In addition, when compared with the
H2O2-treated group, the cell survival rates
in the 10 and 5-mg/l GM1-treated groups were significantly reduced
(P<0.001 and P<0.05, respectively), whereas the difference in
the cell survival rate between the 1-mg/l GM1-treated group and the
control group was not statistically significant. Fig. 2B and C shows the FCM data used for
cell cycle analysis to quantify the ratio of cells in the
G2 and S phase in the various experimental groups. In
the control group, 50.87±4.29% of the total cells were in the G2
and S phases, while in the H2O2-treated
group, only 13.52% of the total cells were in the G2 and S phases,
indicating a significant difference between the two groups
(P<0.001). In the GM1-treated groups, the ratio of cells in the
G2 and S phases was significantly increased when
compared with the H2O2-treated group
(P<0.001; Fig. 2C).
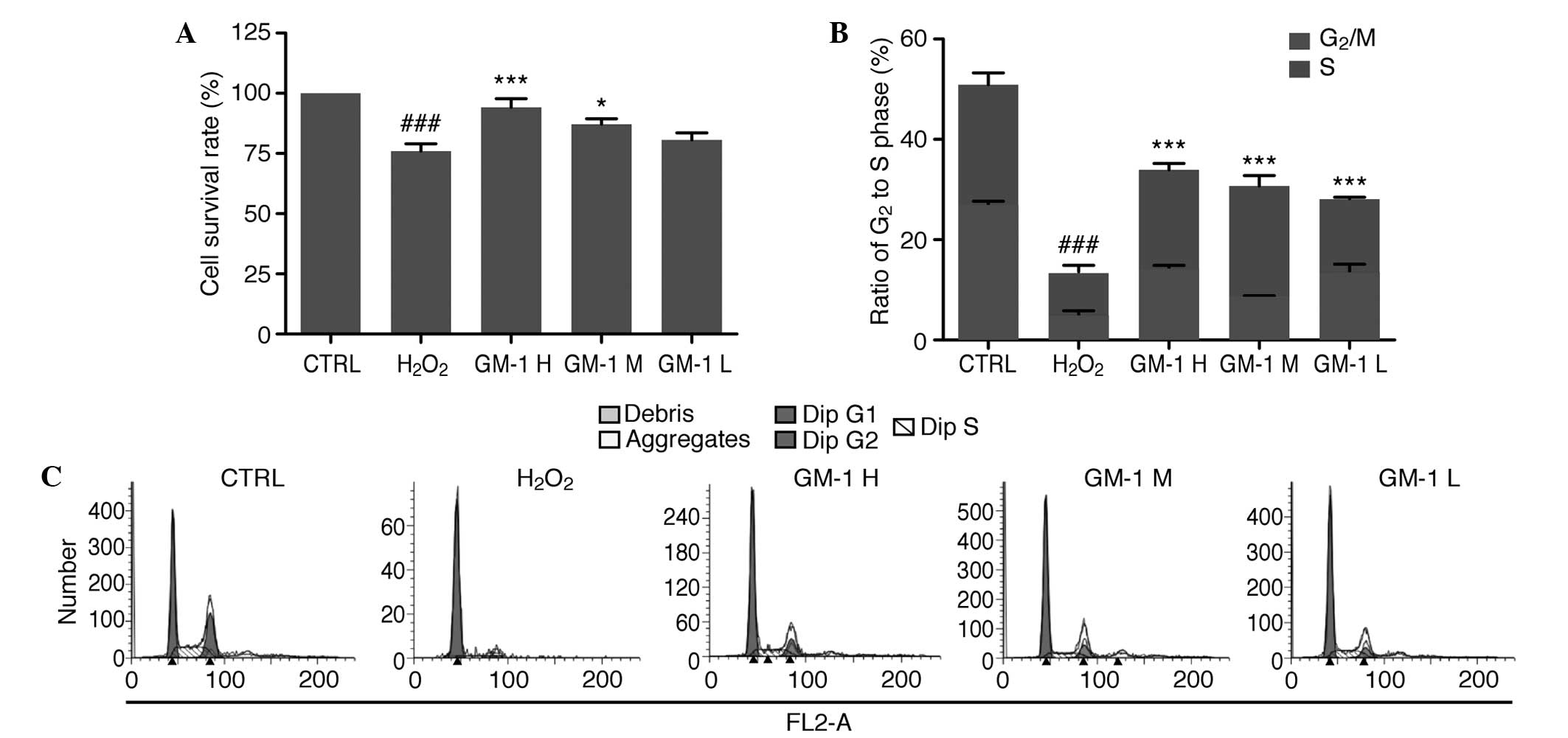 | Figure 2.Protective effects of GM1 on
H2O2-induced human umbilical vein endothelial
cell (HUVEC) lesions. (A) Cell survival rate of the HUVECs in the
H2O2-treated group was significantly
decreased compared with the control group, while treatment with
high and medium concentrations of GM1 significantly increased the
cell survival rate compared with the
H2O2-treated group. (B) Ratio of cells in the
G2 and S phases in the experimental groups. Compared with the
control group, the H2O2-treated group
