Introduction
Adenosine is an endogenous bioactive substance
widely present in the intracellular and extracellular fluid in
mammals. Almost all cells, including Müller cells, can release
adenosine. Müller cells release adenosine through a
calcium-independent facilitated transport method (1,2).
Adenosine is a local hormone that is present at a low level in
physiological conditions, normally at a concentration of 20–200 nM.
Tissue hypoxia or ischemia can lead to adenosine triphosphate
breakdown and the increased generation of adenosine (3). Adenosine, which exerts
anti-inflammatory effects, is released from the retina and has an
important role in pathological conditions, such as ischemia-hypoxia
and glaucoma (4). To a certain
extent, adenosine protects neurons from glutamate toxicity by
suppressing excitatory neurotransmission (5).
Glutamate is a neurotransmitter that can be found in
brain tissue and the retina of the eye (6). In conditions of depolarization, Müller
cells release glutamate and the increased non-vesicular release of
glutamate causes excitotoxic damage to neurons (7). The L-glutamate/L-aspartate transporter
(GLAST) is a primary glial enzyme in the clearance of extracellular
glutamate in physiological conditions (8). In pathological conditions, the
functional downregulation of GLAST induces an increased glutamate
concentration in the retina; however, the association between the
high concentration of adenosine and GLAST downregulation in the
retina is not clear. The aim of the present study, therefore, was
to explore the effects of adenosine and an adenosine receptor
antagonist, SCH442416, on rat retina GLAST expression.
Materials and methods
Rat chronic ocular hypertension (COH)
models
All experimental procedures described were in
accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals. The present study was
approved by the laboratory animal ethics committee of Ruijin
Hospital (Shanghai, China). Male Sprague Dawley rats (weight,
200–250 g) were purchased from Shanghai SIPPR-BK Laboratory Animal
Co., Ltd. (Shanghai, China), and housed in an air-conditioned
animal room at ~23°C under a 12-h light/dark cycle. All efforts
were made to minimize the suffering of the rats in this study.
Prior to all surgeries and procedures, the animals were
anesthetized with an intraperitoneal injection of xylazine and
ketamine hydrochloride (7.4 mg/ml and 5 mg/kg, respectively)
(Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China). Two drops
of 0.3% Ocuflox® solution (Santen Pharmaceutical Co., Ltd., Osaka,
Japan) were topically applied prior to and following the eye
surgeries to prevent infection.
The rat COH model was reproduced following a
procedure described in previous studies (9,10). An
increase in the intraocular pressure (IOP) was induced in 60 rats
by cauterizing three episcleral veins in the right eye of each rat.
Briefly, three veins (including two episcleral veins beside the
superior rectus muscle and one episcleral vein beside the lateral
rectus muscle) were separated and cauterized precisely to avoid
nearby tissue damage. The right eyes of a further 6 rats were
subjected to a sham procedure. IOP measurements were taken weekly
following surgery at the same time in the morning. Proxymetacaine
hydrochloride solution (Alcon Laboratories, Inc., Fort Worth, TX,
USA) was used topically prior to the IOP measurements, which were
conducted with a handheld digital tonometer (TonoPen®; Mentor
Ophthalmics, Inc., Norwell, MA, USA). The probe of the TonoPen was
applied vertically to the cornea surface, and touched the cornea
surface with the same force in all measurements. If the confidence
interval was ≥95%, numerical values were accepted. Ten numerical
values were recorded in each eye.
Drugs and intravitreal injection
Prior to injection, the rat pupil was dilated with a
tropicamide drop (Qianjiang Pharmaceutical Co., Ltd., Qianjiang,
China). A total of 10 µM adenosine (Sigma-Aldrich, St. Louis, MO,
USA) or 10 µM adenosine + 100 nM SCH442416 (Sigma-Aldrich) solution
(2 µl) was injected into the rat vitreous space (n=6 rats/group).
