Introduction
Meningioma is among the most prevalent intracranial
tumors, and has the second highest incidence among all intracranial
tumors (~19.2%) (1). Although
meningiomas are usually classified as benign at early stages,
previous studies have identified malignant meningioma types that
are fast-growing, invasive and prone to recurrence and metastasis
(2,3). According to the standards of the World
Health Organization (WHO), meningiomas may be classified into 3
grades and 15 subtypes based on their malignancy. Grade I
meningiomas are benign with slow growth and grade III meningiomas
are malignant, invasive and prone to recurrence, while the severity
of grade II meningiomas is between that of grades I and III
(4).
In studies investigating tumorigenesis and tumor
treatments, angiogenesis is frequently a key concern (5). Rapid proliferation of tumor tissues and
irregular angiogenesis may cause hypoxia within tumor tissues,
leading to differences in the expression of regulatory factors,
such as hypoxia-inducible factor-1α (HIF-1α) (6). A number of studies have focused on
regulatory factors that target HIF-1α. For example, microRNA-18a
(miR-18a) has been observed to regulate the expression of HIF-1α in
certain tumor tissues (7–9). However, to the best of our knowledge,
the effects of miR-18a on HIF-1α expression in invasive meningiomas
has not previously been reported. In this study, the expression of
miR-18a and HIF-1α was evaluated in patients with invasive
meningiomas, with the aim of elucidating the association between
them.
Materials and methods
Patients
Between February 2011 and June 2014, 69 patients
were confirmed to have meningiomas by pathology, clinical
manifestations and radiology in Laiwu City People's Hospital
(Laiwu, China). The study population included 38 males and 31
females, who were classified into the invasive meningioma group (30
cases) or non-invasive meningioma group (39 cases) according
to-surgical observation and pathological section following surgery.
In the control group, 48 healthy subjects were included. Among the
69 cases, 18 cases were WHO grade I meningiomas, including 6
meningothelial cases, 4 fibrous cases, 4 transitional cases, 3
psammomatous cases and 1 angioblastic case; 21 cases were WHO grade
II meningiomas, including 9 atypical cases, 7 chordoid cases, and 5
clear cell cases; 30 cases were WHO grade III meningiomas,
including 16 anaplastic cases, 8 papillary cases and 6 rhabdoid
cases. The treatment durations ranged between 2 and 56 months, with
an average duration of 26.1 months. No patients had received
previous treatment for meningioma. Prior to surgery, no patient had
received hormones or traditional Chinese medicine, or had any
history of radio-chemotherapy. All procedures were approved by the
Ethics Committee of Laiwu City People's Hospital and written
informed consent was obtained from all patients or their
families.
Samples
Three types of sample were collected from the
patients. First, tumor tissues (invasive and non-invasive) and
normal tissues (control) were collected and stored in liquid
nitrogen. Second, fasting peripheral blood was collected from all
patients on the morning of the day of surgery, followed by storage
in ethylene diamine tetraacetic acid tubes at −20°C. Third, 2 ml
cerebrospinal fluid was collected from all patients during
surgeries, followed by centrifugation and storage at −20°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using RNAqueous® Total RNA
Isolation Kit (AM1912; Invitrogen Life Technologies). RNA purity
was determined using ultraviolet spectrophotometry (NanoDrop 1000;
Thermo Fisher Scientific, Waltham, MA, USA) by determination of the
A260/A280 ratio. cDNA was obtained by RT; qPCR was performed using
a iQ5 real-time PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
The primers for HIF-1α were as follows: Upstream,
5′-GAC AAG CCA CCT GAG GAG AG-3′ (381 bp) and downstream, 5′-GTT
CGC ATC TTG ATA AGG CC-3′. The primers for β-actin were: Upstream,
5′-GGC ATG GGT CAG AAG GAT TCC-3′ (316 bp) and downstream, 5′-ATG
TCA CGC ACG ATT TCC CGC-3′. PCR amplification conditions were as
follows: Initial denaturation at 94°C for 2 min; 45 cycles of
denaturation at 94°C for 30 sec, annealing at 60°C for 1 min, and
elongation at 68°C for 2 min; and final extension at 68°C for 7
min. The 2−ΔΔCt method was used to calculate
HIF-1α/β-actin levels.
