Introduction
In patients with diabetes mellitus type 1, impaired
functional and structural arterial wall (AW) properties, such as
endothelial dysfunction and increased arterial stiffness,
progressively lead to the development of cardiovascular diseases
and their consequences (1,2). These manifestations in patients with
diabetes mellitus are accelerated and usually develop early
(3,4). Despite the intensive control of risk
factors and strenuous treatment strategies, cardiovascular diseases
continue to represent the major cause of morbidity and mortality in
patients with diabetes mellitus type 1 (5,6).
Consequently, a new, effective cardiovascular preventive strategy
is required. The strategy investigated in the present study is
oriented towards improving AW properties per se (7). Such an approach has not previously been
evaluated.
It has previously been shown that a 1-month
treatment with a low-dose fluvastatin and valsartan combination is
effective in improving AW properties in patients with diabetes
mellitus type 1 (8) as well as in
healthy middle-aged individuals (9).
The beneficial effects obtained steadily declined after 1-month
treatment discontinuation, although they remained significantly
present after 3 months. It is well established that improvement of
these AW characteristics (endothelial function and arterial
stiffness) leads to cardiovascular risk imp rovement.
Based on the data from previous studies, it was
hypothesized that a new concept for improving AW characteristics
could be to take advantage of the phenomenon of prolonged efficacy
after treatment discontinuation with cyclic intermittent therapy.
Therefore, testing and potentially introducing this new concept was
the aim of the present study.
Materials and methods
Participants and study design
The same 44 patients of both genders (25 females and
19 males) with diabetes mellitus type 1 that participated in a
previous study (8) were again
recruited in the present study, at 6 months after the
discontinuation of the initial treatment. All participants were
assigned to the same groups and received the same treatment as in
the previous study. The treatment group (n=22) received a low-dose
combination of 10 mg fluvastatin and 20 mg valsartan daily, while
the control group (n=22) received a placebo. The treatment period
was 30 days. Inclusion criteria were: Diagnosis of type 1 diabetes
mellitus lasting ≥5 years, age between 30 and 50 years, stable
insulin dosage in the last 6 months, glycated hemoglobin (HbA1c)
levels < 7.5% (reported in the last month) and no history of
cardiovascular disease (carotid, coronary or peripheral). Exclusion
criteria were smoking, and treatment with statins or any drug from
the family of renin-angiotensin-aldosterone system inhibitors. The
average age of participants at inclusion in the study was 35.6±2.1
years in the control group and 36.3±1.7 years in the treatment
group. The body mass index (23.3±0.5 kg / m2 vs.
25.2±0.9 k g/m2; P=0.10), waist-hip ratio (0.9±0.1 vs.
0.9±0.1; P=0.64), HbA1c values (6.5±0.5 vs. 6.3±0.6; P=0.71) and
duration of diabetes mellitus (15.3±2.1-years vs. 18.4±2.4 years;
P=0.35) did not differ between the control and treatment groups,
respectively. All subjects were informed of the study protocol and
gave their informed consent. Age-matched healthy individuals were
also invited to participate in order to allow for basal comparison
of AW characteristics in both observed groups. The National Medical
Ethics Committee of Slovenia (Ljubljana, Slovenia) approved the
study.
In the patients with diabetes mellitus type 1,
ultrasound measurements were performed 6 months after
discontinuation of the initial treatment and again at the end of
the 1-month treatment repetition period; functional and structural
AW characteristics were measured. Ultrasound measurements were
performed at the initial visit for the age-matched individuals and
the patients with diabetes mellitus type 1. Blood pressure was
measured with an automated sphygmomanometer (Welch Allyn Speidel
& Keller, Skaneateles Falls, NY, USA). Ultrasound measurements
were performed under similar conditions as in the previous study,
that is, in the afternoon after a 6-h fast following their normal
breakfast and morning insulin, in a quiet, temperature-controlled
environment. Patients rested in a supine position for 10 min prior
to ultrasound measurements, which were performed by a single
examiner using an Aloka alfa-10 Prosound ultrasound machine with an
integrated high-resolution eTracking system (Hitachi Aloka,
Wallingford, CT, USA).