exhibited a significantly decreased ratio. Compared with the
H2O2-treated group, all GM1-treated groups
exhibited significantly increased ratios. (C) Cell cycle of HUVECs
in the various groups analyzed by flow cytometry.
###P<0.00, vs. control group; *P<0.05, **P<0.01
and ***P<0.001 vs. H2O2-treated group.
CTRL, control; GM1, monosialotetrahexosylganglioside; H, high dose;
M, medium dose; L, low dose. |
Effects of GM1 on PI3K and GSK3α/β
expression levels in H2O2-induced HUVEC
lesions
Changes in the protein expression levels of PI3K and
GSK3α/β in the H2O2-induced HUVEC lesions
with or without the protective effects of GM1 were assessed using
western blot analysis (Fig. 3).
Densitometric analysis revealed significantly lower expression
levels of PI3K in the H2O2-treated group when
compared with the control group (P<0.01). Furthermore, PI3K
expression levels were significantly increased in the 10 and 5-mg/l
GM1-treated groups when compared with the
H2O2-treated group (P<0.01 and P<0.05,
respectively).
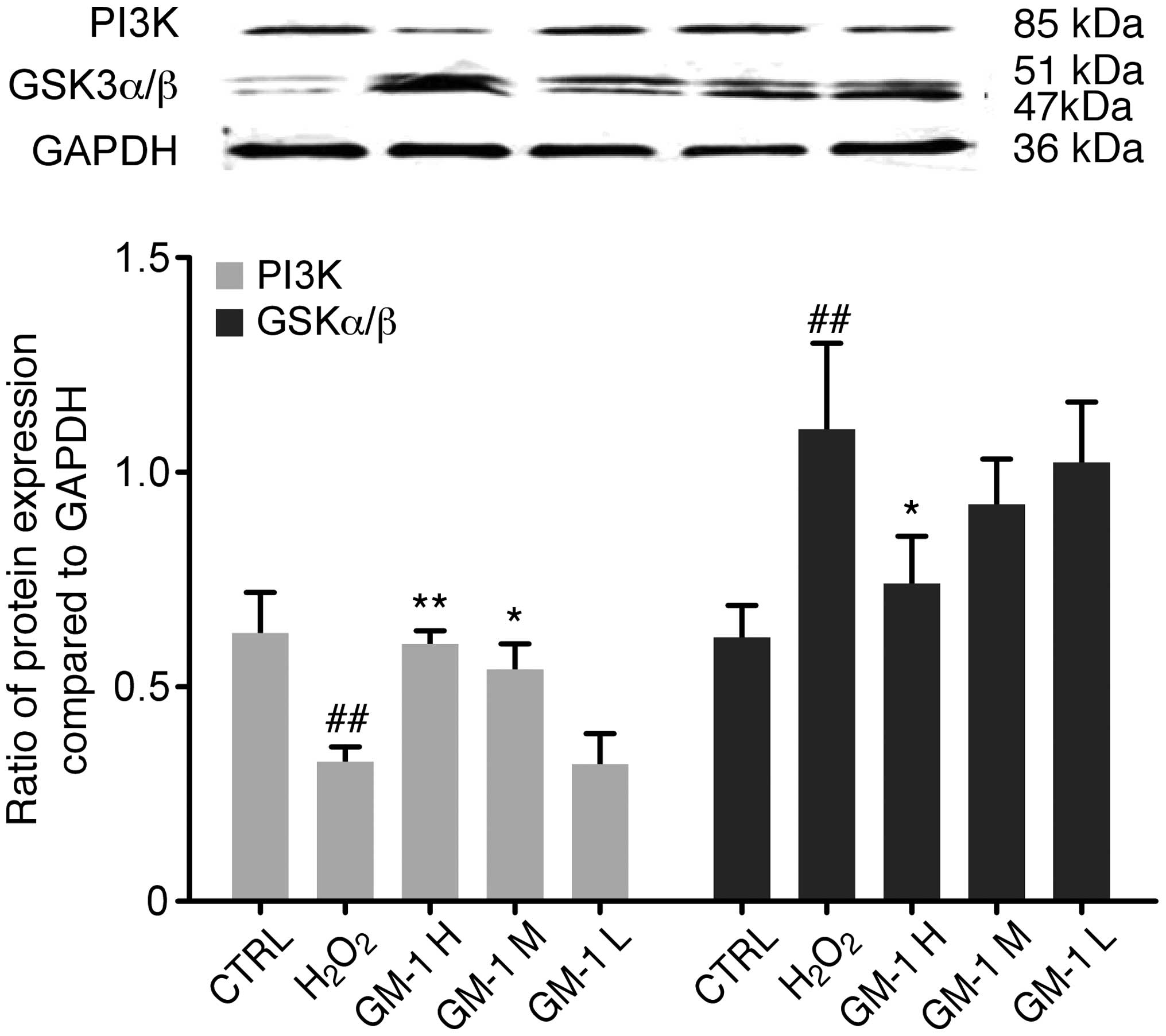 | Figure 3.PI3K and GSK-3 protein expression
levels were determined by western blot analysis. When compared with
the control group, PI3K expression in the
H2O2-treated group was decreased
significantly. When compared with the
H2O2-treated group, the high and medium
concentrations of GM1 significantly increased PI3K expression
levels. Compared with the control group, GSK3α/β expression in the