The upstream primers for miR-18a and U6 small
nuclear RNA (internal control) were 5′-GAT AGC AGC ACA GAA ATA TTG
GC-3′ and 5′-GCG CGT CGT GAA GCG TTC-3′, respectively. The common
downstream primer for miR-18a and U6 was 5′-GTG CAG GGT CCG AGG
T-3′. PCR amplification conditions were as follows: Initial
denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C
for 15 sec, annealing at 60°C for 1 min, and elongation at 72°C for
2 min; and final extension at 72°C for 7 min. The 2−ΔΔCt
method was used to calculate miR-18a/U6 levels.
Western blot analysis
Proteins were extracted and protein concentration
was determined using a bicinchoninic acid protein concentration
determination kit (RTP7102; Real-Times Biotechnology Co., Ltd.,
Beijing, China). Protein samples (20 µg) were then subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Subsequently, the resolved proteins were transferred to
polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked
with 5% skimmed milk at room temperature for 1 h. Then, the
membranes were incubated with polyclonal mouse anti-human HIF-1α
(1:2,000; ab6489) and monoclonal mouse anti-human β-actin
antibodies (1:5,000; ab6276; Abcam, Cambridge, MA, USA) at 4°C
overnight. After extensive washing, the membranes were incubated
with polyclonal anti-mouse IgG-horseradish peroxidase-conjugated
antibody (1:3,000; ab34961; Abcam) for 1 h at room temperature.
Then, the membrane was developed using an enhanced
chemiluminescence detection kit (Sigma-Aldrich, St. Louis, MO, USA)
for imaging. Image Lab software, version 3.0 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used to acquire and analyze imaging
signals. The relative content of HIF-1α protein was expressed as
the HIF-1α/β-actin ratio.
Statistical analysis
All statistical analyses were performed using SPSS
software for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA).
Results are expressed as the mean ± standard deviation for test of
normality. Multi-group measurements were subjected to one-way
analysis of variance. In cases of homogeneity of variance, least
significant difference and Student-Newman-Keuls methods were used,
while in cases of heterogeneity of variance, Tamhane's T2 or
Dunnett's T3 method was used. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical data of patients with
meningioma
In order to evaluate the clinical parameters of the
patients, classification of patients was conducted according to
preoperative symptoms and signs, edema band widths, tumor invasion
and WHO grades. Preoperative symptoms and manifestations were used
to allocate patients into 4 grades (I–IV; Table I). Edema band widths were allocated
to 3 grades (I–III; Table II). In
addition, tumor invasion was graded as A or B (Table III). Clinical pathological
characteristics of tumor patients and control group subjects are
shown in Table IV.
 | Table I.Preoperative symptom grading of
patients. |
Table I.
Preoperative symptom grading of
patients.
| Grade | Symptoms |
|---|
| I | No neurological
symptoms or focal neurological signs |
| II | Mild neurological
deficits |
| III | Neurological deficits
and difficulty in daily life |
| IV | Disturbance of
consciousness |
 | Table II.Grading of edema surrounding tumor
tissues. |
Table II.
Grading of edema surrounding tumor
tissues.
| Grade | Symptoms |
|---|
| I | Edema band <2
cm |
| II | Edema band ≥2 cm that
is restricted within the hemisphere |
| III | Edema band crosses
hemispheres |
 | Table III.Classification of tumor invasion. |
Table III.
Classification of tumor invasion.
| Classification | Symptoms |
|---|
| A | No invasion into
tissues surrounding the tumor |
| B | Invasion into brain
tissues, arachnoid, extracranial soft tissues, and intracranial
venous sinus |
 | Table IV.Clinical pathological characteristics
of menangioma patient and control groups. |
Table IV.
Clinical pathological characteristics
of menangioma patient and control groups.