Ultrasound measurements: Brachial
artery flow-mediated dilation (FMD) measurement
Brachial artery FMD was assessed in accordanc e with
previously reported guidelines (10,11). As
previously d escribed, an echo-machine conti nuously tracked and
recorded the brachial artery diameter and automatically provided
the FMD value. The procedure was the same as that used in the first
study: The diameter of the brachial artery was continuously
visualized and recorded. After monitoring its baseline diameter (1
min), the right forearm blood pressure cuff was inflated 50 mmHg
above systolic pressure (4 min), resulting in arterial occlusion.
This was followed by rapid cuff deflation, which induced reactive
hyperemia. The brachial artery diameter was then recorded.
Assessment of arterial stiffness
parameters
Detailed measurements of arterial stiffness
parameters have also already been described. They were performed on
the right common carotid artery. The ultrasound device
automatically determined the stiffness parameters through analysis
of pulse waves. Pressure waveforms were obtained noninvasively
using arterial diameter change waveforms calibrated automatically
on the basis of systolic blood pressure values. Values of pulse
wave velocity (PWV) and carotid artery local stiffness
(β-stiffness) were then automatically calculated by the ultrasound
machine.
Statistical analysis
All values were expressed as means ± standard error
of the mean and were normally distributed. Any differences between
absolute or relative values during the initial or current treatment
and between the treatment and control groups were assessed through
one-way analysis of variance (ANOVA). When a significant
interaction was present, the Bonferroni post-test was performed. A
P-value of less than 0.05 was considered to indicate a significant
difference. All statistical analyses were performed using GraphPad
Prism software, version 5.0 (GraphPad Inc., La Jolla, CA, USA).
Results
Patient characteristics
Patient characteristics at the beginning (day 0) and