H2O2-treated group was increased
significantly. When compared with the
H2O2-treated group, the highest concentration
of GM1 significantly decreased GSK3α expression levels.
##P<0.01, vs. control group; *P<0.05 and
**P<0.01, vs. H2O2-treated group. CTRL,
control; GM1, monosialotetrahexosylganglioside; H, high dose; M,
medium dose; L, low dose; PI3K, phosphatidylinositol 3-kinase; GSK,
glycogen synthase kinase. |
With regard to GSK-3 expression, one band at 51 kDa
corresponded to p-GSK-3α, and the second band at 47 kDa
corresponded to p-GSK-3β. Densitometric analysis revealed that
p-GSK-3 expression levels in the H2O2-treated
cells were significantly increased compared with the control group
(P<0.01). In the GM1-treated groups, the p-GSK-3α/β expression
levels were decreased when compared with the
H2O2-treated group, and the differences were
statistically significant (P<0.05).
Effects of GM1 on NF-κB expression in
HUVECs
Observations from the indirect immunofluorescence
analysis revealed that NF-κB p65 was expressed predominantly in the
cytoplasm of the cells from the H2O2 group,
with reduced levels of expression observed in the nucleus (Fig. 4). In the control and GM1-treated
groups, the cytoplasm and the nucleus exhibited NF-κB p65
expression. When compared with the H2O2
treatment group, nuclear NF-κB p65 expression levels in the 10 and
5-mg/l GM1 treatment groups were significantly increased
(P<0.05).
