Introduction
Bone tissue is constantly renewed. Bone homeostasis
is maintained with bone formation by osteoblasts (OBs) and
osteoclast (OC)-driven bone resorption. Dysregulation of this
process can result in metabolic bone diseases, including
osteoporosis and osteopetrosis (1).
OCs, which are terminally differentiated multinuclear cells
originating from hematopoietic stem cells, are the only cells with
bone resorption activity and they play an important role in bone
turnover. The occurrence of bone metabolic diseases is closely
associated with the formation and activation of OCs.
As the main active metabolite of vitamin D,
1α,25-dihydroxyvitamin D3
[1α,25-(OH)2D3] displays strong bioactivity,
regulating calcium and phosphorus metabolism and assisting in the
maintenance of calcium homeostasis (2). 1α,25-(OH)2D3 is
involved in the regulation of bone metabolism, not only through a
receptor on the small intestine and kidneys but also by acting on
osteocytes directly (3).
1α,25-(OH)2D3 enhances expression of the
receptor activator for nuclear factor-κB ligand (RANKL), which can
regulate and control bone resorption and the formation of OCs
indirectly by acting on the vitamin D receptor (VDR) on the surface
of OBs. The VDR has been identified on OC precursor cells, and
Kogawa et al (4) reported
that 1α,25-(OH)2D3 induces OC precursor cells
to differentiate into mature OCs that display bone absorption
activity. In contrast to these findings, Uchiyama et al
(5) have demonstrated that
pharmacological doses of active vitamin D3 suppress bone
resorption. This discrepancy in the effects of vitamin D on bones
requires further exploration.
In the present study, OC differentiation was induced
in vitro by the treatment of Wistar rat bone marrow-derived
macrophage cells with 25 µg/l macrophage-colony stimulating factor
(M-CSF) and 45 µg/l RANKL. OCs were treated with
1α,25-(OH)2D3 at concentrations of 0,
10−9, 10−8 or 10−7 mol/l, or with
ethyl alcohol as solvent control. To confirm OC differentiation,
cells were stained with tartrate resistant acid phosphatase (TRAP),
and examination of the absorption lacuna was conducted to study the
effects of 1α,25-(OH)2D3 on the bone
absorption activity of OCs. Additionally, the expression levels of
proteins involved in bone absorption were measured by western
blotting.
Materials and methods
Experimental animals
Three-week-old male Wistar rats of clean grade were
provided by the Comparative Medical Center of Yangzhou University
(Yangzhou, China). This study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Research Council. The animal
care and use committee of Yangzhou University approved all
experiments and procedures conducted on the animals (approval ID:
SYXK (Su) 2007-0005).
Reagents and instruments
α Minimum essential medium (α-MEM) was obtained from
Gibco Life Technologies (Carlsbad, CA, USA). Penicillin and
streptomycin were purchased from Shandong Lukang Pharmaceutical
Ltd. (Shandong, China). Fetal bovine serum (FBS) was purchased from
Thermo Fisher Scientific (Waltham, MA, USA), and M-CSF & RANKL
from PeproTech Inc. (Rocky Hill, NJ, USA).
1α,25-(OH)2D3 and the TRAP staining kit were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Carbonic
anhydrase II (CA II; ab6621), cathepsin K (CK; ab19027) and matrix
metalloproteinase-9 (MMP-9; ab137867) antibodies were purchased
from Abcam (Cambridge, UK) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; FL-335) antibodies were purchased from Santa
Cruz Biotechnology, Inc., (Dallas, TX, USA), while bovine cortical
slices (50 µm thick; 1,600 cryo-cut microtomes) were obtained from
Leica Microsystems GmbH (Wetzlar, Germany). Other reagents used in
this study were of analytical grade and made in China.
Instruments used in this study included an inverted
phase contrast microscope (DMI-3000B; Leica Microsystems GmbH), an
environmental scanning electron microscope (XL-ESEM; Philips,
Amsterdam, Netherlands) and an ultrasonic wave cleaner (KQ-250;
Kunshan Ultrasonic Instruments Co., Ltd., Kunshan, China).
Osteoclastogenesis in
vitro
For osteoclastogenesis from rat bone-marrow-derived
precursor cells, 3-week-old male Wistar rats were used. Animal
experimental protocols were approved by the Committee on the Care
and Use of Animals in Research at Yangzhou University. A total of 3
rats were used per experiment. Whole bone marrow cells, isolated by
flushing the marrow space of femurs and humerus and centrifuging
the resulting suspension at 500 × g for 5 min, were resuspended in
α-MEM containing 100 U/ml penicillin and 100 µg/ml streptomycin.