Adenosine or adenosine + SCH442416 were first dissolved in dimethyl
sulfoxide (DMSO) and then diluted by double-distilled
H2O (final concentration DMSO, 5%). Under a stereoscopic
microscope, a microinjector (Hamilton Robotics, Inc., Reno, NV,
USA) was inserted 2 mm behind the temporal limbus and directed
toward the optic nerve to inject solution into the vitreous space.
Eyes that received only an injection of vehicle solution in the
same manner served as controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
First, total RNA, isolated by TRIzol® (Invitrogen
Life Technologies, Carlsbad, CA, USA), was reverse-transcribed into
cDNA. The PCR solution contained 2 µl cDNA, the specific primer set
(1 µM each) and 11.5 µl QuantiTect SYBR® Green PCR Master Mix from
the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) in a
final volume of 20 µl. The following primer pairs (Invitrogen Life
Technologies) were used: GLAST sense, 5′-CCT ATG TGG CAG TCG TTT-3′
and anti-sense, 5′-CTG TGA TGG GCT GGC TAA-3′, β-actin sense,
5′-GCG CTC GTC GTC GAC AAC GG-3′ and anti-sense, 5′-GTG TGG TGC CAA
ATC TTC TCC-3′. The PCR cycle conditions were as follows: i)
Initial denaturation, one cycle at 94°C for 5 min; ii)
amplification and quantification, 40 cycles at 94°C for 30 sec,
55°C for 30 sec and 72°C for 30 sec; iii) melting curve, 55°C with
the temperature gradually increased up to 95°C.
Western blot analysis
For western blotting, the rat retinas were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) in the presence of protease
inhibitors. The homogenates were centrifuged at 14,000 × g for 30
min at 4°C. The protein concentrations were determined using the
bicinchoninic acid method. Equal quantities of protein (1 µg/µl, 15
µl) were separated by 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. Subsequent to being blocked with
5% skimmed milk at room temperature for 2 h, the membranes were
incubated with rabbit polyclonal antibody against GLAST (1:200
dilution; cat. no. ab416; Abcam, Cambridge, UK) or mouse monoclonal
antibody against GAPDH (1:10,000 dilution; cat. no. KC-5G4 KangChen
Bio-tech Inc., Shanghai, China) overnight at 4°C. The membranes
were then washed three times with Tris-buffered saline-Tween 20 for
10 min and incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:2,000 dilution) for 1 h at room
temperature. Images were captured using the ImageQuant™ Las 4000
mini biomolecular imager (GE Healthcare Life Sciences, Pittsburgh,
PA, USA), and the protein bands were quantitatively analyzed with
Image-Pro Plus version 6.0.0.260 (Media Cybernetics, Inc.,
Rockville, MD, USA) image analysis software.
Immunohistochemistry
Rats were perfused with normal saline and 4%
paraformaldehyde (PFA) solution. The right eyeballs were fixed in
4% PFA solution for 4 h, and then dehydrated with a graded sucrose
solution at 4°C (4 h in 20% sucrose solution and overnight in 30%
sucrose solution). The retinas were vertically sectioned at a
7.5-µm thickness and then mounted on gelatin-coated slides.
Subsequent to being rinsed with 0.01 M phosphate-buffered saline
(PBS), the retina slices were blocked in 4% goat serum (Gibco Life
Technologies, Carlsbad, CA, USA), 0.25% bovine serum albumin
(Sigma-Aldrich) and 0.2% Triton X-100 (Solarbio Science &
Technology Co., Ltd., Shanghai, China) in PBS at room temperature
for 2 h, and incubated with goat polyclonal anti-GLAST primary
antibody (1:200 dilution; cat. no. sc-7758; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C for 48 h.
Following incubation with the primary antibody, the retina slices
were rinsed in 0.01 M PBS and incubated with the fluorescein
isothiocyanate-conjugated donkey anti-goat immunoglobulin G
secondary antibody (1:100 dilution; Jackson ImmunoResearch
Laboratories, West Grove, PA, USA) for 2 h at room temperature.
