Introduction
Fat transplantation is widely used in soft-tissue
augmentation, such as hemifacial atrophy and facial rejuvenation,
as well as nasolabial folds, and lip, chin, jaw and breast
augmentation. Autologous fat tissue, a potentially ideal filler for
soft-tissue defects, is safe and simple to harvest without
producing scars on donor or recipient sites and without
cross-infection or foreign-body reaction. However, the clinical
outcomes of this technique are limited by a low graft survival rate
(1–4). Previously, cytotherapy has been used in
autologous fat transplantation. In one study, a novel strategy was
developed, namely cell-assisted lipotransfer, in which the stromal
vascular fraction containing adipose-drived stem cells (ASCs) was
injected with the fat tissue. The results revealed improved graft
retention compared with the control treatment (1). In addition, mixing ASCs with fat tissue
prior to transplantation has been shown to enhance
neovascularization and increase the survival rate of fat grafts
(5). ASCs are known to secrete
angiogenic growth factors, such as vascular endothelial growth
factor (VEGF), hepatocyte growth factor (HGF) and basic fibroblast
growth factor (bFGF), in response to injury, hypoxia and other
conditions (6). VEGF and bFGF are
considered to mobilize and recruit endothelial (progenitor) cells,
subsequently accelerating the onset of angiogenesis (7). HGF is an additional important
endothelial growth factor with potential angiogenic and mitogenic
effects (8). Cotransplantation of
ASCs with aspirated fat tissue may improve graft retention due to
angiogenic effects. However, in vitro research has shown
that ASCs are able to survive for several days under ischemic
conditions (9). Therefore, the aim
of the present study was to further elucidate the role of ASCs in
the early stages following free fat transplantation.
Materials and methods
Cell isolation and culture
Ten 6 to 8-week-old green fluorescent protein
(GFP)-expressing C57BL/6 mice weighing 18–23 g (Model Animal
Research Center of Nanjing University, Nanjing, China) were
selected, regardless of gender, for collection of ASCs. ASCs were
isolated from the inguinal fat pad of C57BL/6 mice. The fat was
washed with phosphate-buffered saline, excised and digested with
0.125% collagenase (Sigma-Aldrich, St. Louis, MO, USA) on a shaker
at 37°C for 30 min. An equal volume of Dulbecco's modified Eagle's
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA) with 10%
fetal bovine serum (FBS; Gibco) was added to neutralize the
collagenase. Subsequently, the cell suspension was filtered through
a 200-mesh filter (Hebei Hongxia Medicine and Healthcare Product
Co. Ltd., Hebei, China), and the mature adipocytes and connective
tissue were separated from pellets by centrifugation at 1,200 × g
for 5 min. The cell pellets were resuspended, plated at a density
of 1×106 cells per 100-mm dish in DMEM with 10% FBS, and
cultured at 37°C in 5% CO2. Primary cells were cultured
for 7 days and were defined as passage 0. The medium was replaced
every 3 days, and the cells were passaged at a ratio of 1:3 per
week. Only cells that had been cultured for three passages were
used in the subsequent experiments.
Animal model and groups
Forty-six C57BL/6 mice (age, 6–8 weeks; weight,
18–23 g; Southern Medical University, Guangzhou, China) were
selected regardless of gender for use as free fat transplantation
models. The mice were anesthetized with 1% pentobarbital sodium (45
mg/kg). Ten of them were used to harvest inguinal fat pad, which
was cut into pieces, similar to the size of aspirated fat tissue
used for clinical fat injection in humans. The fat tissue samples
were injected subcutaneously into the back of a C57BL/6 mouse using
a 1 ml syringe with a standardized blunt tipped 14 gauge
infiltration cannula [the Coleman technique (10)]. Each C57BL/6 mouse was injected
subcutaneously at two spots with fat tissue (0.2 ml/spot). In the
experimental group, the fat tissue was mixed with 2×105
ASCs, while the control group received fat tissue only. At days 1,
4, 7, 14, 30 and 90 following fat transplantation, the grafts were
excised and analyzed (n=6/time-point). This study was approved by
the Ethics Committee of Nanfang hospital (Southern Medical
University, Guangzhou, China) and conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health, and all
efforts were made to minimize suffering.
Histology
Harvested adipose tissue samples were placed in 4%
formalin and embedded in paraffin. Sections with a thickness of 4
mm were cut from the paraffin blocks. Specimens were stained with
hematoxylin for 20 min, rinsed with Scott's solution for 1 min and
treated with 1% ammonia for 30 sec. Following incubation in 80%
ethanol for 5 min, the samples were stained with eosin for 2 min
and dehydrated with a graded ethanol series. The samples were
assessed under an Olympus BX51 microscope (Olympus, Tokyo, Japan)
and photographed using an Olympus DP71 digital camera.