at the end (day 30) of the current treatment period are presented
in Table I. No significant
differences in systolic and diastolic blood pressure, total,
low-density lipoprotein and high-density lipoprotein cholesterol,
triglycerides and serum glucose levels were found between the
control and treatment groups. Treatment with a low-dose fluvastatin
and valsartan combination did not influence blood pressure,
cholesterol or triglyceride values (Table I).
 | Table I.Patient characteristics in the control
and treatment groups at the beginning (day 0) and after 1 month of
current treatment (day 30). |
Table I.
Patient characteristics in the control
and treatment groups at the beginning (day 0) and after 1 month of
current treatment (day 30).
|
| Control group | Treatment group |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Day 0 | Day 30 | Day 0 | Day 30 | P-value |
|---|
| Systolic BP
(mmHg) |
123.0±1.8 |
123.5±1.9 |
119.8±2.5 |
120.1±2.4 | 0.43 |
| Diastolic BP
(mmHg) |
79.9±1.0 |
79.2±1.0 |
79.2±1.1 |
78.6±1.4 | 0.89 |
| Total cholesterol
(mmol/l) |
4.8±0.2 |
4.7±0.2 |
5.1±0.2 |
5.0±0.2 | 0.39 |
| LDL cholesterol
(mmol/l) |
2.6±0.2 |
2.7±0.2 |
2.6±0.1 |
2.4±0.2 | 0.45 |
| HDL cholesterol
(mmol/l) |
1.7±0.1 |
1.7±0.1 |
1.8±0.1 |
1.8±0.1 | 0.31 |
| Triglycerides
(mmol/l) |
1.0±0.1 |
0.9±0.1 |
0.8±0.1 |
0.8±0.1 | 0.56 |
| Serum glucose
(mmol/l) |
8.0±0.9 |
7.9±0.7 |
7.0±0.7 |
6.3±0.7 | 0.21 |
Comparison of the effectiveness of the
current and initial treatment on the improvement of arterial wall
characteristics
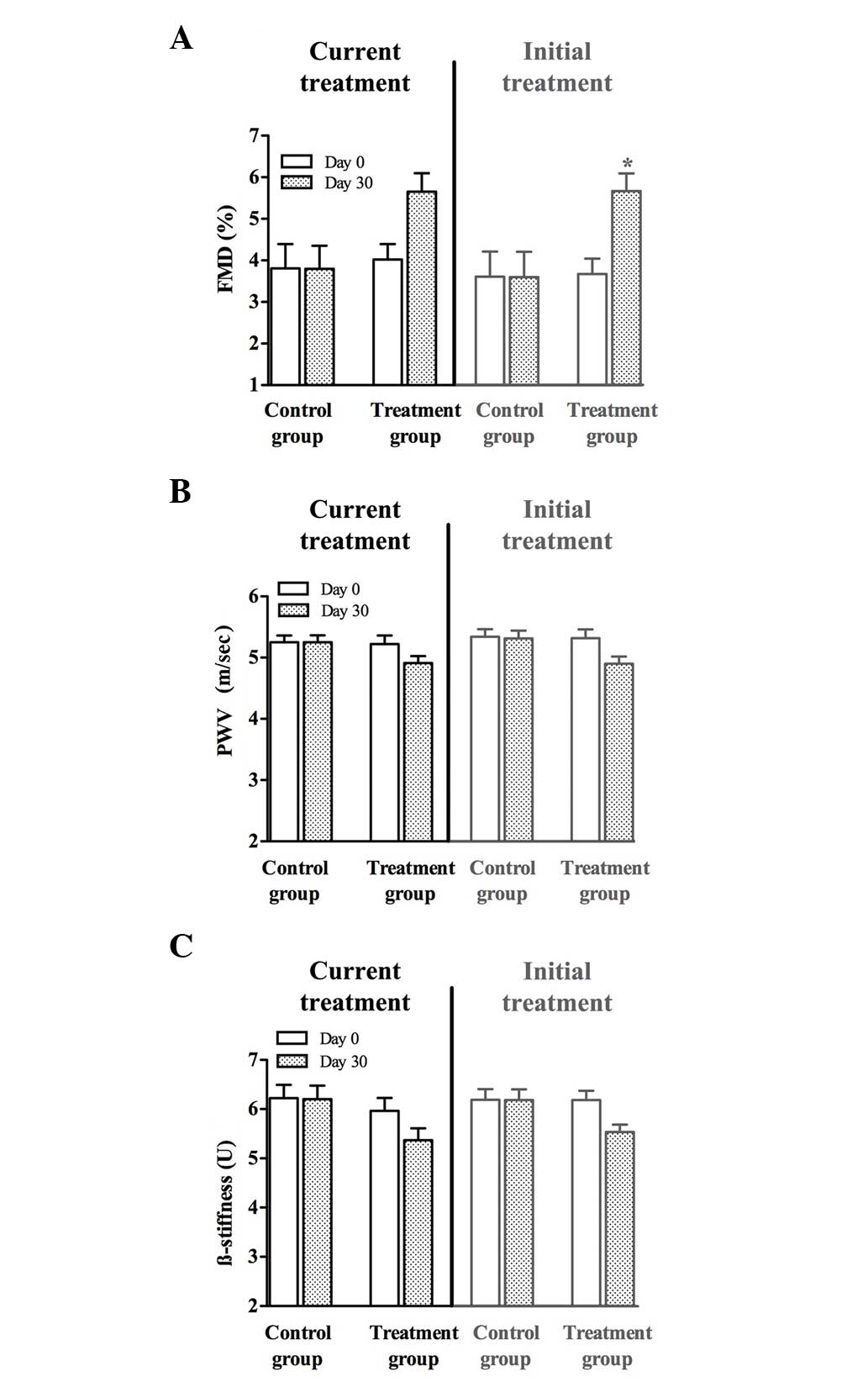
Six months after treatment discontinuation,
participants were again invited to participate in the study. First,
the baseline AW characteristics measurements were repeated, which
were similar to the values at the beginning of the initial
treatment. After 1-month treatment repetition with a low-dose
fluvastatin and valsartan combination, all parameters describing AW
characteristics improved, whereas the absolute values did not reach
the level of significance (Fig. 1).