Finally, the retina slices were mounted with anti-fade mounting
medium with DAPI (Vector Laboratories, West Grove, PA, USA), and
the immunofluorescence images were visualized with a Zeiss Imager
M1 laser-scanning microscope (Carl Zeiss AG, Oberkochen, German)
using a 20X objective lens.
Statistics
Data were analyzed using SPSS 19.0 software (IBM
SPSS, Armonk, NY, USA) and are presented as the mean ± standard
deviation. The paired-samples Student's t-test or one-way analysis
of variance was used to test the data for statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
IOP elevation in the COH models
The mean IOP in the sham-operated animals exhibited
no significant difference between the right (19.03±0.25 mmHg) and
left (19.47±0.69 mmHg) eye in the 8-week observation period (n=6,
P=0.329). In the episcleral vein cauterization group the IOP of the
right eye showed a significant increase from the first week of the
study and continued to show a steady increase until the eighth
week. The mean IOP of the right eye in the episcleral vein
cauterization group was 27.3±1.83 mmHg, compared with 19.03±0.25
mmHg in the sham surgery group (n=6, P=0.001).
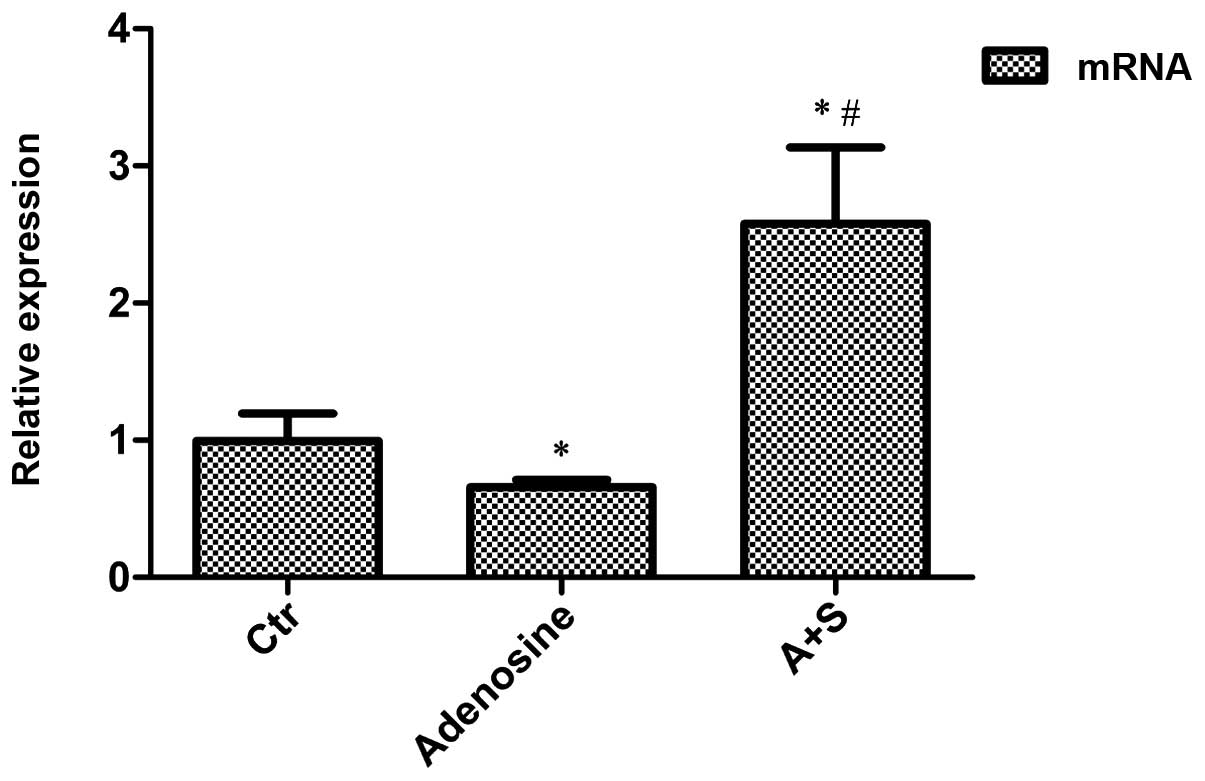
Adenosine- and adenosine
A2A receptor antagonist-induced changes in GLAST mRNA
expression in the rat COH models
Adenosine or adenosine + SCH442416 solution was
intravitreally injected into the right eye of the rats. The rats
were sacrificed two weeks later by cervical dislocation and GLAST
mRNA expression was evaluated through RT-qPCR analysis. Compared
with the control (COH) group, the GLAST mRNA expression was
observed to decrease by 33.6% in the adenosine treatment group
(n=6, P=0.020) and to increase by 159.6% in the group treated with
SCH442416 (n=6, P=0.001) (Fig.