| Patient
characteristic | Control group
(n=48) | Invasive meningioma
group (n=30) | Non-invasive
meningioma group (n=39) |
|---|
| Age (years) |
|
|
|
|
<60 | 22 | 19 | 21 |
| ≥60 | 26 | 11 | 18 |
| Gender |
|
|
|
| Male | 20 | 20 | 19 |
|
Female | 28 | 10 | 20 |
| Invasion
classification |
|
|
|
| A | 48 | 26 | 28 |
| B | 0 | 4 | 11 |
| WHO pathological
grade |
|
|
|
| I | – | 0 | 18 |
| II | – | 0 | 21 |
| III | – | 30 | 0 |
| Tumor diameter
(cm) |
|
|
|
|
<5 | – | 24 | 30 |
| ≥5 | – | 6 | 9 |
| Edema grade |
|
|
|
| I | – | 5 | 16 |
| II | – | 10 | 19 |
| III | – | 15 | 4 |
| Preoperative symptom
grade |
|
|
|
| I | – | 4 | 11 |
| II | – | 7 | 8 |
| III | – | 13 | 15 |
| IV | – | 6 | 5 |
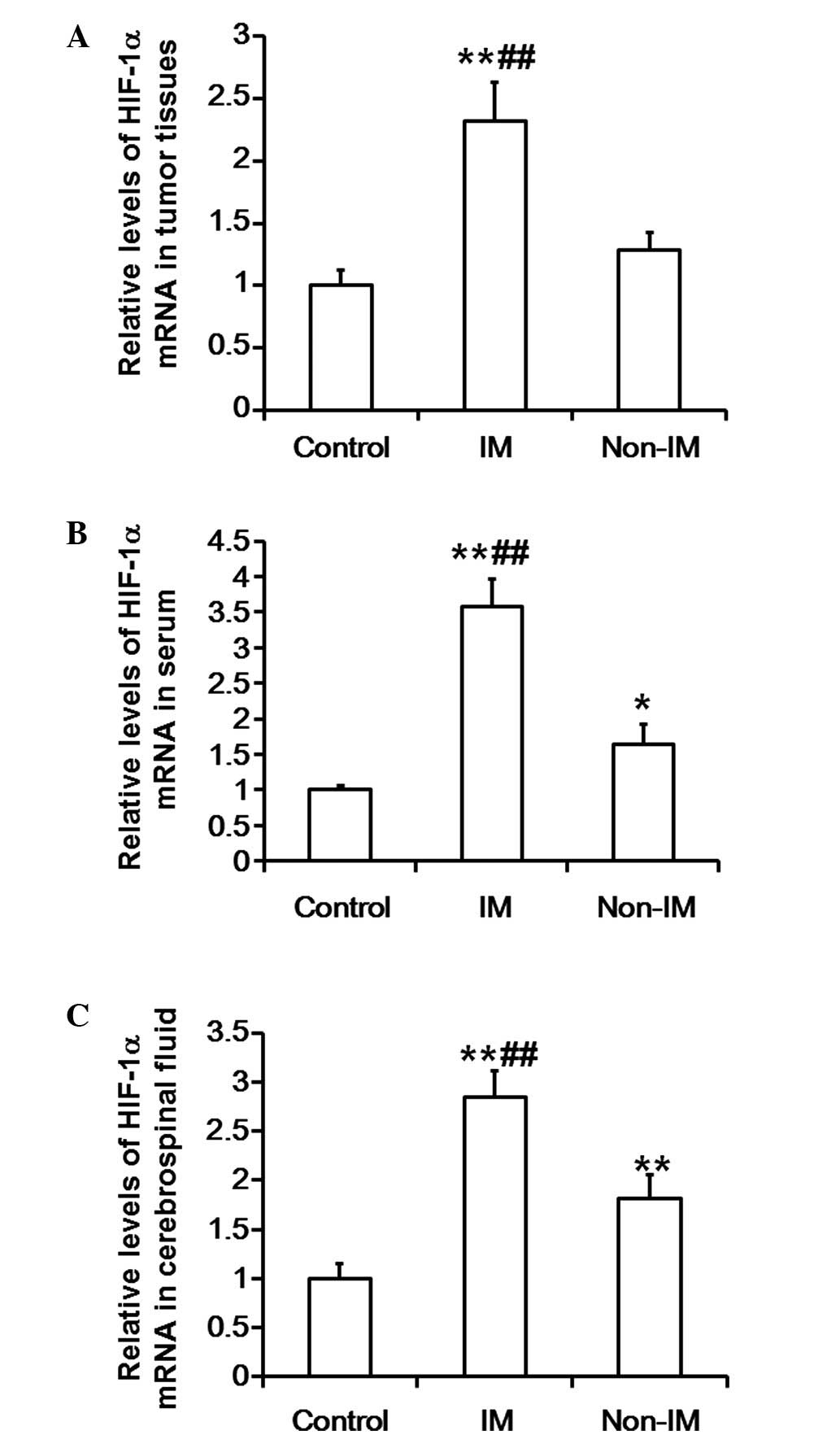
Levels of HIF-1α mRNA in tumor
tissues, serum and cerebrospinal fluid are markedly elevated in
patients with invasive meningiomas
To investigate the levels of HIF-1α mRNA, RT-qPCR
was used to measure the HIF-1α mRNA expression levels in tumor
tissues, serum and cerebrospinal fluid. The results indicate that
the mRNA expression levels of HIF-1α in the tumor tissues of the
invasive meningioma group were significantly elevated compared with
those in the control and non-invasive meningioma groups
(P<0.01). In addition, the mRNA expression levels of HIF-1α in
the tumor tissues of the non-invasive meningioma group were not
significantly different compared with those in the control group
(P>0.05) (Fig. 1A). Similarly,
the mRNA expression levels of HIF-1α in the serum of the invasive
meningioma group were significantly increased compared with those
in the control and non-invasive meningioma groups (P<0.01).
