Introduction
With the continuous development of human society and
increases in the human life span, myopia has become a severe
vision-threatening disease. Furthermore, choroidal
neovascularization (CNV), which is caused by pathological myopia,
has become a common complication resulting in blindness (1). Pathological myopia has exhibited a
trend toward increased incidence in Asian populations, and it is
particularly common in China and Japan (2,3);
therefore, research toward an effective treatment of pathological
myopia-induced CNV would have a profound effect on improving
survival quality. Pathological myopia-induced macular CNV was
previously treated by photodynamic therapy (PDT); however, the
majority of studies showed that the reformation of macular CNV
following PDT led to vision reduction in over one-third of patients
or, even if vision was stable following the treatment, it was not
improved (4,5). The widespread use of anti-vascular
endothelial growth factor (VEGF) drugs in the field of age-related
macular degeneration (AMD) has provided a novel approach for the
treatment of CNV (6). Although the
drug bevacizumab has not yet obtained clinical recognition, it has
received some distinction in the treatment of pathological
myopia-induced CNV (7). By contrast,
ranibizumab (Lucentis®; Novartis Pharma AG, Basel, Switzerland),
which has been clinically registered for the treatment of AMD, has
been described as a treatment for pathological myopia-induced CNV
in only a few reports (8–10).
The aim of the present study was to evaluate the
safety and efficacy of intravitreal injections of ranibizumab in
the treatment of CNV inside the macula lutea of patients with
pathological myopia. The present research was based on the
different mechanisms of ranibizumab in the treatment of macular and
non-macular CNV caused by pathological myopia. The former condition
was evaluated in this study, due to the fact that this condition is
normally associated with smaller lesions than those of AMD CNV, and
the incidence rate of macular CNV caused by pathological myopia is
higher than that of non-macular CNV (11,12). For
the present study, the random prospective method without controls
was adopted. A total of 61 eyes from 61 patients diagnosed with
macular pathological myopia-induced CNV were selected to receive
0.05 ml intravitreal injection(s) of ranibizumab. The monitored
follow-up period was 6 months, and the curative effects were
evaluated.
Materials and methods
General information
A total of 61 eyes from 61 patients, who were
attending the No. 474 Hospital of the Chinese PLA (Urumqi, China)
between August 2012 and January 2013, were included in this study.
The patients had been diagnosed with pathological myopia-induced
macular CNV following fundus fluorescein angiography (FFA) and
optical coherence tomography (OCT) examination. The patients
included 22 men (22 eyes) and 39 women (39 eyes) aged 20.2–49.6
years, with a mean age of 25.2 years. The diopter values ranged
from −6.50 to −16.00 D (average, 10.50±4 D), and the average eye
axis was 27.9 mm.
Patients with systemic and ocular surgery
contraindications were excluded. Intravitreal injections of 0.05 ml
ranibizumab (Novartis Pharma AG) were administered under local
anesthesia to 61 eyes from 61 patients in the hospital; the number
of injections administered to each patient varied between one and
four. This study was conducted in accordance with the Declaration
of Helsinki and with approval from the Ethics Committee of the No.
474 Hospital of the Chinese PLA. Written informed consent was
obtained from all participants.
Intravitreal injection
Prior to the intravitreal injection, the patients
were instructed to use tobramycin and dexamethasone ophthalmic
suspension (TobraDex®; Alcon-Couvreur N.V., Puurs, Belgium) and
levofloxacin eye drops (Shandong Bausch and Lomb Freda
Pharmaceutical Co. Ltd., Jinan, China) four times per day for 3
days. On the day of the procedure, the patient entered the sterile
laminar flow procedure room and their personal information was
checked. Each patient received three local anesthesia treatments
with 0.4% oxybuprocaine hydrochloride eye drops (Towering
Pharmaceutical Co., Ltd., Jiangsu, China) 10 min before the
procedure. Following the disinfection and washing of the eye with
diluted gentamicin liquid (Henan Furen Pharmaceutical Co., Ltd.,
Zhenghou, China), a 1-ml TB needle (Jiangsu Zhengkang Medical
Equipment Co., Ltd., Changzhou, China) was vertically punctured
into the vitreous cavity from 4.0 mm lateral to the lower limbus.