Whole-mount staining of fat
grafts
Visualization of the fat grafts was performed using
the procedure outlined by Eto et al (11). Accordingly, the grafts were cut into
0.5–1-mm pieces and incubated with Hoechst 33342 (Sigma-Aldrich) to
stain all nuclei for 30 min. Subsequently, the samples were
incubated with propidium iodide (PI; S7112; Merck Millipore,
Darmstadt, Germany) for 15 min to stain for dead cells. The samples
were washed and observed directly with a confocal microscope system
(FV1000 confocal microscope; Olympus).
PI-positive cells were counted using four field
images for each sample. Subsequently, the ratio of dead cells was
calculated as follows: Number of PI- and GFP-positive cells/number
of Hoechst- and GFP-positive cells.
Enzyme-linked immunosorbent assay
(ELISA)
Adipose tissue specimens were homogenized in lysis
buffer (CWBiotech, Beijing, China), according to the manufacturer's
instructions, and centrifuged at 12,000 × g for 15 min at 4°C. The
aqueous layer was collected and assessed for VEGF and HGF secretion
using Quantikine ELISA kits (R&D Systems, Minneapolis, MN,
USA), according to the manufacturer's instructions. After washing
and aspirating five times with 400 µl wash buffer, the plates were
incubated for 2 h at room temperature with biotin-conjugated mouse
VEGF (cat. no. MMV00) and mouse/rat HGF (cat. no. MHG00) primary
antibodies, followed by a further five washes with 400 µl wash
buffer and incubation for 30 min at room temperature with
horseradish peroxidase-conjugated streptavidin (Thermo Scientific
Life Technologies, Carlsbad, CA, USA). The color was developed
using the enzymatic substrate, o-phenylenediamine, and the
optical density values of absorbance were measured on a microplate