Notably, the mRNA expression levels of HIF-1α in the serum of the
non-invasive meningioma group were significantly higher compared
with those in control group (P<0.05; Fig. 1B). Furthermore, the mRNA expression
levels of HIF-1α in the cerebrospinal fluid of the invasive
meningioma group were significantly higher compared with those in
the control and non-invasive meningioma groups (P<0.01). The
mRNA expression levels of HIF-1α in the cerebrospinal fluid of the
non-invasive meningioma group were significantly elevated compared
with those in the control group (P<0.01; Fig. 1C). These results suggest that the
levels of HIF-1α mRNA in the tumor tissues, serum and cerebrospinal
fluid of patients with invasive meningioma are markedly
increased.
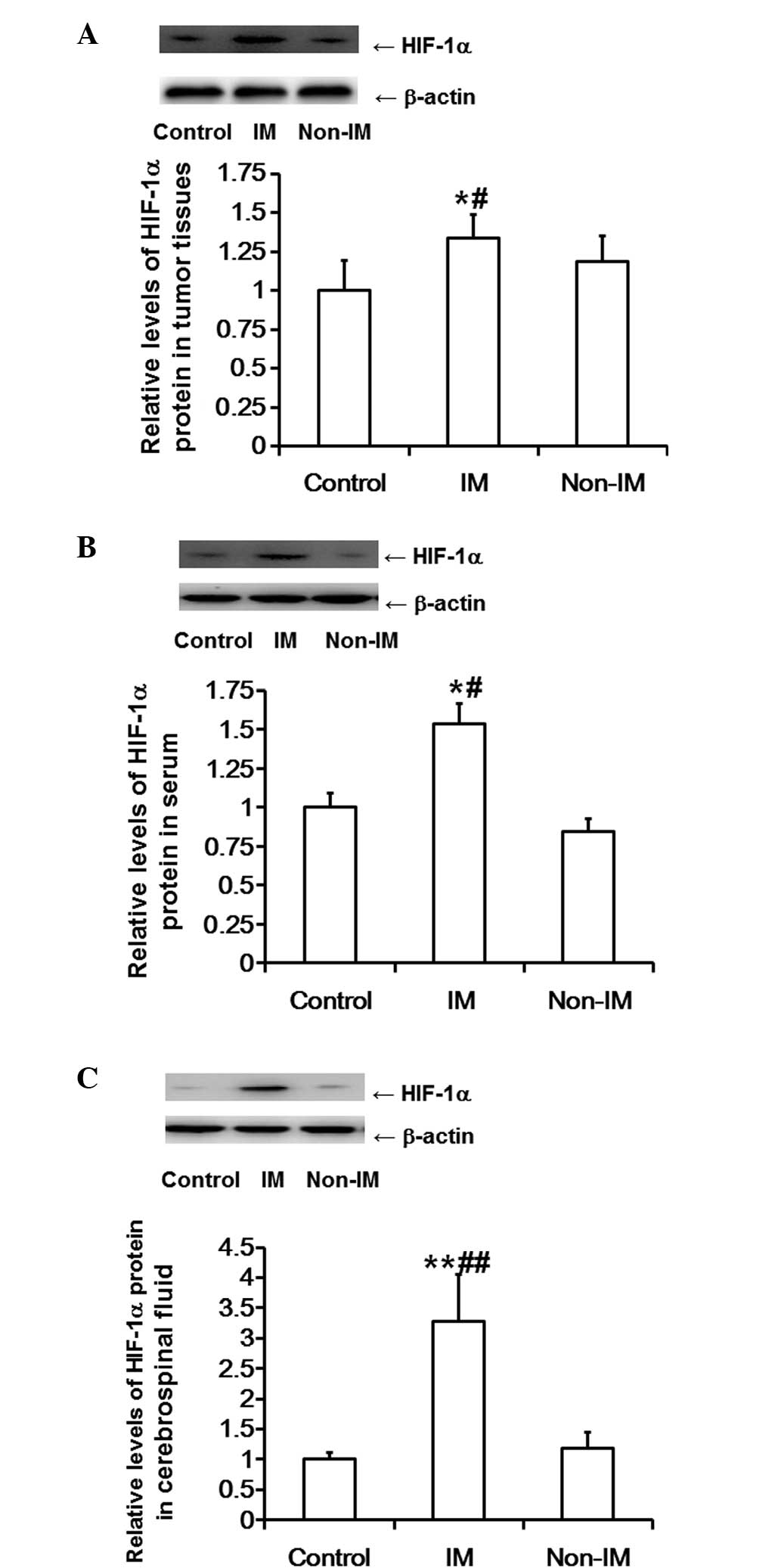
HIF-1α protein expression levels in
tumor tissues, serum and cerebrospinal fluid are markedly increased
in patients with invasive meningiomas, but not in patients with
non-invasive meningiomas
Western blot analysis was employed to determine
HIF-1α protein expression levels in tumor tissue, serum and
cerebrospinal fluid samples. The results showed that the protein
expression levels of HIF-1α in the tumor tissues of the invasive
meningioma group were significantly higher compared with those in
the control and non-invasive meningioma groups (P<0.05).