Following the removal of erythrocytes by lysing in buffer (0.15 M
NH4Cl, 1 mM KHCO3 and 0.1 mM ethylene diamine
tetraacetic acid, pH 7.2), the bone-marrow cells were plated on
100-mm culture dishes and cultured in α-MEM supplemented with 10%
FBS for 24 h in 5% CO2 at 37°C. Non-adherent cells were
collected, plated on 100-mm bacterial dishes and cultured for 3
days in the presence of 25 ng/ml M-CSF. The adherent cells were
considered to be bone marrow-derived macrophages and were used as
OC precursor cells. The macrophages were seeded on 48-well plates
at 4.0×105 cells/ml and cultured with 25 µg/l M-CSF and
45 µg/l RANKL at 37°C in 5% CO2 for 3 days. Fresh medium
was then added and subsequently changed every 2 days.
TRAP staining and OC counting
Bone marrow-derived macrophage cells were incubated
in 48-well plates at a density of 4.0×105 cells/ml (0.5
ml/well) with 25 µg/l M-CSF and 45 µg/l RANKL and different five
groups were created as follows: Group A, control; group B,
10−9 mol/l 1α,25-(OH)2D3; group C,
10−8 mol/l 1α,25-(OH)2D3; group D,
10−7 mol/l 1α,25-(OH)2D3; group E,
0.1% ethyl alcohol (solvent control). Following 8 days of culture,
the medium was removed, the plates were rinsed with
phosphate-buffered saline (PBS) and the cells fixed with 4%
paraformaldehyde for 20 min prior to staining with the TRAP
staining kit according to the manufacturer's instructions. Ten
random fields were selected for examination with an inverted
microscope and the red-stained multinucleated cells containing ≥3
nuclei were counted.
F-actin staining
Cells used for F-actin staining were prepared as
previously described (6), with the
exception that the density of plated cells was increased to
4.0×105 cells/ml (1 ml/well). After 8 days of culture,
the plates were rinsed three times with PBS. Cells were fixed with
4% paraformaldehyde for 20 min then rinsed three times with PBS and
incubated with Triton X-100 (0.5% v/v) for 20 min. They were again
rinsed three times with PBS and treated with 5% bovine serum
albumin for 30 min. Finally, 20 µmol/l
phalloidin-tetramethylrhodamine isothiocyanate (TRITC; Invitrogen
Life Technologies, Carlsbad, CA, USA) was added to the plates
according to the manufacturer's instructions and incubated for 120
min in the dark. The OCs were observed by fluorescence microscopy
(DMI3000B; Leica, Germany) and images captured under the red
channel.
Lacunar absorption analysis
The OC precursor cells were collected and incubated
following a procedure similar to that described in previous
sections with the exception that sterilized bovine cortical slices
were placed at the bottom of 48-well plates prior to incubation. To
remove the cells attached to the osteocomma and absorption lacuna,
bovine cortical slices were removed at day 9 and rinsed three times
with PBS. Bone slices were treated with an ultrasonic wave cleaner
in 0.25 mol/l ammonium hydroxide three times (5 min each time).
Following dehydration in a graded ethanol series of 40, 70, 80, 95
and 100% (v/v), the bone slices were air-dried and gilded using an
ion-plating apparatus (SCD500 Sputter Coater; Leica Microsystems
GmbH) which was followed by observation with an XL30-ESEM scanning
electron microscope. The area of lacunar absorption was measured
using a JD801 image analysis system (Nanjing University, Nanjing,
China).
Western blot analysis
Western blot analysis was carried out as described
previously with minimum alterations (7). The OC precursor cells were collected
and incubated in 100-mm cell culture plates. After 8 days of
culture, cells were collected and total protein extracted using the
Cell Total Protein Extraction kit (Applygen Technologies, Inc.,
Beijing, China). Protein concentration was determined using a
Bicinchoninic Acid Protein kit and samples adjusted to a similar
concentration. Samples were heated for 10 min at 100°C for sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). A
total of 60 µg protein was loaded in each lane for 10–15% SDS-PAGE
and run with a constant voltage of 120 V for 120 min. Proteins were
transferred to nitrocellulose membranes with a constant voltage of
120 V for 90 min. The membrane was blocked at room temperature for
2 h with 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20
(TBST), and then incubated for 12 h at 4°C with the primary
antibodies: Rabbit anti-mouse CA II antibody (1:3,000); rabbit
anti-mouse MMP-9 (1:1,000); rabbit anti-mouse CK (1:500) and
anti-GAPDH as an internal reference. The membranes were rinsed six
times with TBST and incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G fraction
polyclonal antibody (1:5,000) at room temperature for 2 h.
Following additional washes, the membranes were visualized using an
ECL detection kit (Merck Millipore, Billerica, MA, USA) according
to the manufacturer's instructions, and exposed to X-ray film.
Analyses were performed to determine the intensity of western blot
bands using Image Lab software (Bio-Rad Laboratories, Hercules, CA,
USA).
Statistical analysis
Data were analyzed with SPSS t-test software
(version 19.0; IBM SPSS, Armonk, NY, USA) for significance analysis
and are expressed as mean ± standard deviation. P-values <0.05
and <0.01 were considered to indicate results that were
statistically significant and highly statistically significant,
respectively.