1).
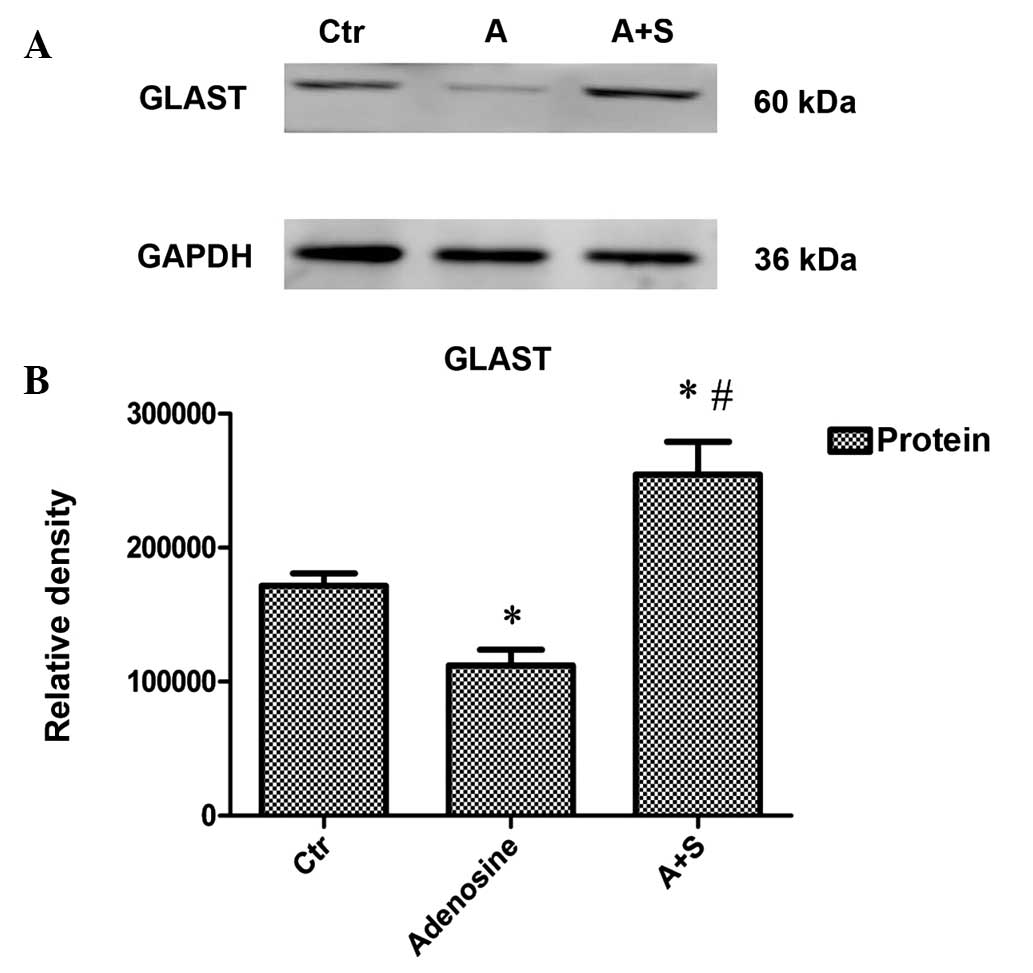
Adenosine- and adenosine
A2A receptor antagonist-induced changes in GLAST protein
expression in the rat COH models
Adenosine or adenosine + SCH442416 solution was
intravitreally injected into the right eye of the rats. The rats
were sacrificed two weeks later, and the retinas were dissected and
used for the experiment. Compared with the control (COH) group, the
administration of adenosine decreased GLAST protein expression in
the retina by 34.7% (n=6, P<0.001), whereas treatment with the
adenosine A2A receptor antagonist SCH442416 increased
GLAST protein expression by 48.3% (n=6, P<0.001) (Fig. 2A and B).