reader (Multiskan MK3; Thermo Scientific Life Technologies) at 450
nm.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software package version 17.0 (SPSS Inc., Chicago, IL, USA)
was used for statistical analysis. A repeated measures analysis of
variance was used to analyze the results. Furthermore, if the test
revealed statistically significant differences, a paired Student's
t-test was used to compare the two groups at one time point, while
one-way analysis of variance was used to compared one group over
four time points. P<0.05 was considered to indicate a
statistically significant difference.
Results
Assessment of fat tissue survival
In the experimental group (group A), fat tissue was
mixed with ASCs, while the control group (group B) received fat
tissue only. Each mouse was injected with the two fat mixtures,
with each of the two designated spots receiving one of the two fat
mixtures in a random fashion. None of the animals succumbed during
the study. At every time-point, the grafts of six mice were
harvested by carefully removing them from the surrounding tissue.
The volume of the grafts was measured using a liquid overflow
method, which was subsequently used to calculate the survival ratio
with the following formula: Survival volume/previous volume. The
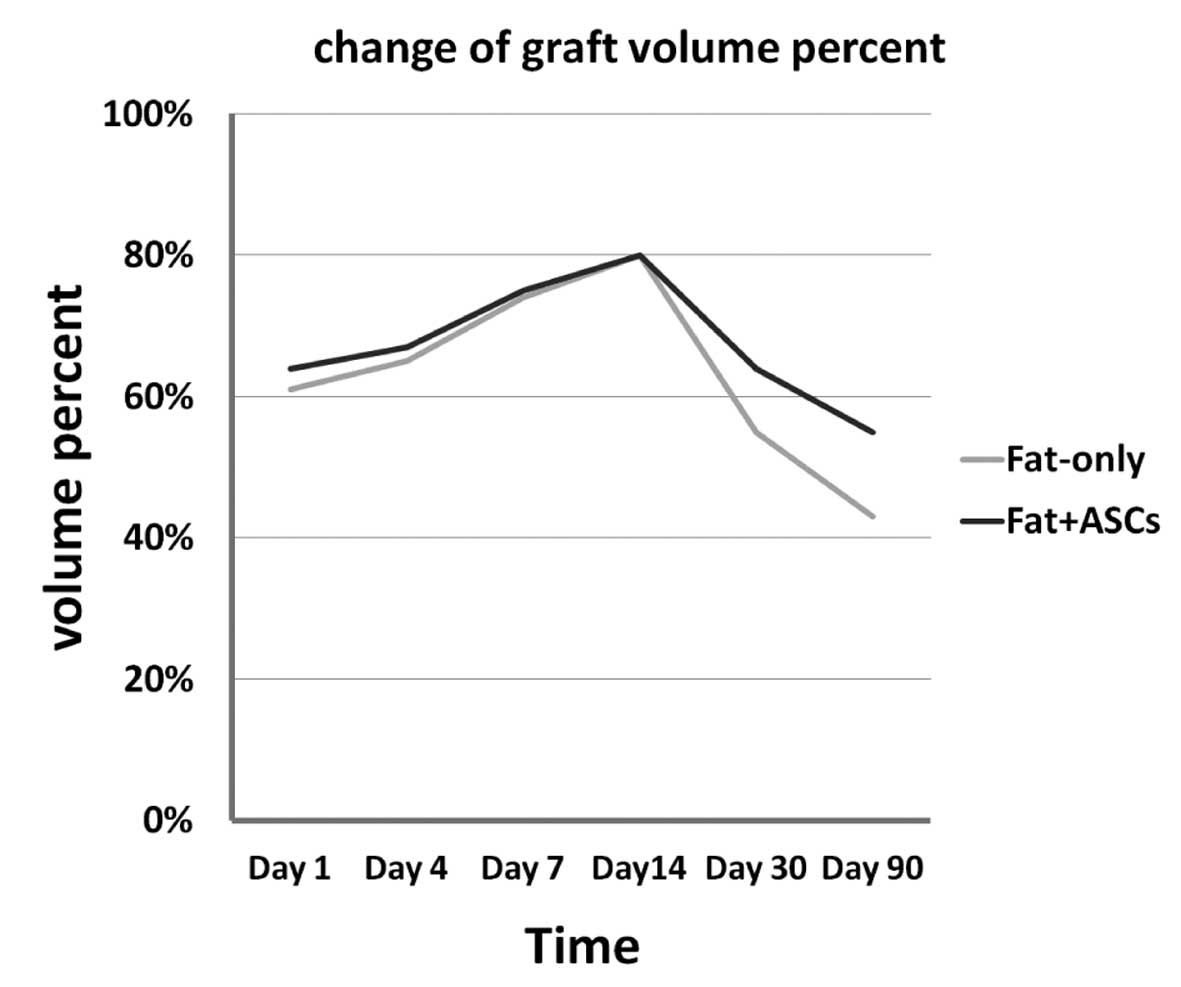
retention of grafts in the two groups is shown in Table I. In addition, the survival ratios
for groups A and B are shown in Fig.
1. The difference in the graft volumes between the ASC and
control groups was not statistically significant until day 14
following the aspirated fat transplantation, although the graft
volumes in the two groups were shown to marginally increase over