Results
Effects of
1α,25-(OH)2D3 on OC formation
In the groups treated with M-CSF and RANKL,
TRAP-positive multinucleated cells were observed by day 8 (Fig. 1). The solvent (ethyl alcohol)-treated
group (group E; 7.7±2.08 cells/field) exhibited no apparent
difference in cells/field as compared with group A (8.0±1.3
cells/field). 1α, 25-(OH)2D3 inhibited the
formation of OCs significantly. The numbers of OCs in groups B
(10−9 mol/l 1α,25-(OH)2D3), C
(10−8 mol/l 1α,25-(OH)2D3) and D
(10−7 mol/l 1α,25-(OH)2D3) were
5.3±0.58, 2.7±1.15 and 0.7±0.56 cells/field, respectively
(*P<0.05 or **P<0.01). The results indicated that
1α,25-(OH)2D3 had inhibitory effects on OC
formation.
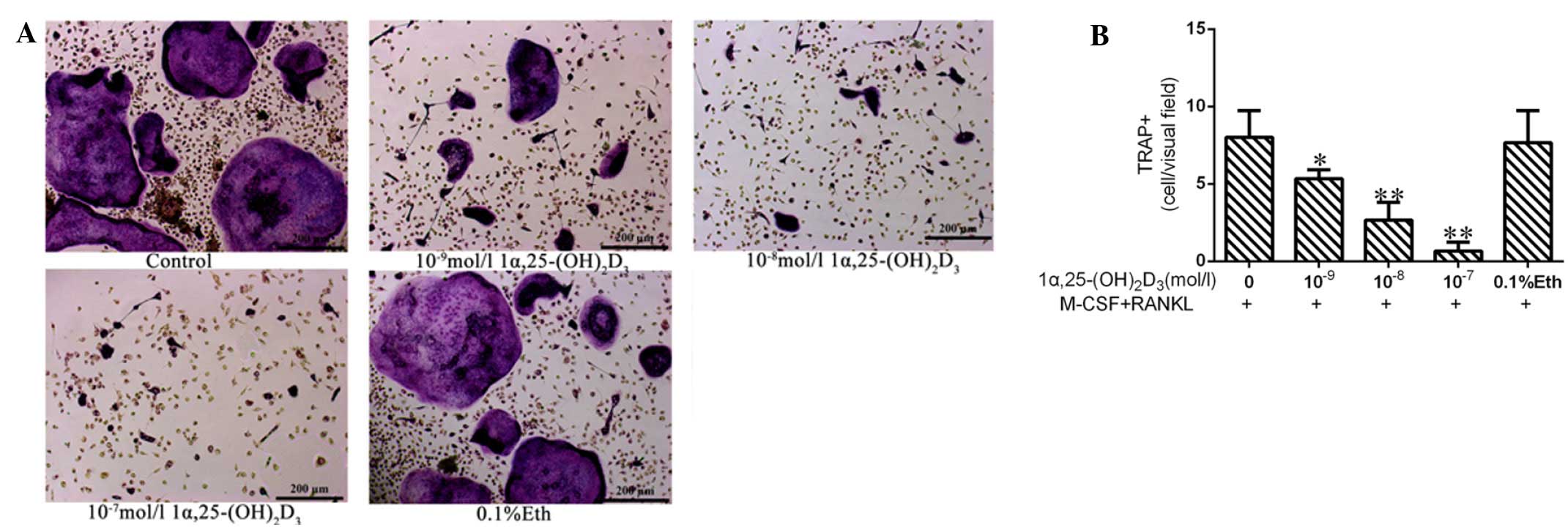 | Figure 1.1α,25-(OH)2D3
inhibits the differentiation of bone marrow-derived mononuclear
cells to osteoclasts in vitro. (A) Red-stained
multinucleated cells following treatment with different
concentrations of 1α,25-(OH)2D3. Scale bars,
200 µm. (B) Numbers of osteoclasts with ≥3 nuclei from 10 random
fields of view were counted. Results are expressed as mean ±
standard deviation. *P<0.05, **P<0.01 vs. the control.