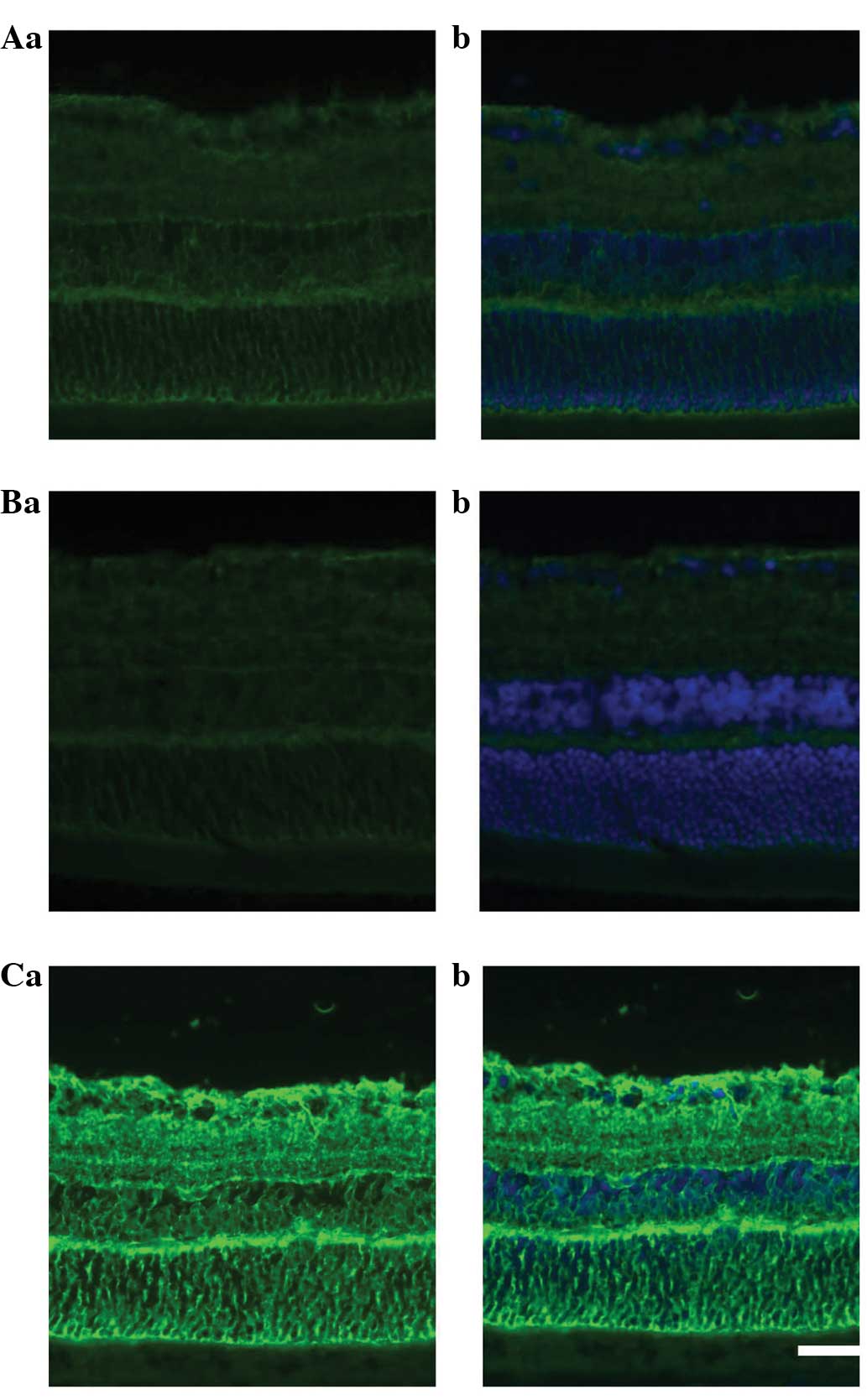
In the immunohistochemical experiments, the
administration of adenosine was shown to decrease GLAST protein
expression relative to that in the control rat retina. By contrast,
the administration of SCH442416 markedly increased the GLAST
protein expression compared with that in the control and adenosine
groups (Fig. 3A–C).