When it was confirmed that the TB needle had entered the vitreous
cavity and had no contact with the lens posterior capsule, 0.05 ml
ranibizumab was slowly injected. Following the withdrawal of the
needle, the needlepoint was gently pressed with a wet cotton swab
for 1–2 min, and the eye was subsequently coated with TobraDex
oculentum and sterile gauze. The day after the surgery, the
patients were evaluated for the presence of subconjunctival
hemorrhage, anterior chamber reaction or endophthalmitis, increased
intraocular pressure, complicated cataract, retinal hole, retinal
detachment and other complications. If the patient was found to
have a complication, it was treated in a timely manner. Following
the procedure, TobraDex and levofloxacin eye drops were used in the
treated eye for 3 consecutive days, and the patients were
followed-up for 6 months thereafter.
Standards for repeated injections
The following standards were used to determine the
requirement for repeated injections: i) The visual acuity decreased
by 0.05 or conscious vision decline occurred; ii) a new hemorrhage
was observed in the macular area; iii) FFA revealed that the
leakage area had increased or a new leakage was found; iv) OCT
revealed an incomplete fibrotic or predominantly fibrotic CNV; and
v) effusion from under or inside the retinal hole was unchanged or
increased. The patient received a repeat injection if any of these
situations occurred.
Observation items
The main observation items were as follows: i) The
number of intravitreal ranibizumab injections; ii) the average
best-corrected visual acuity (BCVA); iii) the macular retinal
thickness (CMT) as observed by OCT; and iv) evaluation of the
presence of apparent macular persistent CNV fluorescent leakage, as
observed using FFA. In addition, the following secondary items were
observed: i) The presence of subconjunctival hemorrhage; ii) the
occurrence of an infectious anterior chamber reaction or
endophthalmitis; iii) an increase in intraocular pressure; iv) the
presence of a cataract complication; and v) retinal breaks or
detachment.
Statistical analysis
Using SPSS software (version 15.0; SPSS Inc.,
Chicago, IL, USA), the mean BCVA and the CMT shown by OCT were
compared using the paired-samples t-test or multivariate analysis
of variance. Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Main observation items
The numbers of intravitreal injections of
ranibizumab were as follows: One injection in 10 eyes, two
injections in 44 eyes, three injections in six eyes and four
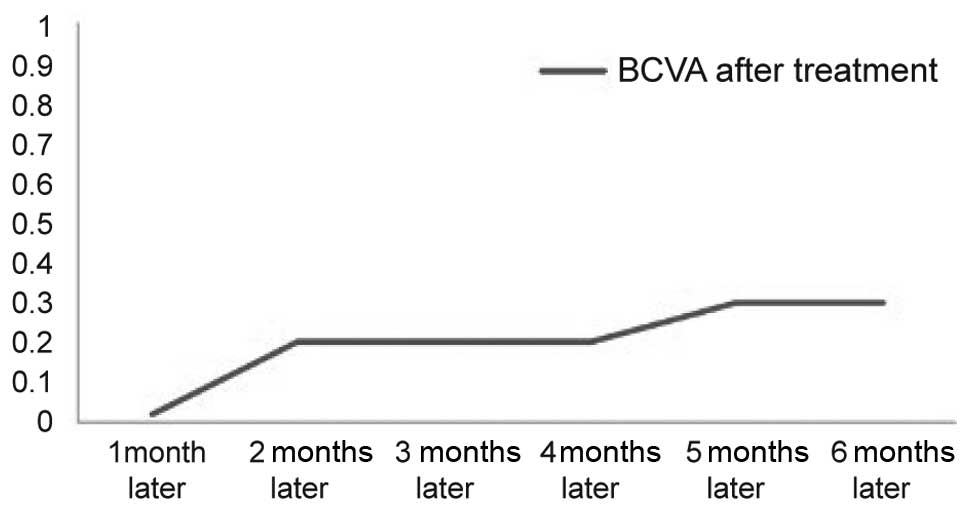
injections in one eye (average, 1.97 injections). The average BCVA
was 0.02±0.01 prior to treatment and 0.30±0.03 subsequent to
treatment, and 88.5% of cases (54/61) showed an improved and stable
visual acuity. OCT revealed that the CMT was reduced by an average
of 45.1 µm among the patients. Using FFA to display macular
fluorescein leakage, 56 cases were found to have no evident CNV
fluorescence leakage following treatment, whereas five cases with
CNV had fluorescence leakage following treatment; however, the
leakage intensity subsequent to treatment was lower compared with
that prior to treatment in these cases (Table I and Fig.