Age-matched controls had FMD 4.8±0.2%; PWV 5.2±0.1 m/sec and
β-stiffness 5.4±0.2 U.
When the relative values (percentage of improvement
after current treatment) of the current treatment were compared, it
was found that all three measured AW characteristics (FMD, PWV and
β-stiffness) significantly improved compared with those in the
control group, as shown in Table
II. No significant changes were observed in the placebo
group.
 | Table II.Relative improvements (% change) of
brachial artery FMD, and common carotid artery PWV and β-stiffness
values for the current treatment in the control and treatment
groups. |
Table II.
Relative improvements (% change) of
brachial artery FMD, and common carotid artery PWV and β-stiffness
values for the current treatment in the control and treatment
groups.
| Variable | Control group | Treatment group |
|---|
| FMD |
1.6±2.7 |
+50.9±14.5a |
| PWV |
0.2±0.3 |
−5.7±1.1a |
| β-stiffness |
0.3±0.2 |
−9.9±1.3b |
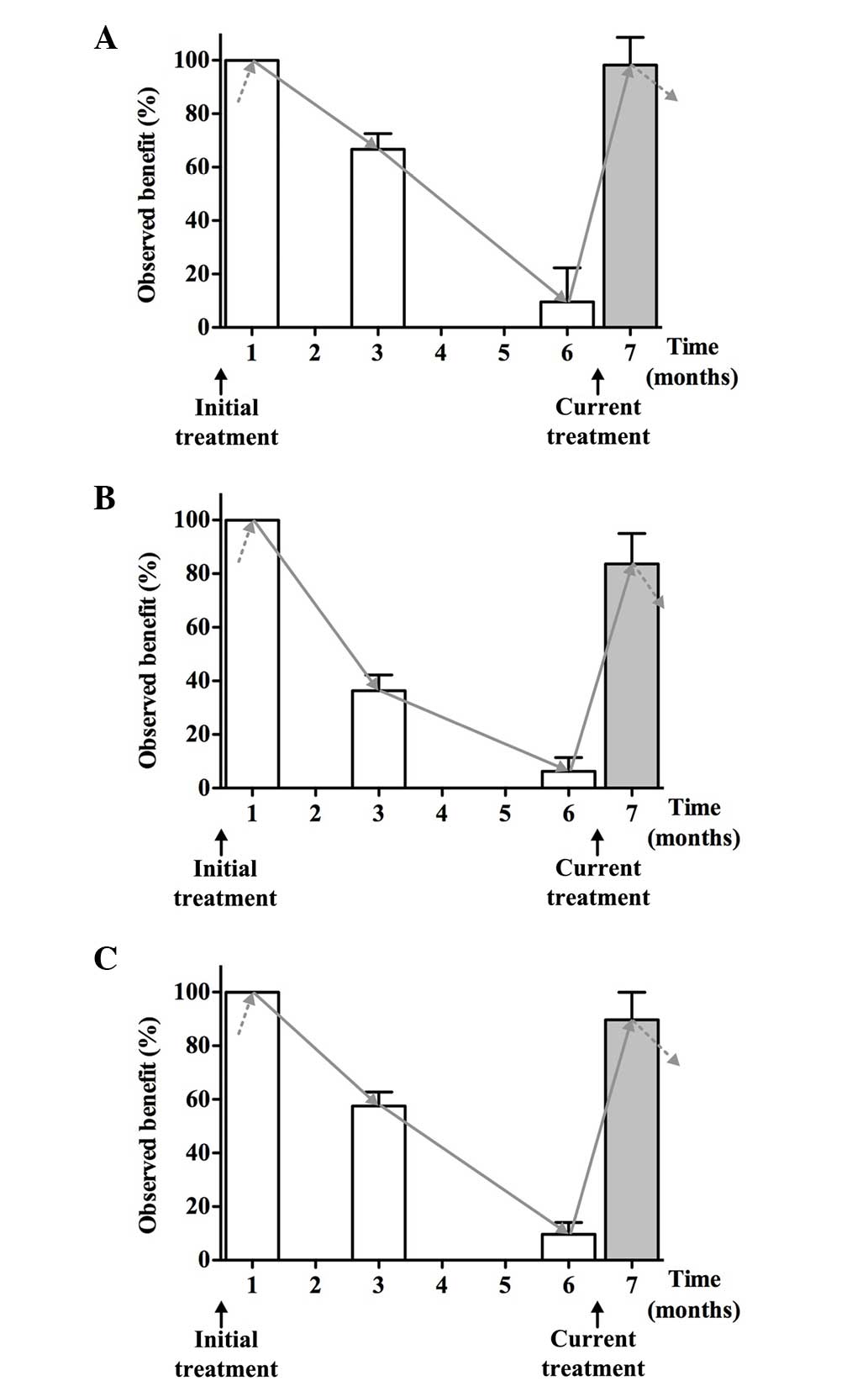
Time dependency of the beneficial
effects of initial and current treatments
The time dependency of the benefits of the initial
and current treatments was studied. All three measured parameters
improved after the initial 1-month treatment with a low-dose
fluvastatin and valsartan combination. Following the
discontinuation of treatment these observed benefits steadily
declined in a time-dependent manner, although they continued to be
present at the level of 66.6% for FMD, 36.1% for PWV and 56.0% for
β-stiffness 3 months after discontinuation of the initial
treatment. Six months after the discontinuation of the initial
treatment the observed benefits were 9.6% for FMD, 6.3% for PWV and
9.5% for β-stiffness. At that time point, treatment was repeated