Discussion
Retinal Müller cells release adenosine and glutamate
in pathological conditions. Glutamate is a major excitatory
neurotransmitter in the mammalian retina and its presence at high
concentrations plays a key role in neuronal damage. Extracellular
glutamate is primarily taken up into the cell via GLAST (~50% of
extracellular glutamate) (11);
however, the effect of adenosine on GLAST function and the
underlying mechanism have yet to be fully elucidated. The results
of the present study have provided the first evidence, to the best
of our knowledge, that treatment with adenosine (10 µM) can
decrease the retinal GLAST protein and mRNA expression. It has been
reported that elevations in IOP to <70 mmHg are conducive to the
maintenance of GLAST activity in rats (12). The present study results were
consistent with this finding. Decreases in the expression of GLAST
protein were accompanied by decreases in the GLAST mRNA level,
suggesting that decreased transcription plays an important role in
the downregulation of GLAST protein expression.
Adenosine, one of the most bioactive substances, is
widely present in intracellular and extracellular fluid (13). Adenosine produces its biological
effect through adenosine receptors. All adenosine receptors are
G-protein-coupled receptors and can be grouped into four subtypes:
A1, A2A, A2B and A3.
Biological and pharmacological studies have found that all four
adenosine receptor are located in the retina. The A1
receptor (A1R), A2AR and A2BR have
been studied extensively, while less focus has been placed on the
A3R. It has been demonstrated that A1R
activation has a protective function in vivo, inhibiting
inflammation and apoptosis in the heart, brain and kidneys
(14). The release of
neurotransmitters, including glutamate, from the synaptic terminals
is inhibited by activation of the A1R. By contrast,
activation of the A2AR promotes the release of
neurotransmitters, including glutamate. The A2BR is
expressed at a low density in almost all tissues and exhibits
low-affinity ligand binding (15).
Less is known about the A3R than about the
A1R, A2AR and A2BR. With regard to
the A2AR, early studies have indicated that
A2AR antagonists can induce a decrease in glutamate
uptake in a mouse model of Alzheimer's disease (15,16). In
the present study, an A2AR antagonist was selected for
the study, since none of the other adenosine receptors tested
(A1R or A3R) modified glutamate uptake.
Although a number of previous studies have shown that
A2AR antagonists increase GLAST expression in cultured
cells (17,18), the results of the present study
provide the first evidence, to the best of our knowledge, that the
blockage of A2ARs can increase the GLAST expression in
the rat retina in vivo. The results of the present study may
provide a novel method for retinal neuron protection.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant no. 81371014) and the Shanghai
‘Science and Technology Innovation Action Plan’ Basic Research Key
Project (grant nos. 11JC1407700 and 11JC1407701).