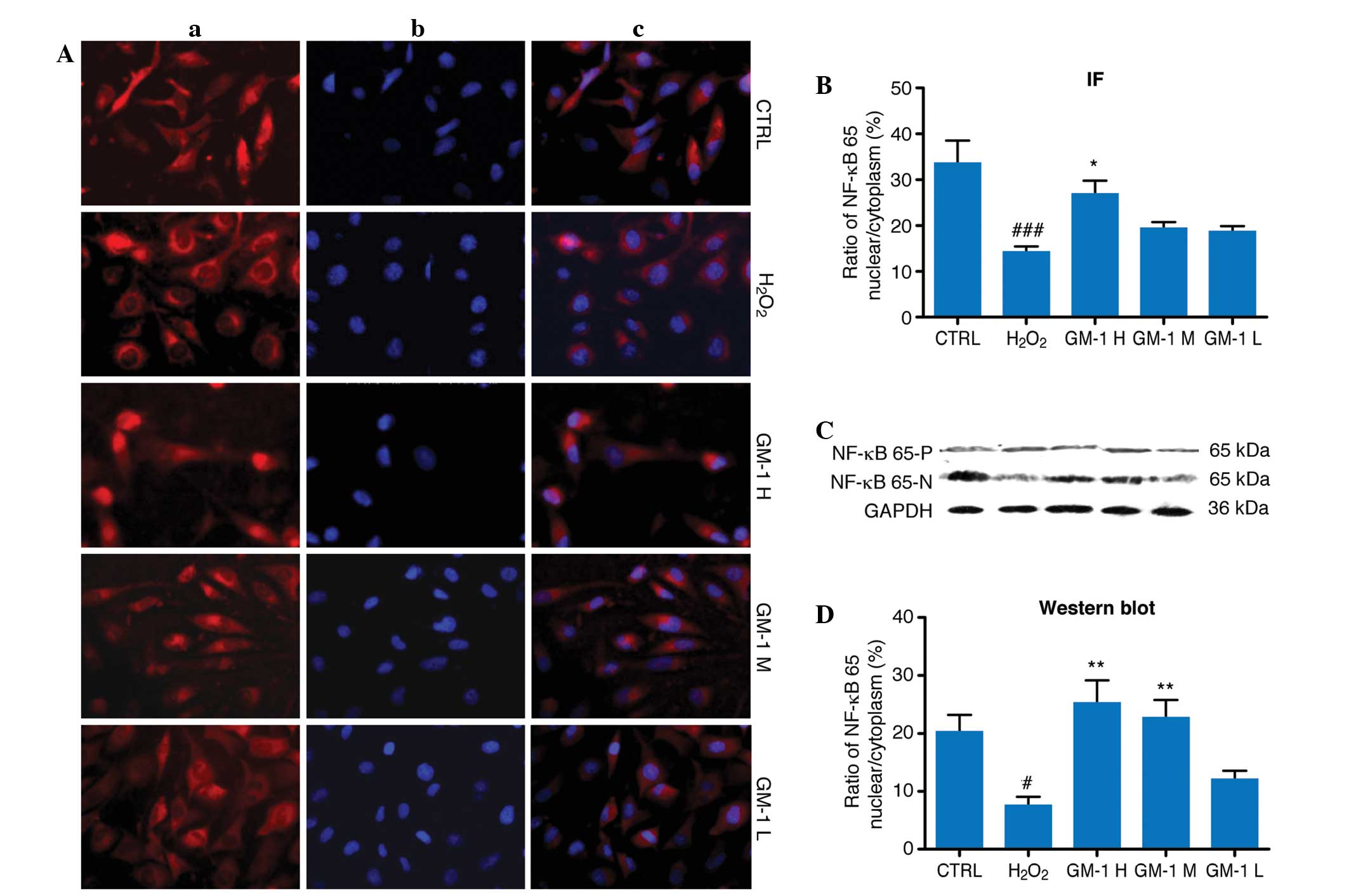 | Figure 4.Effects of GM1 on NF-κB expression in
human umbilical vein endothelial cells (HUVECs). (A) Examples of
the immunofluorescence microscopy images from the various groups:
(a) NF-κB positive signals are shown as red; (b) nuclei are stained
blue with DAPI; (c) composition of the NF-κB and nuclear staining.