However, the protein expression levels of HIF-1α in the tumor
tissues of the non-invasive meningioma group were not significantly
different compared with those in the control group (P>0.05;
Fig. 2A). Similarly, the protein
expression levels of HIF-1α in the serum of the invasive meningioma
group were significantly higher compared with those in the control
and non-invasive meningioma groups (P<0.05); however, the
protein expression levels of HIF-1α in the serum of the
non-invasive meningioma group were not significantly different
compared with those in the control group (P>0.05; Fig. 2B). Furthermore, the protein
expression levels of HIF-1α in the cerebrospinal fluid of the
invasive meningioma group were significantly higher compared with
those in the control and non-invasive meningioma groups
(P<0.01). However, the protein expression levels of HIF-1α in
the cerebrospinal fluid of the non-invasive meningioma group were
not significantly different compared with those in the control
group (P>0.05; Fig. 2C). These
results suggest that HIF-1α protein expression in tumor tissues,
serum and cerebrospinal fluid is markedly enhanced in patients with
invasive meningiomas, but not in patients with non-invasive
meningiomas.
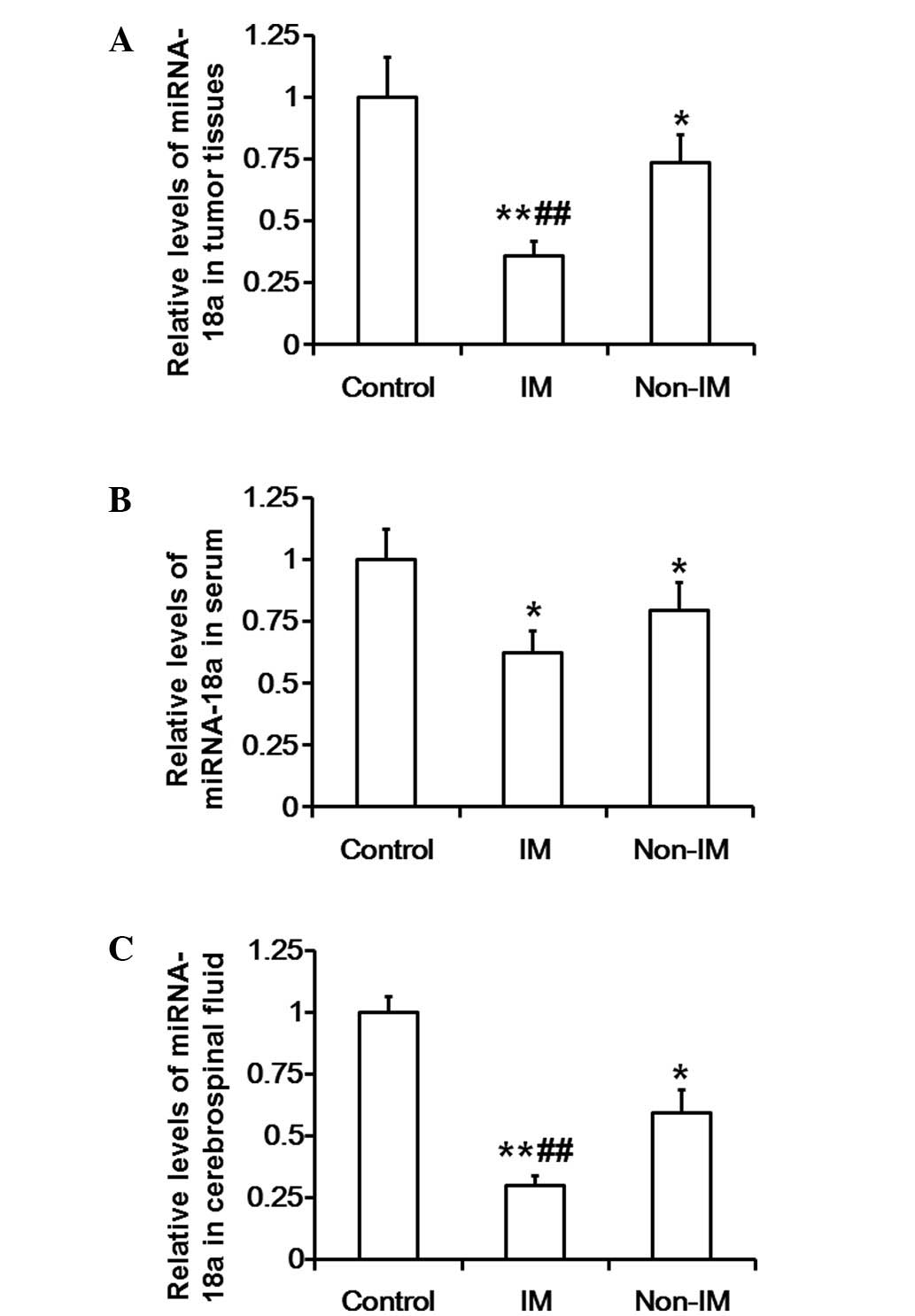
Levels of miR-18a in tumor tissues,
serum and cerebrospinal fluid of patients with invasive meningiomas
and patients with non-invasive meningiomas are notably reduced
RT-qPCR was used to evaluate the levels of miR-18a
in tumor tissue, serum and cerebrospinal fluid samples. The results
show that the levels of miR-18a in the tumor tissues of the
invasive meningioma group were significantly reduced compared with
those in the control and non-invasive meningioma groups
(P<0.01). In addition, the miR-18a expression levels in the
tumor tissues of the non-invasive meningioma group were
significantly lower compared with those in the control group
(P<0.05; Fig. 3A). Furthermore,
miR-18a expression levels in the serum of the invasive meningioma
group were significantly reduced compared with those in the control
group (P<0.05), but were not different from those in the
non-invasive meningioma group (P>0.05). The levels of miR-18a in
the serum of the non-invasive meningioma group were significantly
lower compared with those in the control group (P<0.05; Fig. 3B). The levels of miR-18a in the
cerebrospinal fluid of the invasive meningioma group were