1α,25-(OH)2D3, 1α,25-dihydroxyvitamin D3;
TRAP, tartrate-resistant acid phosphatase; M-CSF, macrophage-colony
stimulating factor; RANKL, receptor activator for nuclear factor-κB
ligand; Eth, ethanol. |
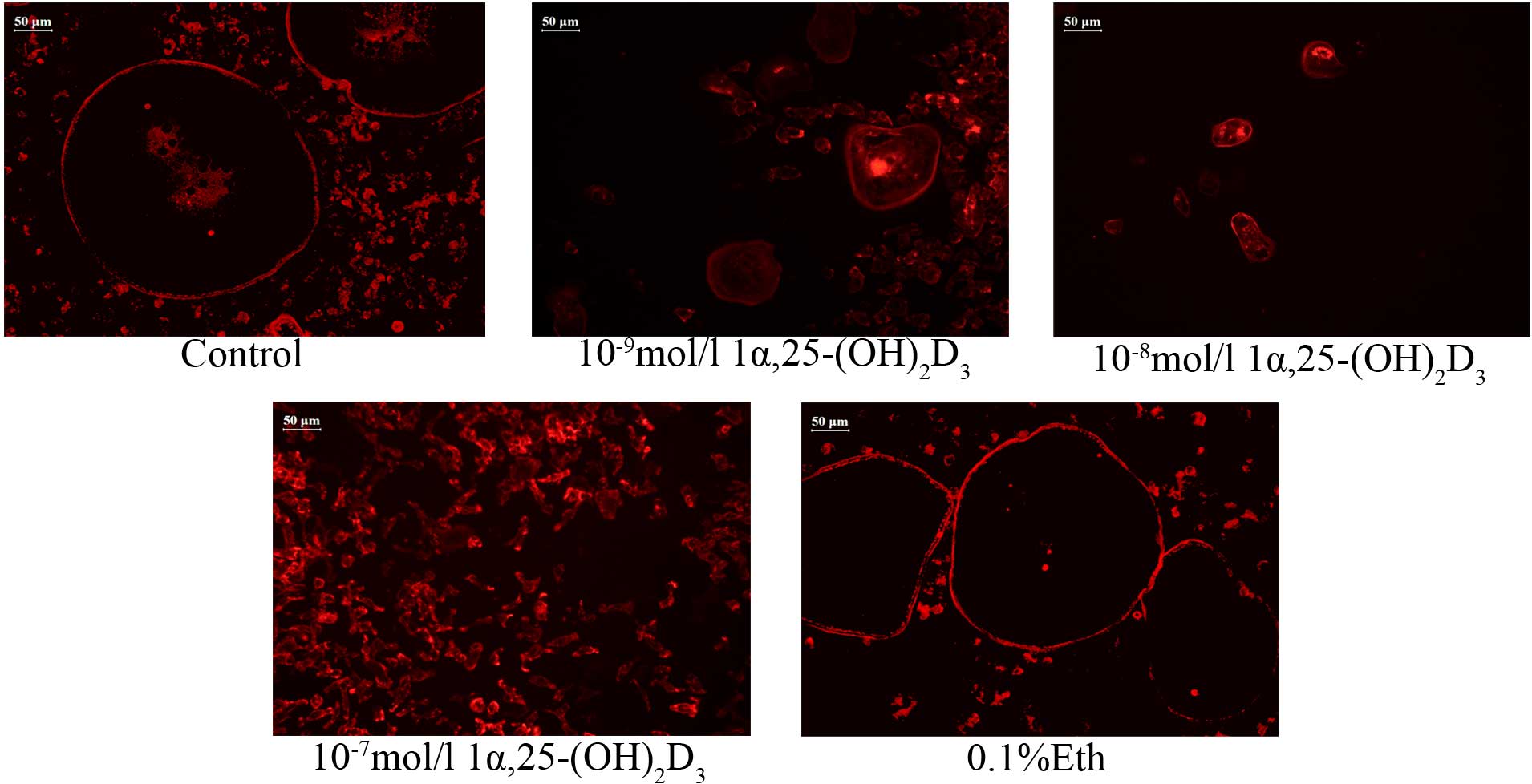
Effects of
1α,25-(OH)2D3 on OC F-actin-based
cytoskeletons
OCs isolated from rat bone marrow-derived macrophage
cells and incubated with M-CSF and RANKL had a regular, clear, red
and rich F-actin-based cytoskeleton (Fig. 2). OCs in the solvent-treated group
(group E) had intense and highly aligned F-actin, which exhibited
no apparent difference compared with control group A (M-CSF and
RANKL only). Following treatment with
1α,25-(OH)2D3, the distribution of F-actin
changed and the levels of expression decreased. The changes were
particularly apparent in group D (10−7 mol/l
1α,25-(OH)2D3), in which the cytoskeleton
could barely be observed. The results show that
1α,25-(OH)2D3) inhibits the formation of the
cytoskeleton in OCs.