(B) Nuclear-to-cytoplasmic ratio of NF-κB of the immunofluorescence
in various groups. Compared with the control group, the
nuclear-to-cytoplasm ratio of NF-κB in the
H2O2-treated group decreased significantly
(P<0.001), while that in the GM1 H group is markedly increased
compared with the H2O2-treated group
(P<0.05). (C) Representative of NF-κB expression in the nucleus
(NF-κB-N) and cytoplasm (NF-κB-P) of the various groups. GAPDH was
used as a positive control against NF-κB expression in the
cytoplasm. (D) Nuclear-to-cytoplasmic ratio of NF-κB of western
blot analysis in various groups. The nuclear-to-cytoplasmic ratio
of NF-κB is decreased in the H2O2-treated
group compared with the control group (P<0.05); while that in
the GM1 H and GM1 M groups were increased markedly compared with
the H2O2-treated group (P<0.05).
#P<0.05 and ###P<0.001 vs. control
group; *P<0.05 and **P<0.01 vs.
H2O2-treated group. CTRL, the control group;
H2O2, H2O2-treated
group; GM1, monosialotetrahexosylganglioside sodium; H, high dose;
M, medium dose; L, low dose; NF, nuclear factor; IF,
immunofluorescence. |
In the western blot assays, NF-κB p65 appeared as a
band with a molecular weight of 65 kDa. No statistically
significant differences were detected between the experimental
groups with regard to the cytoplasmic NF-κB p65 expression levels.
Comparison of the nuclear-to-cytoplasmic ratio of NF-κB p65
expression revealed a statistically significant reduction in the
H2O2-treated group when compared with the
control group. The nuclear-to-cytoplasmic ratios of NF-κB p65 in
the 10 and 5-mg/l GM1-treated groups were significantly increased
compared with the H2O2-treated group
(P<0.01).
Discussion
Vascular endothelial cell injury is a crucial factor
in the pathogenesis of a variety of vascular lesions, and
protecting endothelial function is vital for the prevention and
treatment of vascular disease (9).
H2O2 is a common reactive oxygen species that
promotes free radical generation, which is able to enter and
accumulate within cells, resulting in cellular damage (10). The reported concentrations of
H2O2 required to induce cell lesions varies
widely in the literature (11–13). In
the present study, 500 mmol/l H2O2 was used,
and the results showed that ~10% of the cells were in the DNA
synthesis (S) or mitotic (G2) phase of the cell cycle.
In addition, the cell survival rate was ~75% of that observed in
the control cells. These data indicate that the proliferation of
the HUVECs was inhibited within 24 h of 500 mmol/l
H2O2 treatment. Although GM1 is hypothesized
to function as a neuroprotective agent, the molecule exhibited a
significant protective effect on H2O2-injured
cells. The number of cells in the S and G2 phases
increased to ~35% in the GM1 H group, which was almost twice the
rate observed in the H2O2-treated group.
Although the mechanism underlying this observation remains unclear,
the outcome may be the result of the similarity between the
structures of GM1 and phosphatidylserine, which contributes to cell
entry and the maintenance of cell membrane integrity.
PI3K is a lipid kinase that catalyzes the
phosphorylation of phosphatidylinositol at the D3 position to yield
phosphatidylinositol (3,4,5)-triphosphate (PIP3).
PIP3 is a secondary messenger that transmits
extracellular signals to target proteins downstream of PI3K,
inducing numerous biological effects, including cell proliferation,
apoptosis and differentiation (14,15).
PI3K is composed of the p85 and p110 subunits; p85 lacks PI3K
activity and functions as an adapter, coupling p110 to an activated
protein tyrosine kinase. PI3K may be activated by numerous
extracellular signals that act via receptor tyrosine kinases or G
protein-coupled receptors, including growth factors, cytokines and
hormones (16). In the present
study, western blot analysis was applied to detect the protein
expression of PI3K in HUVECs cultured in DMEM, containing 500
mmol/l H2O2, with or without GM1 treatment.