significantly reduced compared with those in the control and
non-invasive meningioma groups (P<0.01), and the miR-18a
expression in the cerebrospinal fluid of the non-invasive
meningioma group was significantly lower compared with that in the
control group (P<0.05; Fig. 3C).
These results indicate that the levels of miR-18a in the tumor
tissues, serum and cerebrospinal fluid of patients with invasive
meningiomas and patients with non-invasive meningiomas are markedly
reduced.
Discussion
In the present study the expression of miR-18a in
healthy subjects and patients with invasive meningiomas or
non-invasive meningiomas was investigated. In addition, the mRNA
and protein expression levels of HIF-1α, a downstream target gene
of miR-18a, were evaluated.
Invasive meningioma has emerged as a serious health
concern. In contrast with the previously accepted hypothesis that
meningioma is a type of benign tumor, invasive meningioma has been
demonstrated to be a malignant tumor type due to its invasiveness
and metastasis (10,11). The invasiveness of tumors strongly
influences the appropriate treatment method. Clinical diagnostic
methods for meningioma typically include imaging, puncture biopsy
and pathological section biopsy. Imaging inspection depends on the
experience of physicians, and is susceptible to subjective
misdiagnosis. Puncture biopsy may produce differing diagnostic
results due to different sampling sites. Therefore, the gold
standard for determining the malignancy of tumors is the
pathological section of tumor tissues. However, due to their state
of health, or economic circumstances, surgery is not suitable for
all patients, which leads to difficulties in determining the
appropriate tumor treatment strategy. Therefore, it is necessary to
identify more reliable and stable genetic markers for
meningioma.
Uncontrolled vascular growth is a marker for tumors,
in addition to being a target for tumor treatment. In tissues that
are deficient of oxygen, HIF-1α is significantly upregulated, as it
is a key transcription factor for the regulation of oxygen
homeostasis (12,13). The mechanism underlying this function
is the promotion of angiogenesis, which enhances blood supply and
ameliorates oxygen deficiency. To a certain extent, differences in
HIF-1α expression indicate alterations in angiogenesis that are an
auxiliary manifestation of tumor growth.