(current treatment). The improvement reached levels comparable to
those previously obtained with the initial treatment (Fig. 2).
Discussion
In the present study, 1-month treatment with a
low-dose fluvastatin and valsartan combination was repeated 6
months following the discontinuation of the initial treatment in
patients with diabetes mellitus type 1. It was found that 6 months
after initial treatment discontinuation, minimal to slight residual
improvements of AW properties remained (FMD 9.6%, PWV 6.3% and
β-stiffness 9.5%). Notably, repetition of the same treatment again
led to improvement of AW properties when compared with the initial
treatment (significant improvements: FMD +50.9%, PWV −5.7% and
β-stiffness −9.9%). These results strongly support a new approach
to the prevention of cardiovascular disease in patients with
diabetes mellitus type 1, that is, cyclic, intermittent treatment
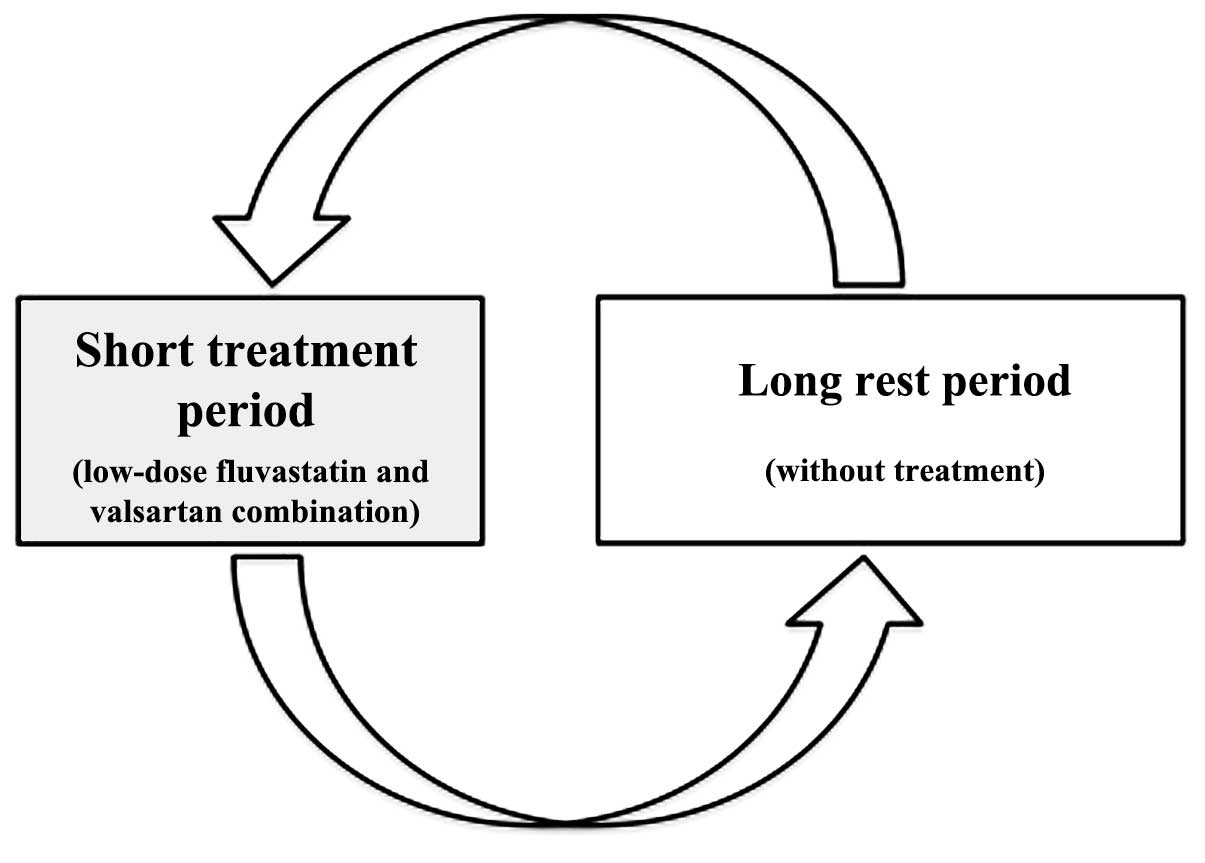
with a low-dose fluvastatin and valsartan combination (Fig. 3).
Overall, the effectiveness of cyclic intermittent
low-dose fluvastatin and valsartan combination treatment as a new
approach with for the long-term improvement of AW properties in
patients with diabetes mellitus type 1, has been demonstrated.
During the rest period (time without treatment) a steady but slow
decline in the benefit obtained was observed. Thus, 6 months after
the discontinuation of the initial treatment, only a slight
improvement of AW parameters was present (FMD 9.6%, PWV 6.3% and
β-stiffness 9.5%). At that particular time point, repetition of the
same treatment (new therapeutic cycle) again lead to improvements
in AW properties comparable to those of the initial treatment