After 24 h, PI3K expression levels were markedly increased in the
GM1-treated cells, indicating that PI3K may be involved in the
protective effects of GM1 on H2O2-treated
cells.
GSK-3 is a multifunctional serine/threonine protein
kinase that is hypothesized to be one of the primary target kinases
downstream of the PI3K signaling pathway. The majority of studies
support the hypothesis that GSK-3 is a key enzyme involved in the
regulation of a variety of cellular functions, although the enzyme
was initially identified as the rate-limiting enzyme in glycogen
metabolism (17). GSK-3 is able to
phosphorylate numerous proteins, including transcription factors;
thus, the enzyme is involved in the regulation of various cellular
functions, including sugar metabolism, the regulation of gene
expression and the maintenance of cytoskeletal integrity, and GSK-3
is particularly associated with apoptosis (18). The kinase activity of GSK-3 is
controlled via differential phosphorylation of the regulatory
serine/threonine residues, which exert an inhibitory effect, and
the regulatory tyrosine residues, which exhibit an activating
effect. There are two key forms of GSK-3: GSK-3α and GSK-3β. The
activity is controlled via the differential phosphorylation of its
regulatory serine/threonine residues, which has an transcription
factors that are responsible for coordinating processes, such as
glycogen synthesis and cell adhesion (19). Growth factor stimulation of mammalian
cells expressing GSK-3α and GSK-3β induces the phosphorylation of
Ser 21 and Ser 9, respectively, via the PI3K-dependent pathway,
which subsequently enhances proliferative signals. Additionally,
GSK-3 physically associates with cAMP-dependent protein kinase A
(PKA) (20), which phosphorylates
Ser 21 of GSK-3α or Ser 9 of GSK-3β, thereby inactivating the two
forms of GSK-3. GSK-3α and GSK-3β are positively regulated by the
phosphorylation of Tyr 279 and Tyr 216, respectively. Activated
GSK-3 participates in energy metabolism, neuronal cell development
and body pattern formation. Tyrosine dephosphorylation of GSK-3 is
involved in the extracellular signal-dependent inactivation of the
kinase (21). In the present study,
GSK-3α and GSK-3β were expressed at markedly higher levels
following H2O2 treatment, while GM1 treatment
decreased the expression levels of these proteins. These results
indicate that the protective effects of GM1 on the proliferation of
damaged HUVECs may involve the mitigation of GSK-3
overexpression.
NF-κB is a nuclear transcription factor that
regulates the expression of a large number of genes, which are
critical for the regulation of apoptosis, viral replication,
tumorigenesis, inflammation and various autoimmune diseases. NF-κB
consists of two subunits, p50 and p65, which are considered to be
involved in a stress response, since they are activated by a
variety of stimuli, including growth factors, cytokines,
lymphokines, ultraviolet radiation, pharmacological agents and
stress. If sequestered in the cytoplasm, NF-κB exists in the form
of a dimer comprised of the NF-κB p50 and p65 subunits, and the
dimer interacts with an inhibitor of κB (IκB), which prevents NF-κB
from exhibiting biological activity. The various stimuli that
activate NF-κB induce the phosphorylation of IκB, which is followed
by its ubiquitination and subsequent degradation. In the nucleus,
NF-κB binds to a consensus nucleotide sequence (5′-GGG ACT TTC
C-3′) within the promoters of various genes, subsequently
activating their transcription (22). The DNA-binding activity of NF-κB is
initiated, followed by rapid transport from the cytoplasm to the
nucleus in cells exposed to mitogens or growth factors (23). cDNAs encoding precursors of two
distinct proteins have been described and designated as p105 and
p100. In cerebral ischemia-reperfusion injury, NF-κB activation is
considered to be a key mediator in the process of inflammation and
the cascade reaction following ischemia (24). Nurmi et al (25) observed that after 24 h of cerebral
ischemia-reperfusion injury in rats, the activity of NF-κB was
increased by ~260% in the ischemic focus and surrounding areas. In
the present study, based on the obtained immunofluorescence
results, NF-κB p65 was shown to be predominantly expressed in the
cytoplasm, exhibiting reduced expression in the nucleus of the
cells exposed to H2O2. This observation
demonstrates that H2O2 treatment may induce a
resting state in the majority of HUVECs, in which
H2O2 inhibits HUVEC proliferation by blocking
NF-κB p65 translocation into the nucleus. Separate analysis of
NF-κB p65 expression in the cytoplasm and nucleus using western
blot analysis revealed that the nuclear-to-cytoplasmic ratio of
NF-κB p65 expression was ~10%, which is half the ratio observed in
the control cells. When GM1 was administered, the
nuclear-to-cytoplasmic ratio of NF-κB p65 expression increased
significantly, indicating that increased quantities of NF-κB p65
had entered the nucleus as a result of the treatment. As
aforementioned, increased rates of NF-κB p65 translocation into the
nucleus result in enhanced rates of transcription. Since NF-κB
translocates into the cytoplasm of resting cells and its activation
does not require other newly transcribed proteins as mediators,
NF-κB is considered to be a rapid reaction switch, enabling the
regulation of early gene expression in response to various
stimuli.