time up to day 14 and, after 14 days, the retention of grafts of
the ASC group was higher than the control group. However, the graft
survival rate notably decreased between days 14 and 30, and
continued to decrease gradually during the 30–90 day period. The
fat + ASCs group exhibited a higher graft survival rate compared
with the fat-only group.
 | Table I.Retention of the grafts in the two
groups at different time points. |
Table I.
Retention of the grafts in the two
groups at different time points.
| Day | Fat + ASCs, ml
(n=6) | Fat only, ml
(n=6) | P-value |
|---|
| 1 |
0.13±0.01 |
0.12±0.00 | 0.272 |
| 4 |
0.13±0.02 |
0.13±0.01 | 0.402 |
| 7 |
0.15±0.01 |
0.15±0.01 | 0.905 |
| 14 |
0.16±0.02 |
0.16±0.02 | 0.898 |
| 30 |
0.13±0.02 |
0.11±0.02 | 0.006 |
| 90 |
0.11±0.01 |
0.09±0.01 | 0.001 |
Histological evaluation of grafts
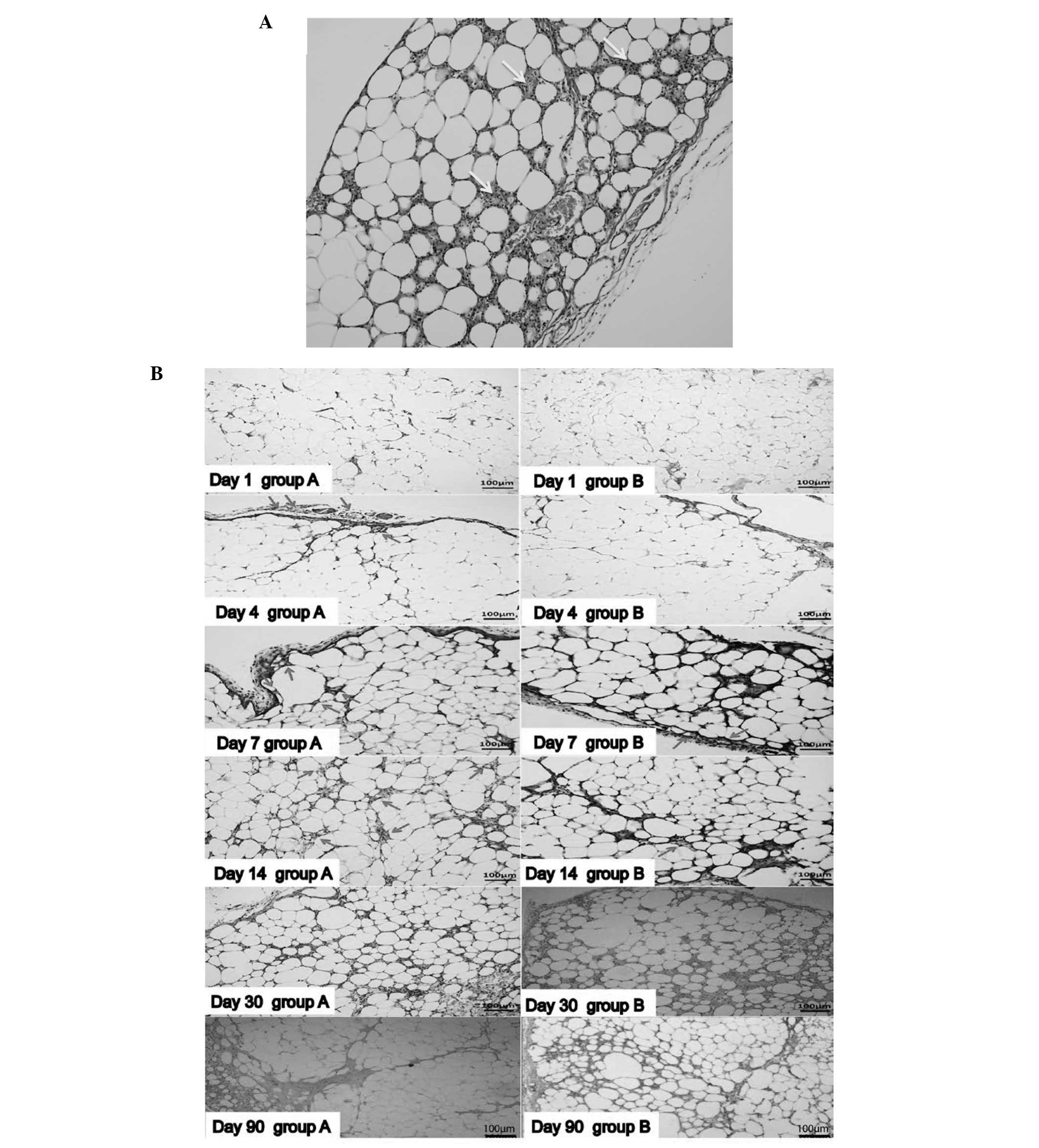
Histological evaluation revealed that a number of
the adipocytes decreased in size and increased in number over time.
On day 14, gaps between the adipocytes were observed, and
infiltrated nucleated cells became immersed in the interstitial
space between adipocytes in the two groups (Fig. 2A). On day 4, blood vessels on the
surface of the fat graft were observed in the fat + ASCs group. In
addition, vessels were shown to grow into the graft from the
surface of the graft. However, in the fat-only group, this
phenomenon appeared on day 7. The number of vessels in the grafts
increased on day 14 in the two groups (Fig. 2B); however, the fat + ASCs group
exhibited an increased number of blood vessels compared with the
control group. There was no evident fibrosis in either group in the
early stages following the aspirated fat transplantation. The two