(significant improvements: FMD +50.9%, PWV −5.7% and β-stiffness
−9.9%). These results confirm the possibility of ‘benefit
repetition’. It is expected that the benefits could be repeated
numerous times, allowing for true long-term treatment.
When comparing the values of measured AW
characteristics in patients with diabetes mellitus type 1 with
those of age-matched controls, it was found, as in other studies
(12–15) that patients with diabetes type 1 have
impaired functional and structural AW characteristics. It seems
that the changes of AW characteristics start to occur early in the
disease course and slowly progress, finally leading to
macrovascular complications. Taking these facts into account, it is
hypothesized that patients with diabetes mellitus type 1 are ideal
targets for an approach directly focusing on impaired AW
properties. Long-term improvement of AW characteristics could, at
least in theory but based on strong data, lead to a reduction of
cardiovascular disorders and events. We consider that a cyclic,
intermittent approach using sub-therapeutic dosages of well-known
drugs is the most suitable, since it enables long-term improvement
of AW characteristics, good compliance of the patients and no drug
side-effects. Since the AW characteristics slowly declined over
time (following treatment discontinuation), the identification of
the period in which the benefits almost disappear was of interest.
Thus, it was found that after 6 months the AW characteristics were
only slightly above the initial values. It may be concluded that at
this time point would be the most logical at which to investigate
the possibility of effect repetition. A 6-month rest period was
therefore selected.