In conclusion, GM1 was demonstrated to exert marked
protective effects on the endothelium, which indicates the novel
application of GM1 for the treatment of brain injury. GM1 is able
to maintain the integrity of the endothelium and increase cell
mitosis, a process in which the PI3K/GSK-3 and NF-κB pathways are
crucially involved.
Acknowledgements
This study was supported by a Jilin province
Department of Health and Family Commission Foundation fellowship
(no. 20140414041GH) and a basic scientific research grant of Jilin
University of Jilin University. The authors thank Drs. Yu Xiaoyan
and Shi Yan for the assessment of pathological slides.
References
|
1
|
Tessitore A, del P Martin M, Sano R, Ma Y,
Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM and
d'Azzo A: GM1-ganglioside-mediated activation of the unfolded
protein response causes neuronal death in a neurodegenerative
gangliosidosis. Mol Cell. 15:753–766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
d'Azzo A, Tessitore A and Sano R:
Gangliosides as apoptotic signals in ER stress response. Cell Death
Differ. 13:404–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mocchetti I: Exogenous gangliosides,
neuronal plasticity and repair and the neurotrophins. Cell Mol Life
Sci. 62:2283–2294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarzmann G, Hofmann P and Pütz U:
Synthesis of ganglioside GM1 containing a thioglycosidic bond to
its labeled ceramide(s). A facile synthesis starting from natural
gangliosides. Carbohydr Res. 304:43–52. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang GZ and Li XG: Cerebral trauma,
Campylobacter jejuni infection, and
monosialotetrahexosylganglioside sodium mediated Guillain-Barré
syndrome in a Chinese patient: A rare case event. J Neuropsychiatry
Clin Neurosci. 26:E16–E17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hawryluk GW, Rowland J, Kwon BK and
Fehlings MG: Protection and repair of the injured spinal cord: A
review of completed, ongoing and planned clinical trials for acute
spinal cord injury. Neurosurg Focus. 25:E142008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masson E, Wiernsperger N, Lagarde M and El
Bawab S: Involvement of gangliosides in glucosamine-induced
proliferation decrease of retinal pericytes. Glycobiology.
15:585–591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frontczak-Baniewicz M, Gadamski R, Barskov
I and Gajkowska B: Beneficial effects of GM1 ganglioside on
photochemically-induced microvascular injury in cerebral cortex and
hypophysis in rat. Exp Toxicol Pathol. 52:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki Y, Nagai N and Umemura K: Novel
situations of endothelial injury in stroke - mechanisms of stroke
and strategy of drug development: Intracranial bleeding associated
with the treatment of ischemic stroke: Thrombolytic treatment of
ischemia-affected endothelial cells with tissue-type plasminogen
activator. J Pharmacol Sci. 116:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Touyz RM and Schiffrin EL: Reactive oxygen
species in vascular biology: Implications in hypertension.
Histochem Cell Biol. 122:339–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Gu L, Ma Q, Zhu D and Huang X:
Resveratrol attenuates hydrogen peroxide-induced apoptosis in human
umbilical vein endothelial cells. Eur Rev Med Pharmacol Sci.