1).
 | Table I.Numerical comparison of pathological
myopic macular choroidal neovascularization before and after
treatment. |
Table I.
Numerical comparison of pathological
myopic macular choroidal neovascularization before and after
treatment.
| Parameter | Before treatment | After the final
treatment | P-value |
|---|
| Average
best-corrected visual acuity (µm)a | 0.02±0.01 | 0.30±0.03 | <0.01 |
| Average macular
retinal thickness (µm)a | 395.9±143.9 | 240.8±71.7 | <0.05 |
| Fundus fluorescein
angiography leakage (n) | 61 | 5 |
|
Secondary observation items
Analysis of the secondary observation items revealed
that no eyes had subconjunctival hemorrhage; one eye had an
anterior chamber reaction with no endophthalmitis; three eyes had
increased intraocular pressure (average, 5 mmHg), including one
case in which the intraocular pressure increased up to 23.2 mmHg;
no cases had a cataract complication; and no cases had retinal
breaks or detachments.
Representative cases
Case 1
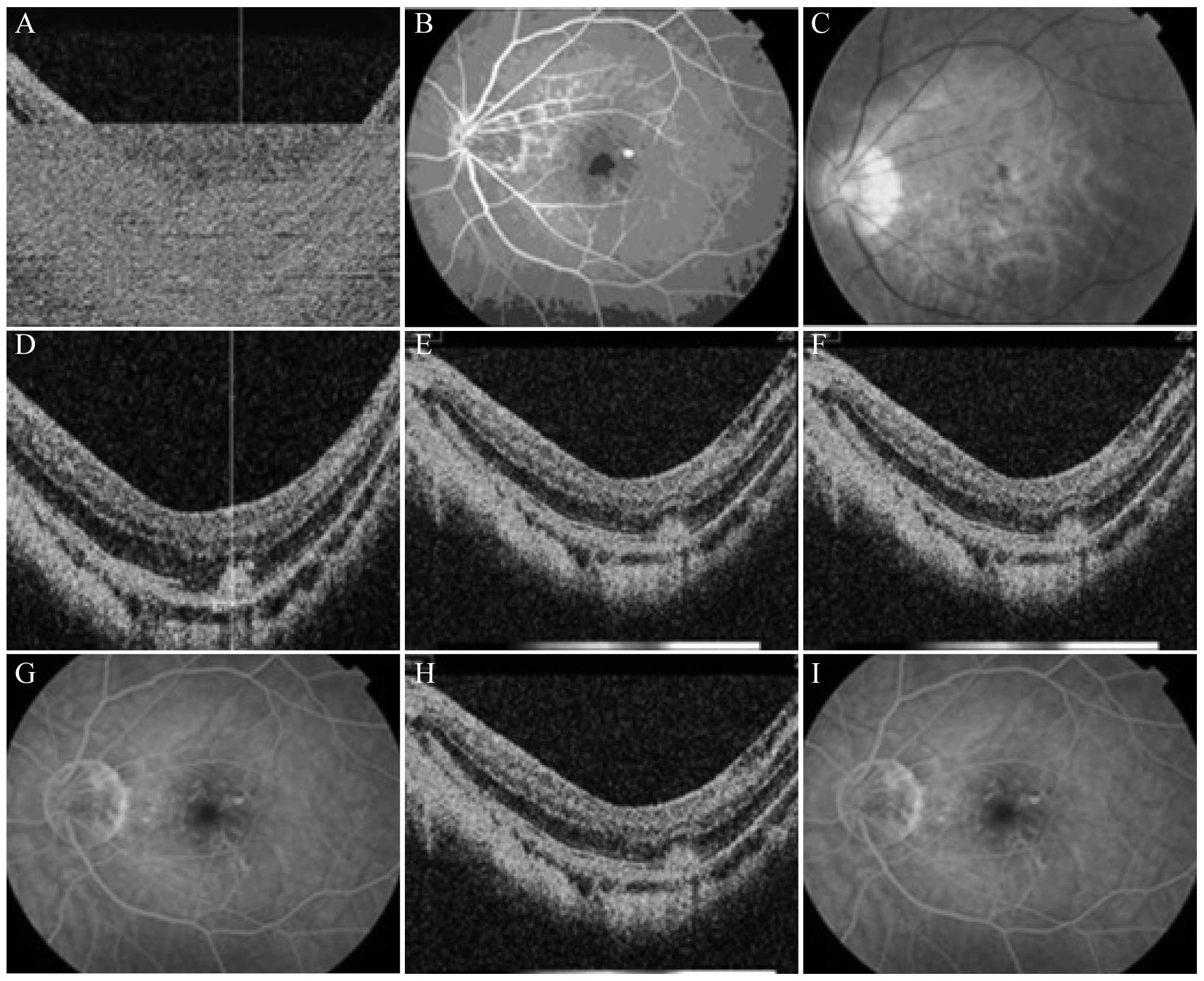
A 40-year-old female patient was admitted to the
hospital in October 2012 with the main complaint that ‘the vision
of the left eye had been decreased for 2 months’. During the
physical examination, the vision of the left eye was checked: Naked
eye, 0.02/correction with the original glasses (−8.00 D)/0.4 and
ocular anterior segment (−). Vitreous opacities were observed.
While the optic disc of the eyeground had a clear boundary and
light color, myopic atrophy was evident. The retina exhibited a
leopard pattern, and thick bleeding lesions were apparent under the