The process of atherosclerosis is widely known to be
accelerated in patients with diabetes mellitus type 1, leading to
increased morbidity and mortality in this particular population
(16). Furthermore, as these changes
are accelerated, atherosclerotic manifestations in this particular
patient group start at an earlier age. Even though there is great
effort in combating risk factors for atherosclerosis in patients
with diabetes mellitus type 1, their residual cardiovascular risk
remains high (17,18). It should be emphasized that effects
of all cardiovascular risk factors converge on the AW, which is the
main focus of pathologic events in atherosclerosis as well as the
main target of the present proposed approach. Furthermore, it is
also known that disrupted or impaired AW properties (endothelial
dysfunction and increased arterial stiffness) have a negative
prognostic value in cardiovascular risk stratification (19,20).
Notably, no study or intervention has yet targeted the AW per
se. Improvement of AW disorders could lead to the lowering of
cardiovascular risk in patients with diabetes mellitus type 1. To
the best of our knowledge, no similar studies have been performed
to date. Therefore, the present study represents a completely new
cardiovascular preventive approach that has not yet been tested
elsewhere.
This study leads to a proposal of a new concept: An
intermittent approach comprising cyclic treatment with low-dose
statin and sartan combination. The use of a low, sub-therapeutic
dose of a combination of fluvastatin and valsartan is proposed. The
administration of these drugs combined is proposed in a cyclical
manner, with therapy repeating in certain time cycles (6 months in
patients with diabetes mellitus type 1). Furthermore, the approach
is intermittent, with therapy being administered for 1 month,
followed by a so-called free-of-treatment or ‘rest’ period (when
the beneficial effects remain present, but gradually decline). The
free-of-treatment period is followed by another cycle (7). The main advantages of the approach
described lie particularly in the low doses used, so there are no
side-effects, while the short-term regimen improves patient
compliance. Multiple repetitions, for example biannually, present
repeated improvements of preclinical atherosclerosis, thus leading
to postponement of its presentation in a naturally evolving
process.
The major limitation of this study is that only a
small group of patients was included. However, larger studies are
planned in the future, as well as multiple repetition evaluation.
In addition, the present study was not focused on exploring the
mechanistic background of the observed beneficial effects. This is
planned for larger future studies. However, it is hypothesized that
the expression of vasoactive genes, and anti-inflammatory and
anti-oxidative effects are at least partially involved, as shown in
previous studies (21,22).
In conclusion, the present study has confirmed and
expanded the authors' previously established concept of low-dose
intermittent treatment with fluvastatin and valsartan combination.
Its effectiveness has been demonstrated in patients with diabetes
mellitus type 1 and showed that after a rest period of 6 months,
during which the level of benefit obtained declines, treatment
repetition leads to improvements in AW properties similar to those
of the initial treatment. The level of AW improvements can be again
achieved after 6 months of ‘rest’. These results give further
support to the proposed new approach in cardiovascular disease
prevention, since the combination of short ‘treatment periods’ and
longer ‘rest periods’, which constitute ‘cyclic treatment’, would
enable continuous/long-term arterial function improvement.
References
|
1
|
Davel AP, Wenceslau CF, Akamine EH, Xavier
FE, Couto GK, Oliveira HT and Rossoni LV: Endothelial dysfunction
in cardiovascular and endocrine.metabolic diseases: An update. Braz
J Med Biol Res. 44:920–932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milan A, Tosello F, Fabbri A, Vairo A,
Leone D, Chiarlo M, Covella M and Veglio F: Arterial stiffness:
From physiology to clinical implications. High Blood Press
Cardiovasc Prev. 18:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christen AI, Armentano RL, Miranda A, et
al: Arterial wall structure and dynamics in type 2 diabetes
mellitus methodological aspects and pathophysiological findings.
Curr Diabetes Rev. 6:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schram MT, Chaturvedi N, Fuller JH and
Stehouwer CD: EURODIAB Prospective Complications Study Group: Pulse
pressure is associated with age and cardiovascular disease in type
1 diabetes: The Eurodiab Prospective Complications Study. J
Hypertens. 21:2035–2044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mangiapane H: Cardiovascular disease and
diabetes. Adv Exp Med Biol. 771:219–228. 2012.PubMed/NCBI
|
|
6
|
Sheikh-Ali M, Raheja P and Borja-Hart N:
Medical management and strategies to prevent coronary artery
disease in patients with type 2 diabetes mellitus. Postgrad Med.