17:88–94. 2013.PubMed/NCBI
|
|
12
|
Suo R, Zhao ZZ, Tang ZH, Ren Z, Liu X, Liu
LS, Wang Z, Tang CK, Wei DH and Jiang ZS: Hydrogen sulfide prevents
H2O2-induced senescence in human umbilical
vein endothelial cells through SIRT1 activation. Mol Med Rep.
7:1865–1870. 2013.PubMed/NCBI
|
|
13
|
Liu DH, Chen YM, Liu Y, Hao BS, Zhou B, Wu
L, Wang M, Chen L, Wu WK and Qian XX: Ginsenoside Rb1 reverses
H2O2-induced senescence in human umbilical
endothelial cells: Involvement of eNOS pathway. J Cardiovasc
Pharmacol. 59:222–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong SW, Jung KH, Lee HS, Choi MJ, Son MK,
Zheng HM and Hong SS: SB365 inhibits angiogenesis and induces
apoptosis of hepatocellular carcinoma through modulation of
PI3K/Akt/mTOR signaling pathway. Cancer Sci. 103:1929–1937. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi F, Wang YC, Zhao TZ, Zhang S, Du TY,
Yang CB, Li YH and Sun XQ: Effects of simulated microgravity on
human umbilical vein endothelial cell angiogenesis and role of the
PI3K-Akt-eNOS signal pathway. PLoS One. 7:e403652012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu C, Ha T, Wang X, Liu L, Zhang X,
Kimbrough EO, Sha Z, Guan M, Schweitzer J, Kalbfleisch J, Williams
D and Li C: The TLR9 ligand, CpG-ODN, induces protection against
cerebral ischemia/reperfusion injury via activation of PI3K/Akt
signaling. J Am Heart Assoc. 3:e0006292014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Zhang J, Chai S and Wang X:
Progesterone alleviates hypoxic-ischemic brain injury via the
Akt/GSK-3β signaling pathway. Exp Ther Med. 8:1241–1246.
2014.PubMed/NCBI
|
|
18
|
Choi SE, Kang Y, Jang HJ, Shin HC, Kim HE,
Kim HS, Kim HJ, Kim DJ and Lee KW: Involvement of glycogen synthase
kinase-3beta in palmitate-induced human umbilical vein endothelial
cell apoptosis. J Vasc Res. 44:365–374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doble BW and Woodgett JR: Role of glycogen
synthase kinase-3 in cell fate and epithelial-mesenchymal
transitions. Cells Tissues Organs. 185:73–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu HM, Lee CG, Hwang SJ and Kim SG:
Mitigation of carbon tetrachloride-induced hepatic injury by
methylene blue, a repurposed drug, is mediated by dual inhibition
of GSK3β downstream of PKA. Br J Pharmacol. 171:2790–2802. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eigler T, Ben-Shlomo A, Zhou C, Khalafi R,
Ren SG and Melmed S: Constitutive somatostatin receptor subtype-3
signaling suppresses growth hormone synthesis. Mol Endocrinol.
28:554–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JM, Lee EK, Park G, Kim MK, Yokozawa
T, Yu BP and Chung HY: Morin modulates the oxidative stress-induced
NF-kappaB pathway through its anti-oxidant activity. Free Radic
Res. 44:454–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urban MB and Baeuerle PA: The role of the
p50 and p65 subunits of NF-kappaB in the recognition of cognate
sequences. New Biol. 3:279–288. 1991.PubMed/NCBI
|
|
24
|
Howard EF, Chen Q, Cheng C, Carroll JE and
Hess D: NF-kappa B is activated and ICAM-1 gene expression is
upregulated during reoxygenation of human brain endothelial cells.
Neurosci Lett. 248:199–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nurmi A, Lindsberg PJ, Koistinaho M, Zhang
W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N,
Schwaninger M and Koistinaho J: Nuclear factor-kappaB contributes
to infarction after permanent focal ischemia. Stroke. 35:987–991.
2004. View Article : Google Scholar : PubMed/NCBI
|