central fovea of the macula. The FFA and OCT findings of the fundus
oculi are shown in Fig. 2A–C. The
left eye was administered a 0.05-ml intravitreal injection of
ranibizumab, and a physical examination was performed 1 month
later. At this examination, the eyesight in the left eye was as
follows: Naked eye, 0.04/correction with the original glasses
(−8.00 D)/0.5 and ocular anterior segment (−). Vitreous opacities
were observed. Although the optic disc of the eyeground had a clear
boundary and light color, myopic atrophy was apparent. The leopard
pattern of the retina remained but the range of the thick bleeding
lesions under the central fovea of the macula was reduced. The FFA
and OCT findings of the fundus oculi are shown in Fig. 2D and E. The vision of the left eye
was rechecked 3 and 6 months later: Naked eye, 0.04/correction with
the original glasses (−8.00 D)/0.6 and ocular anterior segment (−).
Vitreous opacities were apparent. The eyeground FFA indicated that
the neovascular leakage of the macula lutea had disappeared, as the
high fluorescence leakage observed previously had become a weak
fluorescence leakage. OCT revealed that the macular CNV was
basically fibrotic, and the reflective optical bands had changed
from low and middle signals to high signals (Fig. 2F–I).
Case 2
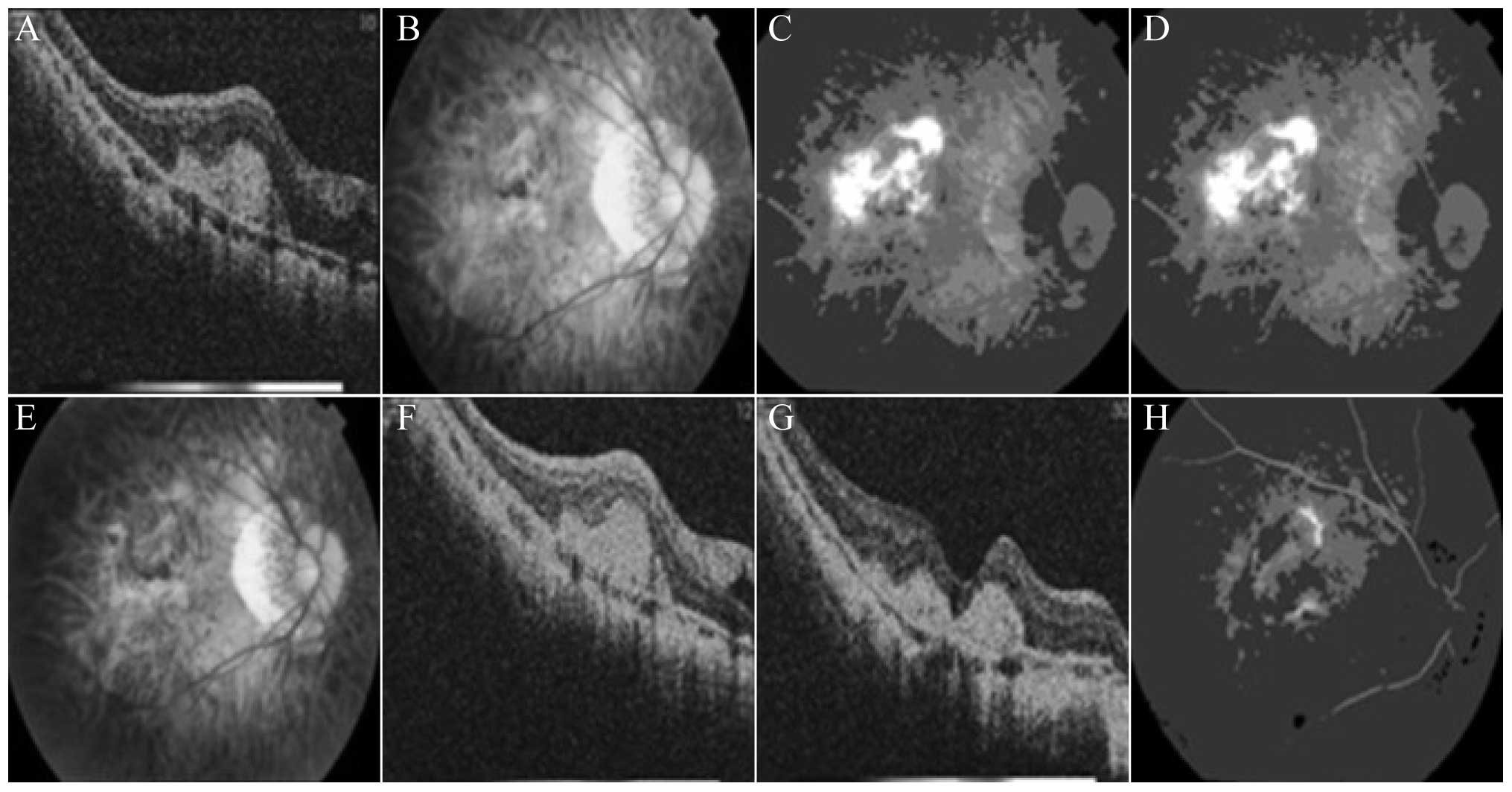
A 27-year-old male patient was admitted to the
hospital in November 2012 with the main complaint that ‘the
substances seen by the right eye had been deformed for 1 month’.
The physical examination upon admission revealed that the vision of