125:17–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janić M, Lunder M and Sabović M: A new
anti-ageing strategy focused on prevention of arterial ageing in
the middle-aged population. Med Hypotheses. 80:837–840. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Savić V, Eržen B, Janić M, Lunder M,
Boncelj M, Kanc K, Janež A and Šabović M: Improvement of arterial
wall characteristics by the low-dose fluvastatin and valsartan
combination in type 1 diabetes mellitus patients. Diab Vasc Dis
Res. 10:420–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lunder M, Janić M, Jug B and Sabović M:
The effects of low-dose fluvastatin and valsartan combination on
arterial function: A randomized clinical trial. Eur J Intern Med.
23:261–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corretti MC, Anderson TJ, Benjamin EJ,
Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H,
Gerhard-Herman M, Herrington D, et al: Guidelines for the
ultrasound assessment of endothelial-dependent flow-mediated
vasodilation of the brachial artery: A report of the International
Brachial Artery Reactivity Task Force. J Am Coll Cardiol.
39:257–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Celermajer DS, Sorensen KE, Gooch VM,
Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK and Deanfield
JE: Non-invasive detection of endothelial dysfunction in children
and adults at risk of atherosclerosis. Lancet. 340:1111–1115. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cherney DZ and Montanari A: Gender,
clamped hyperglycemia and arterial stiffness in patients with
uncomplicated type 1 diabetes mellitus. Clin Exp Hypertens.
36:187–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llaurado G, Ceperuelo-Mallafre V,
Vilardell C, Simo R, Freixenet N, Vendrell J and Gonzalez-Clemente
JM: Arterial stiffness is increased in patients with type 1
diabetes without cardiovascular disease: A potential role of
low-grade inflammation. Diabetes Care. 35:1083–1089. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Elderen SG, Westenberg JJ, Brandts A,
van der Meer RW, Romijn JA, Smit JW and de Roos A: Increased aortic
stiffness measured by MRI in patients with type 1 diabetes mellitus
and relationship to renal function. AJR Am J Roentgenol.
196:697–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pareyn A, Allegaert K, Asscherickx W,
Peirsman E, Verhamme P and Casteels K: Impaired endothelial
function in female adolescents with type 1 diabetes measured by
peripheral artery tonometry. Eur J Pediatr. 172:1017–1022. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanter JE and Bornfeldt KE: Inflammation
and diabetes-accelerated atherosclerosis: Myeloid cell mediators.
Trends Endocrinol Metab. 24:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slim IB: Cardiovascular risk in type 1
diabetes mellitus. Indian J Endocrinol Metab. 17 (Suppl 1):S7–S13.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Snell-Bergeon JK and Nadeau K:
Cardiovascular disease risk in young people with type 1 diabetes. J
Cardiovasc Transl Res. 5:446–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ben-Shlomo Y, Spears M, Boustred C, May M,
Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH,
Cruickshank JK, et al: Aortic pulse wave velocity improves
cardiovascular event prediction: An individual participant
meta-analysis of prospective observational data from 17,635
subjects. J Am Coll Cardiol. 63:636–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruggiero D, Paolillo S, Ratta GD,
Mariniello A, Formisano T, Pellegrino AM and Filardi PP:
Endothelial function as a marker of pre-clinical atherosclerosis:
Assessment techniques and clinical implications. Monaldi Arch Chest
Dis. 80:106–110. 2013.PubMed/NCBI
|
|
21
|
Lunder M, Drevenšek G, Černe D, Marc J,
Janić M and Šabović M: Treatment with low-dose atorvastatin,
losartan and their combination increases expression of
vasoactive-related genes in rat aortas. J Cardiovasc Pharmacol
Ther. 18:177–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Janic M, Lunder M, Prezelj M and Šabović
M: A combination of low-dose fluvastatin and valsartan decreases
inflammation and oxidative stress in apparently healthy middle-aged
males. J Cardiopulm Rehabil Prev. 34:208–212. 2014. View Article : Google Scholar : PubMed/NCBI
|