References
|
1
|
Linnertz R, Wurm A, Pannicke T, et al:
Activation of voltage-gated Na+ and Ca2+
channels is required for glutamate release from retinal glial cells
implicated in cell volume regulation. Neuroscience. 188:23–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brückner E, Grosche A, Pannicke T,
Wiedemann P, Reichenbach A and Bringmann A: Mechanisms of VEGF- and
glutamate-induced inhibition of osmotic swelling of murine retinal
glial (Müller) cells: Indications for the involvement of vesicular
glutamate release and connexin-mediated ATP release. Neurochem Res.
37:268–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mubagwa K and Flameng W: Adenosine,
adenosine receptors and myocardial protection: An updated overview.
Cardiovasc Res. 52:25–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribelayga C and Mangel SC: A circadian
clock and light/dark adaptation differentially regulate adenosine
in the mammalian retina. J Neurosci. 25:215–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark BD, Kurth-Nelson ZL and Newman EA:
Adenosine-evoked hyperpolarization of retinal ganglion cells is
mediated by G-protein-coupled inwardly rectifying K+ and
small conductance Ca2+-activated K+ channel
activation. J Neurosci. 29:11237–11245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hertz L: The glutamate-glutamine (GABA)
cycle: Importance of late postnatal development and potential
reciprocal interactions between biosynthesis and degradation. Front
Endocrinol (Lausanne). 4:592013.PubMed/NCBI
|
|
7
|
Reichenbach A and Bringmann A: New
functions of Müller cells. Glia. 61:651–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Isbikawa M, Yoshitomi T, Zorumski CF and
Izumi Y: Downregulation of glutamine synthetase via GLAST
suppression induces retinal axonal swelling in a rat ex vivo
hydrostatic pressure model. Invest Ophthalmol Vis Sci.
52:6604–6616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shareef SR, Garcia-Valenzuela E, Salierno
A, Walsh J and Sharma SC: Chronic ocular hypertension following
episcleral venous occlusion in rats. Exp Eye Res. 61:379–382. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Miao Y, Wang XH and Wang Z:
Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal
ganglion cell apoptosis in a rat experimental glaucoma model.
Neurobiol Dis. 43:455–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarthy VP, Pignataro L, Pannicke T, et al:
Glutamate transport by retinal Müller cells in glutamate/aspartate
transporter-knockout mice. Glia. 49:184–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holcombe DJ, Lengefeld N, Gole GA and
Barnett NL: The effects of acute intraocular pressure elevation on
rat retinal glutamate transport. Acta Ophthalmol. 86:408–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong Y, Yang Z, Huang WC and Luo X:
Adenosine, adenosine receptors and glaucoma: An updated overview.
Biochim Biophys Acta. 1830:2882–2890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim M, Chen SW, Park SW, et al:
Kidney-specific reconstitution of the A1 adenosine receptor in A1
adenosine receptor knockout mice reduces renal ischemia-reperfusion
injury. Kidney Int. 75:809–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stone TW, Ceruti S and Abbracchio MP:
Adenosine receptors and neurological disease: Neuroprotection and
neurodegeneratioon. Handb Exp Pharmacol. 193:535–587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi RN, Pamplona FA and Prediger RD:
Adenosine receptor antagonists for cognitive dysfunction: A review
of animal studies. Front Biosci. 13:2614–2632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Zhong Y, Shen X, Cheng Y, Qi J and
Wang J: In vitro effect of adenosine A2A receptor antagonist
SCH 442416 on the expression of glutamine synthetase and glutamate
aspartate transporter in rat retinal Müller cells at elevated
hydrostatic pressure. Oncol Rep. 27:748–752. 2012.PubMed/NCBI
|
|
18
|
Valadas JS, Batalha VL, Ferreira DG, et
al: Neuroprotection afforded by adenosine A2A receptor blockade is
modulated by corticotrophin-releasing factor (CRF) in glutamate
injured cortical neurons. J Neurochem. 123:1030–1040. 2012.
View Article : Google Scholar : PubMed/NCBI
|