the left eye was as follows: Naked eye, index(10 cm)/correction
with the original glasses (−12.00 D)/0.02 and ocular anterior
segment (−). The crystal cortex exhibited slight turbidity, and
vitreous opacities were apparent. The optic disc of the eyeground
had a clear boundary and light color, but myopic atrophy was
evident. The retina exhibited a leopard pattern, with apparent
chorioretinal atrophy in the macula lutea. The eyeground FFA and
OCT findings are shown in Fig. 3A–D.
The left eye was administered a 0.05-ml intravitreal injection of
ranibizumab, and a physical examination was performed 1 month
later. This examination revealed the following in the left eye:
Naked eye, index(10 cm)/correction with the original glasses
(−12.00 D)/0.08 and ocular anterior segment (−). The crystal cortex
exhibited slight turbidity, and vitreous opacities were apparent.
While the optic disc of the eyeground had a clear boundary and
light color, myopic atrophy was evident; additionally, the retina
exhibited a leopard pattern, and chorioretinal atrophy was apparent
in the macula lutea. No significant differences were detected in
best-corrected visual, FFA and OCT during subsequent 5-month
follow-up period (Fig. 3E–H).
The aforementioned two cases were treated with an
intravitreal injection of ranibizumab. Subsequently, FFA and OCT
revealed that the active lesions had become inactive, vision was
increased, the degree of fibrosis of the CNV was strengthened or
the CNV was completely fibrotic and the retinal thickness of the
macula lutea was decreased.