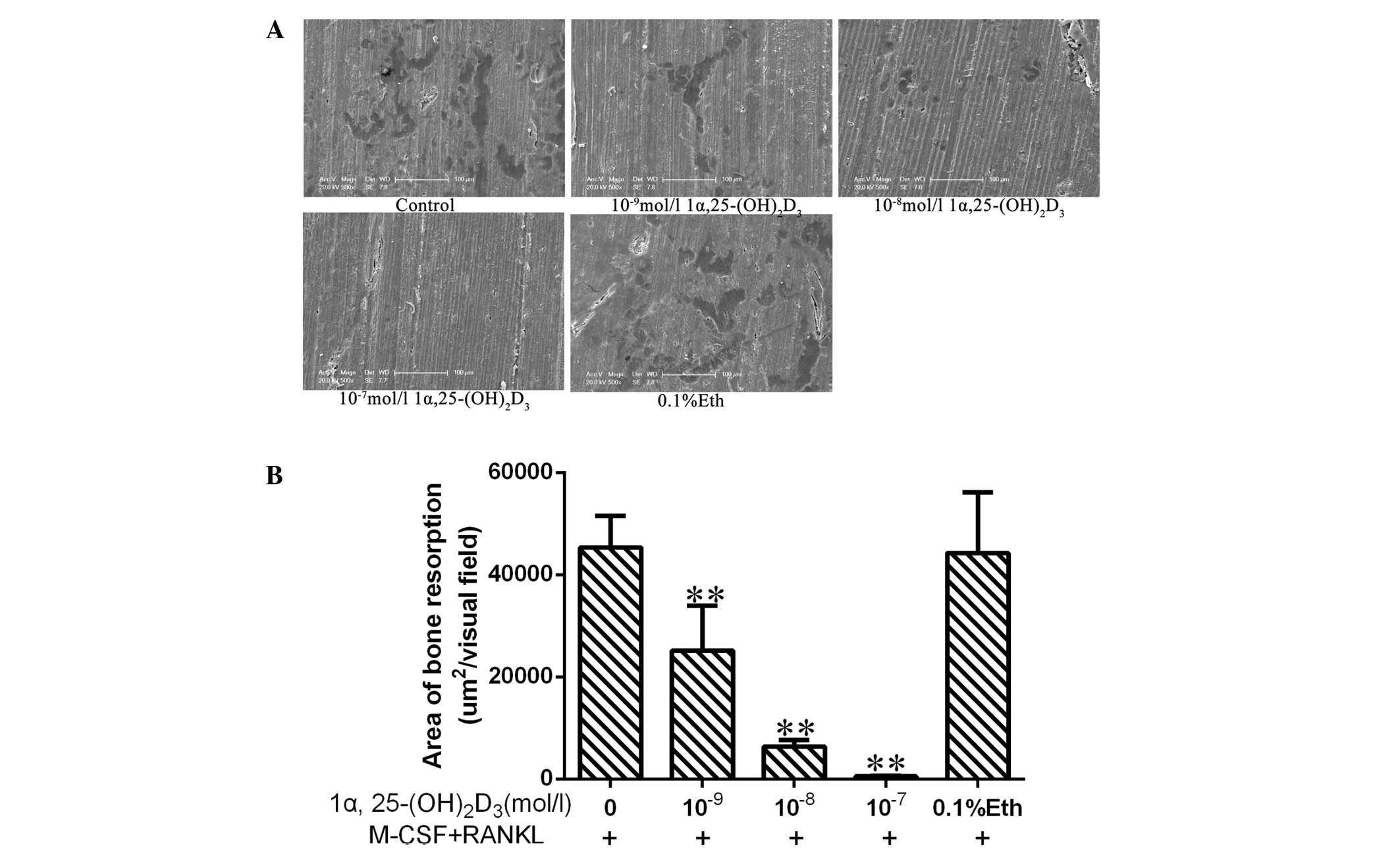
Effects of
1α,25-(OH)2D3 on OC bone resorption
Bone marrow-derived macrophage cells incubated with
M-CSF and RANKL differentiated into OCs, which exhibited bone
absorption activity. Absorption lacunae were observed on bovine
cortical slices following 8 days of culture (Fig. 3A). The lacunae in bone in the
solvent-treated group (group E) were no different to those in
control group A. By contrast, 1α,25-(OH)2D3
inhibited the formation of lacunae.
The area of the lacunae in the groups treated with
1α,25-(OH)2D3 was smaller than those in the
control group, with the areas of the lacunae in groups B, C and D
measured as 25,174±8,754, 6,336±1,346 and 514±169
µm2/visual field, respectively (Fig. 3B). The results showed that
1α,25-(OH)2D3 reduced the area of lacunae in
a dose-dependent manner and inhibited the bone resorption activity
of OCs.
1α,25-(OH)2D3
regulates the expression of proteins associated with bone
absorption
OCs that had differentiated from bone marrow-derived
macrophage cells after treatment with M-CSF and RANKL expressed CA
II, CK and MMP-9, as shown by western blotting (Fig. 4). The expression levels of these