As tumor blood vessels may be considered to be a
potential treatment target, it is necessary to identify the type of
mRNA that regulates abnormal vascular growth, and the upstream
genes that regulate this type of mRNA. In the current literature
regarding gene target therapy for tumors, miRNA regulation pathways
are the best understood mechanism. miRNA is a type of non-coding
RNA molecule that is able to affect the function of its target
genes by inhibiting or enhancing their mRNA and protein expression
levels (14,15). miR-18a belongs to the miR17-92 gene
cluster, of which HIF-1α is a target gene (16). It has been reported that miR-18a
serves a crucial function in the occurrence and development of
myocardial ischemia (17), colonic
neoplasm (18), liver tumors
(19), prostate cancer (20) and malignant gliomas (21). Furthermore, a previous study suggests
that miR-18a may be a specific biomarker for the early diagnosis
and treatment of tumors (22).
Another study observed that miR-18a negatively regulates HIF-1α and
inhibits angiogenesis in tumor tissues (6). Consistent with this finding, the
results of the present study indicate that the expression of HIF-1α
in patients with invasive meningioma was significantly increased
compared with that in control subjects and patients with
non-invasive meningioma, while the expression of miR-18a in
patients with invasive meningioma was significantly reduced
compared with that in control subjects and patients with
non-invasive meningioma. Therefore, the present data suggest that
miR-18a serves an indicative function in invasive meningiomas, and
acts by regulating HIF-1α expression.
As meningiomas occur in the brain, the most
complicated region of the human body, it is impractical to obtain
samples of meningiomas for the evaluation of HIF-1α expression.
However, meningiomas are able to enter the blood circulation and
cerebrospinal fluid, which facilitates the indirect determination
of the vascular growth and invasiveness of the tumor. In the
present study an approximately fixed trend was observed in the
association among meningioma grades, HIF-1α expression and miR-18a
expression. Therefore, the determination of the expression of
HIF-1α and miR-18a in the blood and cerebrospinal fluid may be
useful for clinical diagnosis and the evaluation of patient
condition.
In contrast with imaging investigation, puncture
biopsy and pathological section biopsy, gene markers exhibit unique
indicative characteristics, in addition to providing novel targets
for the prevention and treatment of diseases. However, the activity
and regulatory mechanisms of miR-18a are not fixed across various
pathological and physiological conditions, or in different types of
tumors, and factors that are associated with invasive meningiomas
are not limited to HIF-1α (23–25). In
addition, there are abundant miRNAs that may potentially regulate
these factors. Therefore, the exact effect and mechanism of action
of miR-18a require further investigation in cell, animal and
clinical experiments. In conclusion, the results of the present
study demonstrate that miR-18a may negatively regulate HIF-1α
expression in invasive meningiomas.
Acknowledgements
This study was supported by Dr Quanxiang Wang of
Laiwu City People's Hospital.
References
|
1
|
Chamberlain MC, Glantz MJ and Fadul CE:
Recurrent meningioma: Salvage therapy with long-acting somatostatin
analogue. Neurology. 69:969–973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frydrychowicz C, Holland H, Hantmann H,
Gradistanac T, Hoffmann KT, Mueller W, Meixensberger J and Krupp W:
Two cases of atypical meningioma with pulmonary metastases: A
comparative cytogenetic analysis of chromosomes 1p and 22 and a
review of the literature. Neuropathology. 35:175–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marguet F, Proust F, Crahes M, Basset C,
Joly-Helas G, Chambon P and Laquerrière A: Malignant meningioma
with adenocarcinoma-like metaplasia: A rare entity to be not
misdiagnosed. Ann Pathol. 34:223–227. 2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan X: Angiogenesis: A promising strategy
for tumor therapy. Sheng Wu Wu Li Xue Bao. 26:180–193. 2010.(In
Chinese).
|
|
6
|
Brahimi-Horn MC, Chiche J and Pouysségur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo F and Hu T: Overexpression of miR-18a
suppresses tumor angiogenesis in colon cancer. Zhong Guo Xian Dai
Yi Xue Za Zhi. 23:37–41. 2013.(In Chinese).
|
|
8
|
Liu FJ, Kaur P, Karolina DS, Sepramaniam
S, Armugam A, Wong PT and Jeyaseelan K: MiR-335 regulates Hif-1α to
reduce cell death in both mouse cell line and rat ischemic models.