Discussion
Anti-VEGF therapy has become an important treatment
for CNV, and evidence for its usefulness has been increasing
(13). At present, six main types of
medical treatment are used for pathological myopia-induced macular
CNV: Retinal laser photocoagulation, surgical resection, macular
translocation (14), PDT, anti-VEGF
drugs and Traditional Chinese Medicine. Compared with the other
treatments, anti-VEGF drugs have significant advantages. Retinal
laser photocoagulation and surgical treatment are likely to cause
irreversible structural damage to the macular tissues. Ladas et
al (15) stated that PDT is not
ideal for improving eyesight and that it may induce choroidal
capillary non-perfusion. In addition, certain individual cases may
exhibit direct or indirect lacquer crack-like lesions following PDT
treatment, and these lesions can easily cause CNV (15). Although improvements have been
reported following treatment with Traditional Chinese Medicine,
large-sample observations and reproducibility are lacking in these
studies. The anti-VEGF drugs currently on the market include
pegaptanib, bevacizumab, triamcinolone and ranibizumab, but
ranibizumab (Lucentis) has significant advantages compared with the
other drugs, and it is the only anti-VEGF drug that has been
approved for intraocular injection. Lucentis, which is a VEGF
inhibitor co-developed by Genentech and Novartis, was designed as a
Fab fragment, and this fragment can penetrate the retinal layer
more easily than the full-length antibody (16). It can thus bind to VEGF-A with higher
affinity, consequently blocking the cascade reaction caused by the
combination of VEGF-A and VEGF receptors R1/R2 on the vascular
endothelium, and achieving the most beneficial biological treatment
effect (17). Histopathologically,
ranibizumab inhibits the proliferation of endothelial cells and
vascular permeability and reduces inflammation and leakage, thereby
preventing CNV formation and macular edema (18).
In the present study, the intravitreal injection of
ranibizumab improved vision and reduced macular retinal thickness
and fluorescein leakage in 60 out of 61 eyes. A slight visual
improvement occurred in one eye only following four intravitreal
injections of ranibizumab, and the best correction in vision was
from 0.02 to 0.04. The reason for this small improvement is unclear
and further research is required to determine why certain eyes may
not respond to this injection. Previous studies have assessed the
number of treatments required and the post-treatment visual
outcomes following intravitreal ranibizumab injection (8,19).
Figurska and Stankiewicz (19)
reported an Early Treatment Diabetic Retinopathy Study (ETDRS)
visual acuity improvement of two lines in a 55-year-old patient who
received two intravitreal injections of ranibizumab; furthermore,
the ETDRS visual acuity was improved by three lines in another
25-year-old patient who received three intravitreal ranibizumab
injections. A similar study (8)
reported that the average number of intravitreal ranibizumab
injections required to treat pathological myopia-induced macular
CNV was 2.2, and the average BCVAs were 0.01 (prior to the
treatment) and 0.30 (subsequent to the treatment). In the present
study, the average number of injections was 1.97, and the average
BCVAs were 0.02±0.01 and 0.30±0.03 prior to and subsequent to
treatment, respectively. Compared with the aforementioned previous
studies, fewer injections were administered and better vision
improvements were obtained following the treatment. Different
research programs and ethnic differences, as well as the
differences among the patients and other conditions, may explain
these variations in results between the present and prior studies.
Although the suitability of the age-grouping method has been
questioned (20), we postulated that
age grouping would reflect the effect of age on the treatment
effect. Regarding the injection number option, we adopted the pro
re nata method, similar to Wakabayashi et al (13), in which the administration of further
injections was determined based on the first injection curve
effect. Few researchers have selected the classic treatment, which
is suitable for exudative AMD; in the classic treatment, the
decision of whether to re-treat is based upon the evaluation
following three consecutive injections (21–23).
Different treatment options in the previous studies may have been
based on the facts that the leakage, edema and lesion areas of
pathological myopia-induced CNV lesions are lower than those of
exudative AMD, and the lesions are mainly located under the macula
lutea. Regarding the association between pathological
myopia-induced macular CNV lesion locations and the fovea
centralis, the CNV lesions of 44 eyes were located under the
macular fovea centralis, accounting for 72.1% (44/61) of cases in
the present study.