proteins in group E (solvent control) and group A were not
significantly different. By contrast, the expression levels of CA
II, CK and MMP-9 decreased significantly in the groups treated with
1α,25-(OH)2D3 (*P<0.05 or
**P<0.01).
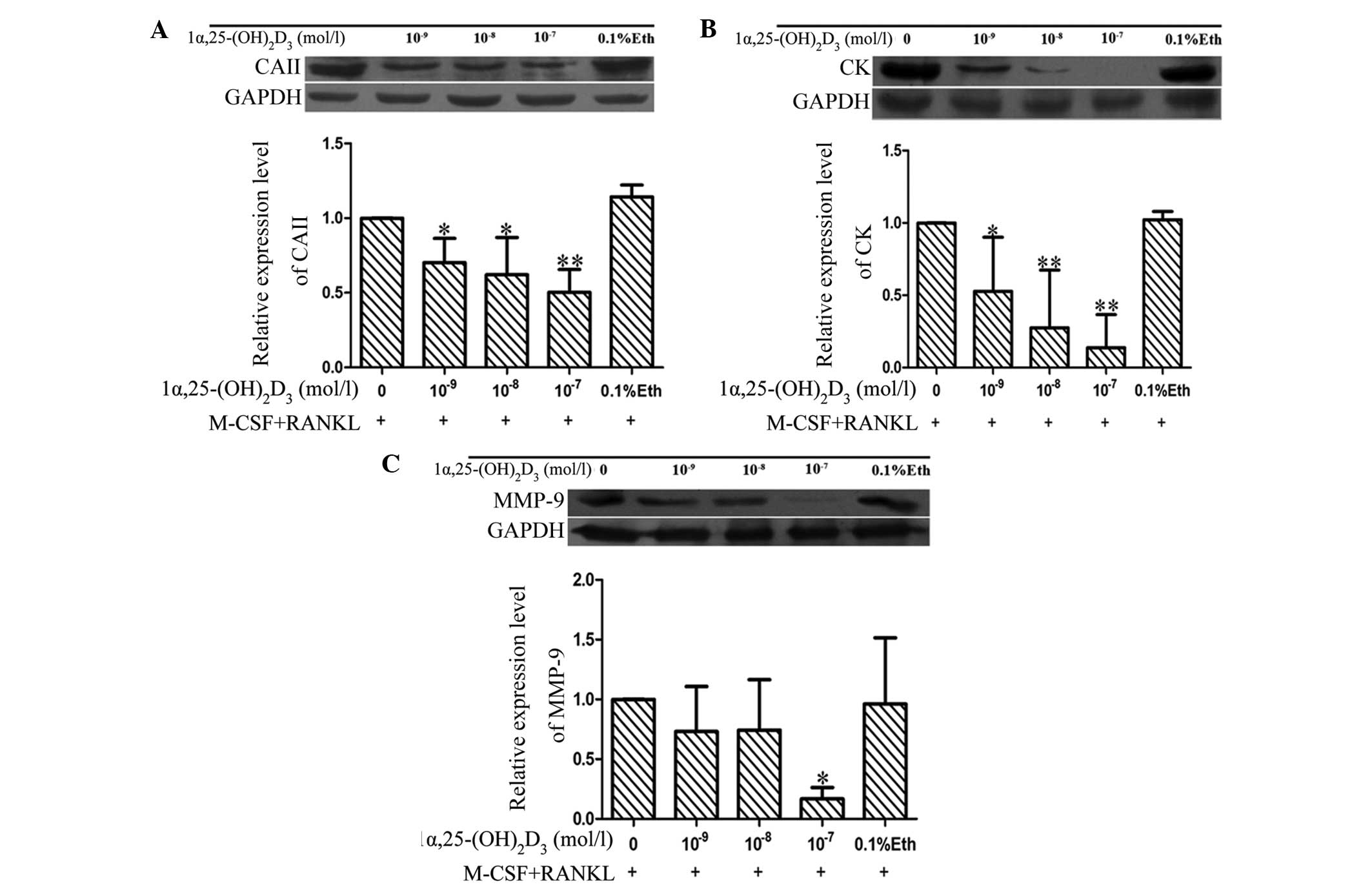 | Figure 4.1α,25-(OH)2D3
reduces the expression of (A) CA II, (B) cathepsin K and (C) MMP-9.
Results are expressed as mean ± standard deviation. *P<0.05,
**P<0.01 vs. the control. 1α,25-(OH)2D3,
1α,25-dihydroxyvitamin D3; CA II, carbonic anhydrase II; CK,
cathepsin K; MMP, matrix metalloproteinase; M-CSF,
macrophage-colony stimulating factor; RANKL, receptor activator for
nuclear factor-κB ligand; Eth, ethanol. |
Discussion
OCs are terminally differentiated multinuclear
cells. As the only type of cells displaying bone absorption
activity, OCs play an important role in bone turnover (8). As it is demanding to isolate, purify
and culture OCs in vitro, and subcultures cannot be
established, the study of OCs is challenging. Currently, RAW264.7
cells, which are OC precursors (9),
are the main cells used to obtain OCs through differentiation. The
RAW264.7 cell line was established from a tumor induced by Abelson
murine leukemia virus; therefore, biological discrepancies may
exist and results obtained using this cell line may be
controversial (10). In the present
study, OCs obtained from bone marrow-derived mononuclear cells
isolated from Wistar rats and differentiated by M-CSF and RANKL
treatment displayed typical characteristics of OCs, such as
TRAP-positive staining and bone absorption activity. The biological
features of OCs obtained in this study are similar to those of OCs
in vivo, indicating that the method can be used to obtain
results that are accurate and relevant.