groups presented an increasing number of infiltrated nucleated
cells and a number of vacuoles on day 30. The size of the
adipocytes varied. In the fat + ASCs group, the grafts were divided
into a regular grid by fibrous tissue on day 90; however, the
fat-only group exhibited an irregular structure with increased
fibrous tissue.
Whole-mount staining of the fat
grafts
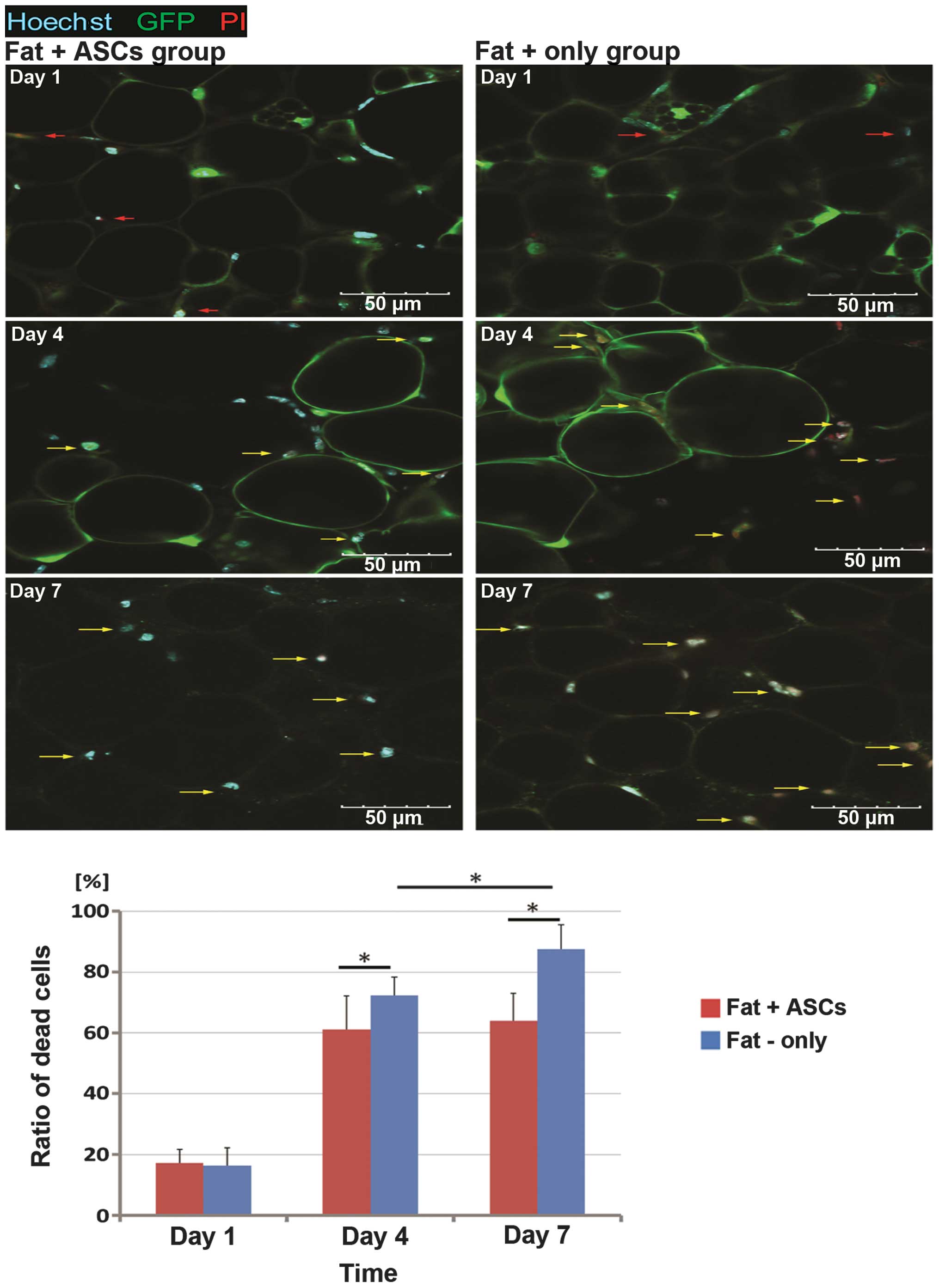
The ratio of PI-positive dead cells to the total
cells (without transplant ASCs) increased from day 1 (16.40±5.79%)
following the transplantation, with a sharp increase at day 4
(72.4±11.11%) and further increments at later time points (day 7,
87.48±8.11%) in group B (Fig. 3B).
The ratio of dead cells showed no statistically significant
difference between the two groups on day 1 (group A, 17.22±4.44%).
However, fewer dead cells were observed in group A compared with
group B at the later time points (day 4, 61.07±5.94%; day 7,
64.05±9.03%). No statistically significant difference in the dead
cell ratio was observed between days 4 and 7 in group A (Fig. 3A). The green fluorescence became
weaker over time in the two groups.
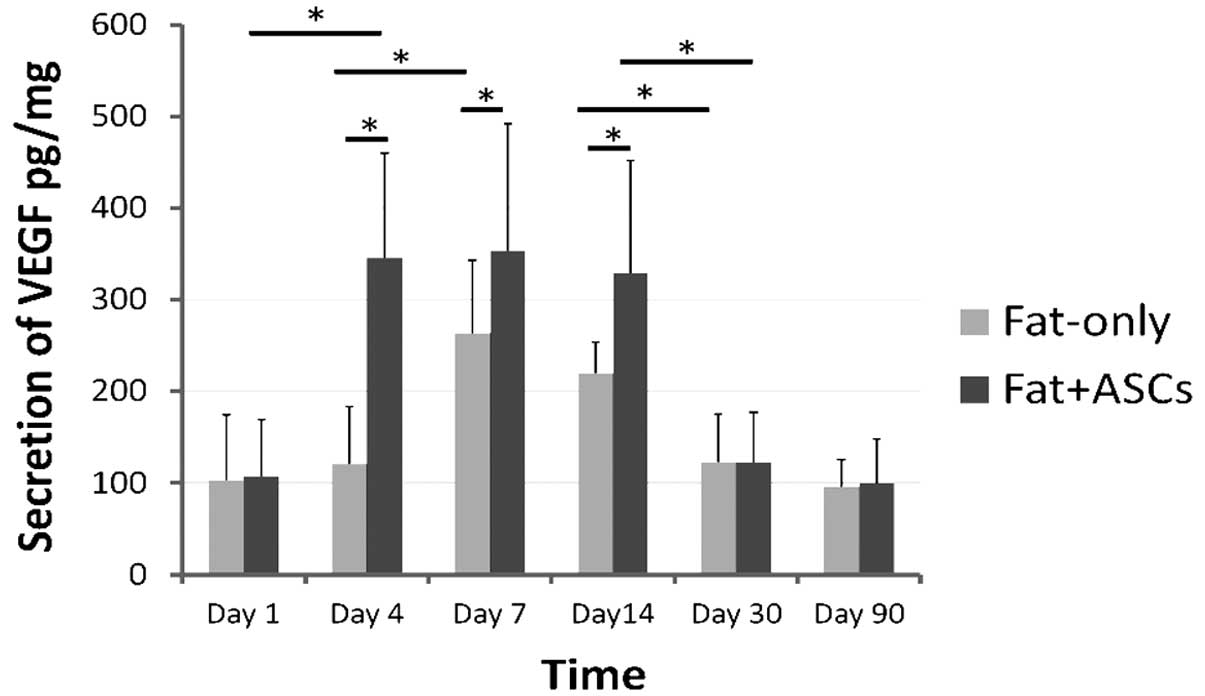
Expression of VEGF and HGF
In group B, the ELISA results demonstrated that the
secretion of VEGF increased significantly on day 7, after which the
levels decreased gradually. By contrast, VEGF protein levels peaked
between days 4 and 14 following transplantation in group A
(Fig. 4), which indicated that the
secretion of VEGF by the ASCs had an earlier peak time and higher
level, as shown by the comparison between group A and group B. The
two groups exhibited low expression levels of VEGF after 30
days.