The lacquer crack-like lesion represents the unique
damage of pathological myopia-induced eyeground disease, and it is
the marker of macular degeneration. Comparative observations of
indocyanine-green angiography (ICGA) and FFA have shown that the
two techniques have a discovery rate of lacquer crack-like lesions
of 89 and 38%, respectively, indicating that ICGA can detect these
lesions more effectively than can FFA (8). In the present study, lacquer cracks
were detected in 40 out of 61 eyes (65.6%) with pathological
myopia-induced macular CNV. Among the 40 eyes, lacquer cracks were
detected in 15 out of the contralateral eyes (37.5%). This result
has prompted us to pay increased attention to lacquer cracks in the
treatment and research of pathological myopia. Patients in whom
lacquer cracks are found in one eye should be considered to be at a
high risk of lacquer cracks in the contralateral eye, and early
intervention may be necessary. This subject is worthy of further
investigation.
Regarding adverse reactions, 10 eyes exhibited
subconjunctival thick hemorrhage with the bleeding being
self-absorbed ~1 week later. An infectious weak anterior chamber
reaction without the occurrence of endophthalmitis was observed in
one eye, and the anterior chamber reaction was resolved following
treatment with TobraDex and levofloxacin eye drops four times per
day for 7 consecutive days. Increased intraocular pressure occurred
in three cases, with an average increase of 5 mmHg. The eye
pressures dropped to normal levels following treatment with timolol
eye drops twice per day for 3 consecutive days, and the subsequent
follow-up revealed no further increases in intraocular pressure. No
cases of complicated cataract and retinal tear or detachment were
observed, which may be primarily associated with the skill and
proficiency of the surgeons.
In conclusion, intravitreal ranibizumab treatment
showed high efficacy for pathological myopia-induced macular CNV;
this treatment required few injections and resulted in few side
effects, while achieving improvements in visual acuity and organ
structure. This treatment therefore appears to be useful for this
condition. The present study had several limitations, however:
First, the sample size of 61 eyes was small, and further studies
with a larger sample size are required; secondly, the follow-up
period of 6 months was short, and controlled studies with a
follow-up period of 1 or 2 years will be necessary; thirdly, this
study lacked a control group, and a comparative study including
other treatment methods would provide results that are more robust.
In the previous discussions, although no comparative research was
suggested regarding which treatment was optimal, there were studies
that outlined the limitations of other methods (14,15).
Currently, only ranibizumab has been approved in China for the
treatment of wet AMD, while it has not been approved for the
treatment of pathological myopia-induced CNV, it is used outside of
the specified indications. Although a previous study indicated that
ranibizumab should become the first-line treatment for pathological
myopia-induced CNV (24), a full
explanation of the treatment should be provided and approval should
be obtained from the Medical Ethics Committee and from the patient
when the treatment is applied. Furthermore, the side effects of
ranibizumab in treating pathological myopia-induced CNV in Asian
populations require further study and confirmation. Since the
follow-up times in this study were inconsistent and the average
follow-up time was short, both the sample size and the follow-up
time should be extended in the future, and an in-depth study should
be performed with the purpose of obtaining information regarding
recurrence and injection times at different periods, among other
observations. The present study and its recommendation, which may
be novel for some, arose from questions that have emerged from
developments in medical science. Thus, a novel method that is more
effective, safe and reliable for the treatment of pathological
myopia-induced CNV may be possible in the near future.
References
|
1
|
Silva R: Myopic maculopathy: A review.
Ophthalmologica. 228:197–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawada A, Tomidokoro A, Araie M, Iwase A
and Yamamoto T: Tajimi Study Group: Refractive errors in an elderly
Japanese population: The Tajimi study. Ophthalmology. 115:363–370.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng CY, Hsu WM, Liu JH, Tsai SY and Chou
P: Refractive errors in an elderly Chinese population in Taiwan:
The Shihpai Eye Study. Invest Ophthalmol Vis Sci. 44:4630–4638.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenfeld PJ, Brown DM, Heier JS, et al:
MARINA Study Group: Ranibizumab for neovascular age-related macular
degeneration. N Engl J Med. 355:1419–1431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pece A, Vadalà M, Isola V and Matranga D:
Photodynamic therapy with verteporfin for juxtafoveal choroidal
neovascularizzation in pathologic myopia: A long-term follow-up
study. Am J Ophthalmol. 143:449–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin DF, Maguire MG, Ying GS, Grunwald
JE, Fine SL and Jaffe GJ: CATT Research Group: Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. N
Engl J Med. 364:1897–1908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gharbiya M, Allievi F, Mazzeo L and
Gabrieli CB: Intravitreal bevacizumab treatment for choroidal
neovascularization in pathologic myopia: 12-month results. Am J
Ophthalmol. 147:84–93, e1. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vadalà M, Pece A, Cipolla S, et al: Is
ranibizumab effective in stopping the loss of vision for choroidal
neovascularisation in pathologic myopia? A long-term follow-up
study. Br J Ophthalmol. 95:657–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otsuka K, Imai H, Shimoyama T, Nagai T,
Honda S and Azumi A: Recurrence of macular hole retinal detachment
after intravitreal ranibizumab injection for the treatment of
choroidal neovascularization from the remaining macular hole edge.