Vitamin D is important in calcium homeostasis and
bone metabolism, although differences in the regulation of OCs
cultured in vitro or in vivo have been reported
(10). Certain studies have shown
that 1α,25-(OH)2D3 inhibits the formation of
OCs from mouse monocyte-macrophage cells (11,12),
while Medhora et al (13)
reported enhanced OC formation and increases in the expression of
integrin β3 when 1α,25-(OH)2D3 was added in
an early study of an OC culture model in vitro (14). Vitamin D was initially identified as
being useful for the treatment of rickets. A lack of vitamin D can
lead to the onset of rickets in younger animals and chondropathy in
adult animals (15). The present
study showed that different concentrations of
1α,25-(OH)2D3 may inhibit the formation and
activity of OCs, as well as downregulating the expression levels of
CA II, CK and MMP-9. These results are similar to those reported by
Arai et al (16) who found
that colony-stimulating factor-1 (c-Fms) was the key signaling
molecule controlling RANK expression and
1α,25-(OH)2D3 inhibited OC formation by
downregulating the expression of c-Fms to reduce RANK
expression.
Due to the ease of use, TRAP staining was used to
identify OCs in the present study. TRAP has the highest expression
levels in normal bone tissue, and is not only regarded as a marker
of OCs, but also participates in bone absorption and the maturation
of OCs (17). In the present study,
TRAP staining demonstrated that bone marrow-derived mononuclear
cells from Wistar rats may be induced to differentiate into OCs
with M-CSF and RANKL treatment. The OCs had typical features,
including red particles in the cytoplasm and >3 nuclei,
following staining with TRAP. Compared with the control group,
different concentrations of 1α,25-(OH)2D3
inhibited OC formation in a dose-dependent manner. OCs displayed
F-actin-based cytoskeletons, which appeared red with clear-cut
edges following staining with phalloidin-TRITC. Different
concentrations of 1α,25-(OH)2D3 inhibited the
formation of the F-actin-based cytoskeleton, findings that are
similar to those from Kim et al (18). The results of bone resorption lacunae
analysis showed that the area of lacuna decreased following
treatment with 1α,25-(OH)2D3 in a
dose-dependent manner. Together, the results show that
1α,25-(OH)2D3 reduces the formation of OCs
from Wistar rat bone marrow-derived mononuclear cells in
vitro, and inhibits the activity of bone absorption.
It is well known that CA II, CK and MMP-9 proteins
are involved in the OC bone resorption process (19). CA II, a metalloenzyme whose active
site contains Zn2+, participates in numerous important
physiological changes in the body. It has a critical regulatory
function in bone resorption: Not only does it control the secretion
of H+, but it can also affect OC differentiation and
movement, and plays a role in changing intracellular
Ca2+ concentration and pH in absorption lacunae
(20). The main function of CA II in
OC resorption is to catalyze the reaction of CO2 and
H2O to form H+; H+ secretion into
the resorption cavity is important for OC activity in bone
resorption. The acidic environment dissolves bone hydroxypropyl
phosphate and OCs can further degrade bone matrix components,
mainly through the secretion of cathepsins and MMPs, a process in
which CK and MMP-9 play significant roles (21). CK, abundantly expressed in OC, is a
lysosomal cysteine protease that can degrade protein components of
the demineralized bone matrix, including degradation of bone matrix
of type I collagen, osteopontin and osteonectin. Type I collagen
protein is the main component of the bone matrix and can be
degraded by CK at multiple sites (22). MMP-9 is a type of zinc-dependent
protease which has a highly conserved structure and is important in
the early stages of OC differentiation and bone resorption
(23). In the present study, OCs
were obtained from bone marrow-derived mononuclear cells of rats by
inducing differentiation via treatment with M-CSF and RANKL. OCs
express high levels of CA II, CK and MMP-9. Treatment with
1α,25-(OH)2D3 inhibited the expression levels
of CA II and CK proteins significantly. There was no significant
effect on the expression levels of MMP-9 when treated with
10−9 or 10−8 mol/l
1α,25-(OH)2D3; however, at 10−7
mol/l 1α,25-(OH)2D3 inhibited the expression
of MMP-9 significantly. The results demonstrated that
1α,25-(OH)2D3 is able to downregulate the
expression of CA II, which reduces calcium salt-induced dissolution
of bone tissue by decreasing the concentration of H+ in
the sealing zone and inhibiting the secretion of CK and MMP-9
proteins to prevent the degradation of bone matrix proteins during
bone resorption. Ultimately, this inhibits the bone resorption
activity of OCs.
In conclusion, within the 10−9 to
10−7 mol/l concentration range,
1α,25-(OH)2D3 inhibits OC formation from rat
bone marrow-derived mononuclear cells, and inhibits bone resorption
activity by downregulating expression levels of CA II, CK and
MMP-9. These results indicated that
1α,25-(OH)2D3 can directly regulate bone
metabolism by inhibiting the formation and maturation of
osteoclasts.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant nos. 31172373 and 31372495), the
National Science Foundation for Distinguished Young Scholars of
China (Grant no. 31302154), the Natural Science Foundation of the
Jiangsu Higher Education Institutions of China (Grant no.
13KJB230002), the Specialized Research Fund for the Doctoral
Program of Higher Education (Grant no. 20133250120002) and the
Project Funded by the Priority Academic Program Development of
Jiangsu Higher Education Institutions. In addition, the authors
would like to thank the Testing Center of Yangzhou University for
providing technical support for the ESEM used in this project.