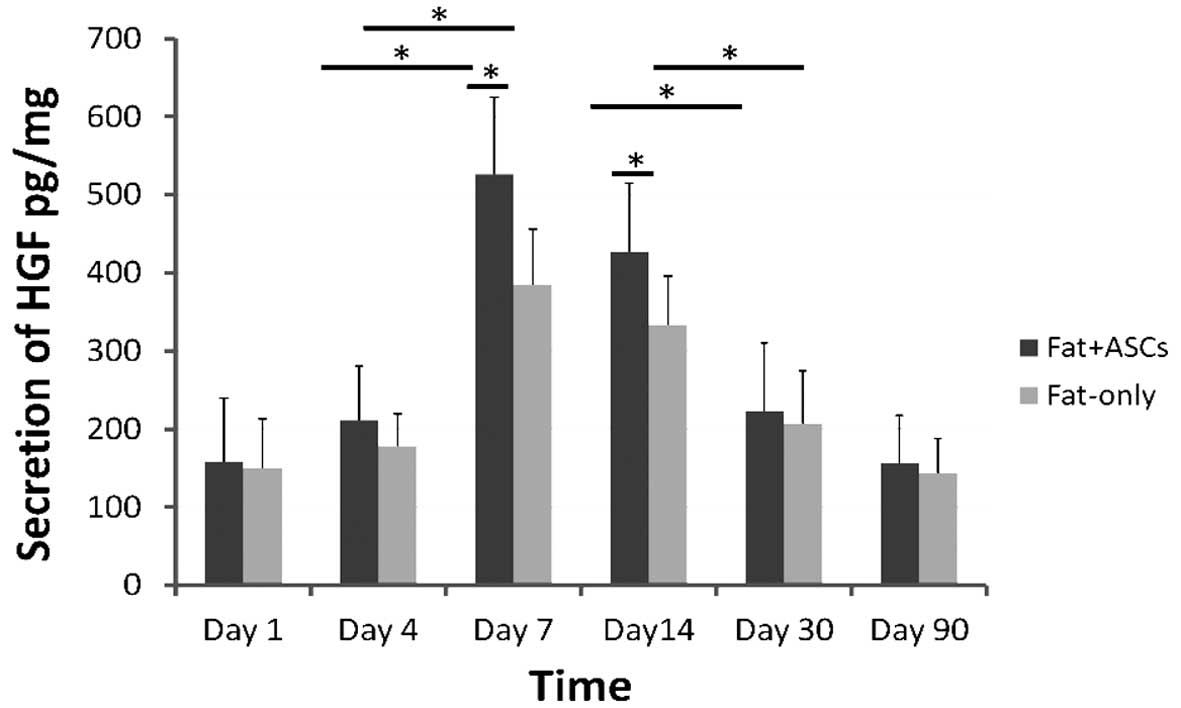
Following transplantation, the secretion of HGF
peaked on day 7 and decreased gradually between days 14 and 90 in
the two groups (Fig. 5). However,
higher levels of HGF secretion were observed in group A when
compared with group B.
Discussion
A number of studies have focused on ASCs as helper
cells in autologous fat transplantation. Lu et al (5) reported that ASCs significantly increase
the transplant survival rate (60.1±17.6%), as compared with a
control group (27.1±8.2%). Furthermore, in a clinical study,
Yoshimura et al (1) used
ASC-assisted lipotransfer for facial lipoatrophy and obtained
improved clinical results.
However, in contrast to these studies, the present
study revealed that there was no statistically significant
difference between the fat-only and the fat + ASCs groups in the
graft survival rate immediately following the aspirated fat
transplantation (days 1–14). The results indicated that the
difference between the two groups was observed in the graft tissue
structure rather than the graft survival rate. With regard to the
histology examination, group A exhibited increased vascularization
compared with group B at days 4, 7 and 14.