Case Rep Ophthalmol. 3:424–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lorenzo D, Arias L, Alcubierre R, et al:
Intravitreal ranibizumab for choroidal neovascularization secondary
to pathological myopia: 12-month follow-up. Ophthalmologica.
226:103–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida T, Ohno-Matsui K, Yasuzumi K, et
al: Myopic choroidal neovascularization: A 10-year follow-up.
Ophthalmology. 110:1297–1305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayashi K, Shimada N, Moriyama M, Hayashi
W, Tokoro T and Ohno-Matsui K: Two-year outcomes of intravitreal
bevacizumab for choroidal neovascularization in Japanese patients
with pathologic myopia. Retina. 32:687–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakabayashi T, Ikuno Y and Gomi F:
Different dosing of intravitreal bevacizumab for choroidal
neovascularization because of pathologic myopia. Retina.
31:880–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mateo C, Moreno J, Rosales G, et al:
Two-year results of macular translocation with scleral infolding in
myopic choroidal neovascularisation. Semin Ophthalmol. 19:29–42.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ladas ID, Moschos MM, Rouvas AA,
Karagiannis DA and Kokolakis SN: Lacquer crack formation after
photodynamic therapy. Eur J Ophthalmol. 13:729–733. 2003.PubMed/NCBI
|
|
16
|
Ferrara N, Damico L, Shams N, Lowman H and
Kim R: Development of ranibizumab, an anti-vascular endothelial
growth factor antigen binding fragment, as therapy for neovascular
age-related macular degeneration. Retina. 26:859–870. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Wiesmann C, Fuh G, et al:
Selection and analysis of an optimized anti-VEGF antibody: Crystal
structure of an affinity-matured Fab in complex with antigen. J Mol
Biol. 293:865–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krzystolik MG, Afshari MA, Adamis AP, et
al: Prevention of experimental choroidal neovascularization with
intravitreal anti-vascular endothelial growth factor antibody
fragment. Arch Ophthalmol. 120:338–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Figurska M and Stankiewicz A: Anti-VEGF
therapy in the treatment of myopic macular choroidal
neovascularization - case report. Klin Oczna. 110:387–391. 2008.(In
Polish). PubMed/NCBI
|
|
20
|
Ng DS, Kwok AK and Chan CW: Anti-vascular
endothelial growth factor for myopic choroidal neovascularization.
Clin Experiment Ophthalmol. 40:e98–e110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu TT and Kung YH: The 12-month outcome of
three consecutive monthly intravitreal injections of ranibizumab
for myopic choroidal neovascularization. J Ocul Pharmacol Ther.
28:129–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai TY, Chan WM, Liu DT and Lam DS:
Intravitreal ranibizumab for the primary treatment of choroidal
neovascularization secondary to pathologic myopia. Retina.
29:750–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calvo-Gonzalez C, Reche-Frutos J, Donate
J, Fernandez-Perez C and Garcia-Feijoo J: Intravitreal ranibizumab
for myopic choroidal neovascularization: Factors predictive of
visual outcome and need for retreatment. Am J Ophthalmol.
151:529–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neelam K, Cheung CM, Ohno-Matsui K, Lai TY
and Wong TY: Choroidal neovascularization in pathological myopia.
Prog Retin Eye Res. 31:495–525. 2012. View Article : Google Scholar : PubMed/NCBI
|