References
|
1
|
Qu X, Zhai Z, Liu X, et al: Dioscin
inhibits osteoclast differentiation and bone resorption through
down-regulating the Akt signaling cascades. Biochem Biophys Res
Commun. 443:658–665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lieben L, Carmeliet G and Masuyama R:
Calcemic actions of vitamin D: Effects on the intestine, kidney and
bone. Best Pract Res Clin Endocrinol Metab. 25:561–572. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lieben L and Carmeliet G: Vitamin D
signaling in osteocytes: Effects on bone and mineral homeostasis.
Bone. 54:237–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kogawa M, Anderson PH, Findlay DM, et al:
The metabolism of 25-(OH)vitamin D3 by osteoclasts and their
precursors regulates the differentiation of osteoclasts. J Steroid
Biochem Mol Biol. 121:277–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchiyama Y, Higuchi Y, Takeda S, et al:
ED-71, a vitamin D analog, is a more potent inhibitor of bone
resorption than alfacalcidol in an estrogen-deficient rat model of
osteoporosis. Bone. 30:582–588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song R, Gu J, Liu X, Zhu J, Wang Q, Gao Q,
Zhang J, Cheng L, Tong X, Qi X, et al: Inhibition of osteoclast
bone resorption activity through osteoprotegerin-induced damage of
the sealing zone. Int J Mol Med. 34:856–862. 2014.PubMed/NCBI
|
|
7
|
Gu JH, Tong XS, Chen GH, et al: Regulation
of matrix metalloproteinase-9 protein expression by
1α,25-(OH)2D3 during osteoclast
differentiation. J Vet Sci. 15:133–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teitelbaum SL: Osteoclasts: What do they
do and how do they do it? Am J Pathol. 170:427–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinkmann J, Hefti T, Schlottig F, et al:
Response of osteoclasts to titanium surfaces with increasing
surface roughness: An in vitro study. Biointerphases.
7:342012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suda T, Takahashi F and Takahashi N: Bone
effects of vitamin D - Discrepancies between in vivo and
in vitro studies. Arch Biochem Biophys. 523:22–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takasu H, Sugita A, Uchiyama Y, et al:
c-Fos protein as a target of anti-osteoclastogenic action of
vitamin D and synthesis of new analogs. J Clin Invest. 116:528–535.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu K, Gläser R and Mrowietz U: Vitamin
D(3) and analogues modulate the expression of CSF-1 and its
receptor in human dendritic cells. Biochem Biophys Res Commun.
297:1211–1217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Medhora MM, Teitelbaum S, Chappel J, et
al: 1 alpha, 25-dihydroxyvitamin D3 up-regulates expression of the
osteoclast integrin alpha v beta 3. J Biol Chem. 268:1456–1461.
1993.PubMed/NCBI
|
|
14
|
Takahashi N, Yamana H, Yoshiki S, et al:
Osteoclast-like cell formation and its regulation by osteotropic
hormones in mouse bone marrow cultures. Endocrinology.
122:1373–1382. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeLuca HF: History of the discovery of
vitamin D and its active metabolites. Bonekey Rep. 3:4792014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arai F, Miyamoto T, Ohneda O, et al:
Commitment and differentiation of osteoclast precursor cells by the
sequential expression of c-Fms and receptor activator of nuclear
factor κB (RANK) receptors. J Exp Med. 190:1741–1754. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Price CP, Kirwan A and Vader C:
Tartrate-resistant acid phosphatase as a marker of bone resorption.
Clin Chem. 41:641–643. 1995.PubMed/NCBI
|
|
18
|
Kim TH, Lee B, Kwon E, et al:
1,25-Dihydroxyvitamin D3 inhibits directly human osteoclastogenesis
by down-regulation of the c-Fms and RANK expression. Joint Bone
Spine. 80:307–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Väänänen HK, Liu YK, Lehenkari P and
Uemara T: How do osteoclasts resorb bone? Mater Sci Eng C.
6:205–209. 1998. View Article : Google Scholar
|
|
20
|
Grüneberg S, Stubbs MT and Klebe G:
Successful virtual screening for novel inhibitors of human carbonic
anhydrase: Strategy and experimental confirmation. J Med Chem.
45:3588–3602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Väänänen K: Mechanism of osteoclast
mediated bone resorption - rationale for the design of new
therapeutics. Adv Drug Deliv Rev. 57:959–971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie L, Moroi Y, Hayashida S, et al:
Cathepsin K-upregulation in fibroblasts promotes matrigel invasive
ability of squamous cell carcinoma cells via tumor-derived IL-1α. J
Dermatol Sci. 61:45–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishibashi O, Niwa S, Kadoyama K and Inui
T: MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect
on osteoclastic bone resorption by suppressing cell migration. Life
Sci. 79:1657–1660. 2006. View Article : Google Scholar : PubMed/NCBI
|