The graft survival rate of the two groups decreased
markedly during days 14–30, and continued to decrease gradually
between days 30 and 90. However, the fat + ASCs group had a higher
survival rate compared with the fat-only group, which resulted from
the difference in the histological structure between the two groups
prior to day 14. Necrosis of the fat tissue is primarily observed
on days 3 and 7 under ischemic conditions (12). Therefore, the decrease in the graft
volume between days 14 and 90 may be due to the absorption of
necrotic tissue and fibrosis following necrosis. Adipogenesis and
angiogenesis are exerted by activated adipose stem/progenitor
cells, and completion of these processes requires several months. A
number of the dead adipocytes are replaced with new next-generation
adipocytes during the first three months while others are not
(13). Lipid droplets are absorbed
by macrophage phagocytosis, although the absorption is very slow
and the absorption period is dependent on the diameter of the lipid
droplets; when the lipid droplet diameter was large (e.g. 10 mm),
the cyst wall was formed before completing absorption and the cyst
wall calcified over time.
A previous study offered insight into the
histological changes of graft fat (14). On day 1 following the fat injection,
the number of viable adipocytes is markedly decreased, and the
viable zone is restricted to the peripheral area (<300 µm from
the edge) (9). In the present study,
the whole-mount stained images show a degree of cell death on day
1, with the nucleus of the dead cells localized within the
ring-like GFP-positive area. A previous study indicated that
excised adipose tissue contained 10–15% adipocytes (11). The majority of the dead cells on day
1 may represent the dead adipocytes. The green fluorescence became
weaker over time, which may be due to an increase in adipocyte
necrosis, leading to GFP degradation.
On day 4, graft angiogenesis occurs from the host
bed vascular system (15). A similar
result was obtained in the present study, and the histological
evaluation of the fat graft indicated that the mice that received
ASCs had a more highly vascularized fat graft compared with the
control mice at days 4, 7 and 14. In addition, an increased number
of inflammatory cells were observed in the area of high-density
neovascularization. This phenomenon may be partly associated with
macrophages, which promote angiogenesis. Additional studies have
indicated that macrophages appear to undergo marked phenotypic
changes in tumors when exposed to hypoxia, and activate the
expression of a number of mitogenic and proangiogenic cytokines and
enzymes (16). Regenerative
adipogenic changes, such as an increasing number and size of new
adipocytes, initiate from the peripheral dead adipocyte area at day
7 following fat transplantation (9).
In the present study, smaller adipocytes, as compared with normal,
were observed after day 7. The small adipocytes were hypothesized
to be derived from the surviving interstitial cells (including the
graft-resident ASCs that contribute to adipogenesis), host-derived
precursors or the dedifferentiated adipocytes; however, further
research is required to indicate the origin of the small
adipocytes. In the later stages following fat transplantation,
fibrous tissue growth occurred instead of adipocyte necrosis.
Nevertheless, there was no evident fibrosis in either group during
the early stages following the fat transplantation.
The results of the present study demonstrated that
ASCs enhanced the density of neovascularization, although this had
no effect on the survival rate of the graft fat in the early stages
following transplantation. Angiogenic growth factors secreted by
ASCs, such as VEGF and HGF, may be responsible for this finding.
The injection force mechanically injures the fat, and
non-vascularization causes ischemic injury. ASCs can secrete
various angiogenic growth factors, such as VEGF, HGF and bFGF, in
response to injury, hypoxia and other conditions (6). Lee et al (17) indicated that ASC proliferation and
secretion of angiogenic growth factors, such as VEGF and bFGF, were
significantly increased under hypoxic conditions (2%
O2). Similarly, Rubina et al (18) reported that the mRNA expression
levels of angiogenic growth factors, such as VEGF, HGF and bFGF,
increased from 1.7 to 4.1-fold in response to hypoxia. Furthermore,
an additional study that used a Wister rat model of free fat grafts
indicated that VEGF was expressed in the interstitial mononuclear
cells, predominantly on day 7, after which the levels decreased
(19), which was confirmed in the
present experiments. However, in the current study, the ASCs caused
VEGF secretion to peak earlier in the fat + ASCs group on day 4.
HGF is an additional important endothelial growth factor with
potential angiogenic and mitogenic effects (8). ASCs maintained the secretion of HGF at
a high level following transplantation. Following mechanical injury
and ischemia-reperfusion injury to human adipose tissue, fibroblast
growth factor-2, epidermal growth factor, transforming growth
factor-β and platelet-derived growth factor are secreted during the
early stages of wound healing (days 0–1). Thereafter, as the levels
of these growth factors decrease, VEGF and HGF secretion gradually
increases between days 5 and 7 post-injury (19). In the present study, the
transplantation of ASCs resulted in increased VEGF and HGF
secretion, which caused VEGF levels to peak earlier and maintain
the secretion of HGF at a higher level. ASCs may contribute to
angiogenesis by secreting angiogenic growth factors, which appear
in the early stages following aspirated fat transplantation. In
vitro studies have revealed that ASCs remain viable for only
three days under ischemic conditions (9). Since free fat transplantation causes an
ischemic condition, cell death occurs in the majority of the
transplanted ASCs during the first week following transplantation.