PLoS One. 10:e01284322015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Xu D, Xie H, Tang J, Liu R, Li J,
Wang S, Chen X, Su J, Zhou X, et al: miR-33a functions as a tumor
suppressor in melanoma by targeting HIF-1α. Cancer Bio Ther.
16:846–855. 2015. View Article : Google Scholar
|
|
10
|
Jacob JT, Link MJ and Pollock BE: Role of
stereotactic radiosurgery in meningiomas and vestibular
schwannomas. Curr Treat Options Neurol. 16:3082014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Tu Y, Wang S, Xu S, Xu L, Xiong Y,
Mei J and Wang C: Role of HER-2 activity in the regulation of
malignant meningioma cell proliferation and motility. Mol Med Rep.
May 21–2015.(epub ahead of print). View Article : Google Scholar
|
|
12
|
Morfoisse F, Kuchnio A, Frainay C,
Gomez-Brouchet A, Delisle MB, Marzi S, Helfer AC, Hantelys F, Pujol
F, Guillermet-Guibert J, et al: Hypoxia induces VEGF-C expression
in metastatic tumor cells via a HIF-1α-independent
translation-mediated mechanism. Cell Rep. 6:155–167. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bakirtzi K, West G, Fiocchi C, Law IK,
Iliopoulos D and Pothoulakis C: The neurotensin-HIF-1α-VEGFα axis
orchestrates hypoxia, colonic inflammation and intestinal
angiogenesis. Am J Pathol. 184:3405–3414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ayala de la Peña F, Kanasaki K, Kanasaki
M, Tangirala N, Maeda G and Kalluri R: Loss of p53 and acquisition
of angiogenic microRNA profile are insufficient to facilitate
progression of bladder urothelial carcinoma in situ to
invasive carcinoma. J Biol Chem. 286:20778–20787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Q, Wang X, Cui J, Wang P, Xiong M,
Jia C, Liu L, Ning B, Li L, Wang W, et al: Bidirectional regulation
of angiogenesis and miR-18a expression by PNS in the mouse model of
tumor complicated by myocardial ischemia. BMC Complement Altern
Med. 14:1832014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yau TO, Wu CW, Dong Y, Tang CM, Ng SS,
Chan FK, Sung JJ and Yu J: MicroRNA-221 and microRNA-18a
identification in stool as potential biomarkers for the
non-invasive diagnosis of colorectal carcinoma. Br J Cancer.
111:1765–1771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CL, Yeh KH, Liu WH, Chen CL, Chen DS,
Chen PJ and Yeh SH: Elevated p53 promotes the processing of miR-18a
to decrease estrogen receptor-α in female hepatocellular carcinoma.
Int J Cancer. 136:761–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu TI, Hsu CH, Lee KH, Lin JT, Chen CS,
Chang KC, Su CY, Hsiao M and Lu PJ: MicroRNA-18a is elevated in
prostate cancer and promotes tumorigenesis through suppressing STK4
in vitro and in vivo. Oncogenesis. 3:e992014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Wang P, Zhao W, Yao Y, Liu X, Ma
J, Xue Y and Liu Y: MiR-18a regulates the proliferation, migration
and invasion of human glioblastoma cell by targeting neogenin. Exp
Cell Res. 324:54–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komatsu S, Ichikawa D, Takeshita H,
Morimura R, Hirajima S, Tsujiura M, Kawaguchi T, Miyamae M, Nagata
H, Konishi H, et al: Circulating miR-18a: A sensitive cancer
screening biomarker in human cancer. Vivo. 28:293–297. 2014.
|
|
23
|
Bertozzi D, Marinello J, Manzo SG, Fornari
F, Gramantieri L and Capranico G: The natural inhibitor of DNA
topoisomerase I, camptothecin, modulates HIF-1α activity by
changing miR expression patterns in human cancer cells. Mol Cancer
Ther. 13:239–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chai ZT, Kong J, Zhu XD, Zhang YY, Lu L,
Zhou JM, Wang LR, Zhang KZ, Zhang QB, Ao JY, et al: MicroRNA-26a
inhibits angiogenesis by down-regulating VEGFA through the
PIK3C2α/Akt/HIF-1α pathway in hepatocellular carcinoma. PLoS One.
8:e779572013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lemaire J, Mkannez G, Guerfali FZ, Gustin
C, Attia H, Sghaier RM, Sysco-Consortium, Dellagi K, Laouini D and
Renard P: MicroRNA expression profile in human macrophages in
response to Leishmania major infection. PLoS Negl Trop Dis.
7:e24782013. View Article : Google Scholar : PubMed/NCBI
|