Subsequently, angiogenesis is initiated at ~day 4 when the majority
of adipocytes have undergone cell death (15). Due to the greater hypoxia tolerance
of ASCs compared with adipocytes and the earlier initiation of
angiogenesis caused by the transplanted ASCs, there were ~43% of
interstitial cells surviving at day 4 in group A. However, <30%
of interstitial cells had survived at day 4 in group B, and the
number of living interstitial cells continued to decrease to day 7.
The transplanted ASCs prevented the further increase in
interstitial cell death and promoted angiogenesis from the host
sooner by secreting angiogenic growth factors, which contributed to
the survival of interstitial cells (including the graft-resident
ASCs).
In conclusion, exogenous ASCs may not directly
participate in angiogenesis and adipogenesis following free fat
transplantation, but instead may promote the survival ratio of the
graft-resident interstitial cells via a paracrine effect, which are
involved in angiogenesis and adipogenesis. The preliminary results
suggest that exogenous ASCs are effective in fat transplantation,
which provides an experimental basis for further research and
clinical work.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81171834 and
81372083) and the Science and Technology Key Program of Guangzhou,
China (grant no. 11C32120716).
References
|
1
|
Yoshimura K, Sato K, Aoi N, et al:
Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of
clinical use of adipose-derived stem cells. Dermatol Surg.
34:1178–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshimura K, Asano Y, Aoi N, et al:
Progenitor-enriched adipose tissue transplantation as rescue for
breast implant complications. Breast J. 16:169–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longobardi G, Pellini E, Diana G and
Finocchi V: Rhytidectomy associated with autologous fat
transplantation in Parry-Romberg syndrome. J Craniofac Surg.
22:1031–1034. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sykes JM, Tapias V and Pu LL: Autologous
fat grafting viability: Lower third of the face. Facial Plast Surg.
26:376–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu F, Li J, Gao J, et al: Improvement of
the survival of human autologous fat transplantation by using
VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg.
124:14372009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshimura K, Suga H and Eto H:
Adipose-derived stem/progenitor cells: Roles in adipose tissue
remodeling and potential use for soft tissue augmentation. Regen
Med. 4:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirschi KK, Skalak TC, Peirce SM and
Little CD: Vascular assembly in natural and engineered tissues. Ann
N Y Acad Sci. 961:223–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morishita R, Sakaki M, Yamamoto K, et al:
Impairment of collateral formation in lipoprotein(a) transgenic
mice: Therapeutic angiogenesis induced by human hepatocyte growth
factor gene. Circulation. 105:1491–1496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eto H, Kato H, Suga H, et al: The fate of
adipocytes after non-vascularized fat grafting: Evidence of early
death and replacement of adipocytes. Plast Reconstr Surg.
129:1093–1095. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coleman SR: Facial recontouring with
lipostructure. Clin Plast Surg. 24:347–367. 1997.PubMed/NCBI
|
|
11
|
Eto H, Suga H, Matsumoto D, et al:
Characterization of structure and cellular components of aspirated
and excised adipose tissue. Plast Reconstr Surg. 124:1087–1097.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suga H, Eto H, Aoi N, et al: Adipose
tissue remodeling under ischemia: Death of adipocytes and
activation of stem/progenitor cells. Plast Reconstr Surg.
126:1911–1923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshimura K, Eto H, Kato H, et al: In
vivo manipulation of stem cells for adipose tissue
repair/reconstruction. Regen Med. 6:33–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rieck B and Schlaak S: Measurement in
vivo of the survival rate in autologous adipocyte
transplantation. Plast Reconstr Surg. 111:2315–2323. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Billings E Jr and May JW Jr: Historical
review and present status fo free fat graft autotransplantation in
plastic and reconstructive surgery. Plast Reconstr Surg.
83:368–381. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murdoch C, Giannoudis A and Lewis CE:
Mechanisms regulating the recruitment of macrophages into hypoxic
areas of tumors and other ischemic tissues. Blood. 104:2224–2234.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee EY, Xia Y, Kim WS, et al:
Hypoxia-enhanced wound-healing function of adipose-derived stem
cells: Increase in stem cell proliferation and up-regulation of
VEGF and bFGF. Wound Repair Regen. 17:540–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubina K, Kalinina N, Efimenko A, et al:
Adipose stromal cells stimulate angiogenesis via promoting
progenitor cell differentiation, secretion of angiogenic factors,
and enhancing vessel maturation. Tissue Eng Part A. 15:2039–2050.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishimura T, Hashimoto H, Nakanishi I and
Furukawa M: Microvascular angiogenesis and apoptosis in the
survival of free fat grafts. Laryngoscope. 110:1333–1338. 2000.
View Article : Google Scholar : PubMed/NCBI
|