Introduction
A-kinase anchor protein 95 (AKAP95) binds to the RII
subunit of protein kinase A (PKA) and catalyzes the phosphorylation
of target proteins; thus, ensuring the signal transduction process
of the cyclic adenosine monophosphate (cAMP) pathway (1). AKAP95 has been found to strongly
interact with all D-type cyclins and regulate cyclin
D3-cyclin-dependent kinase (CDK)4 activity in cotransfected Chinese
hamster ovary cells (2). Cyclin D/E
is able to form a cyclin D/E-AKAP95-PKA complex with the RII
subunit of PKA via the AKAP95 protein during the G1/S phase to
subsequently promote cell proliferation (3). Cyclin D3, a subtype of D-type cyclins,
has been reported to be overexpressed in non-Hodgkin lymphoma and
primary invasive breast cancer (4,5). AKT, a
proteohormone with multiple functions, is not only involved in cell
growth, metabolism and apoptosis, but is also associated with the
initiation and development of cancer. A previous study reported
that AKT activated CDK4 through regulating the expression of cyclin
D1, cyclin D2, p21 and p57 in the G1 phase, and subsequently
promoting pancreatic β-cell proliferation in mice (6). Therefore, it was hypothesized that
correlations may exist between the expression of AKAP95, cyclin D3
and AKT. In the present study, the correlations among these three
proteins and their association with pathology indicators was
investigated by analyzing the expression of these proteins in 51
lung cancer tissue samples.
Materials and methods
Sample collection
A total of 51 lung cancer tissue samples were
collected surgically from lung cancer patients, aged between 38 and
79 years (average age, 59±12 years), in Shengjing Hospital of China
Medical University (Shenyang, China) between 2007 and 2009. The
specimens were pathologically diagnosed by pathology experts, and
were grouped according to the following diagnostic results:
Differentiation grade, histological type and presence of lymph node
metastasis. Furthermore, 15 samples of pericarcinoma tissue, which
were located 3 cm away from the cancerous tissue and did not
contain any cancer cells (pathologically identified), were used as
a control. The present study was approved by the ethics committee
of Xiamen University (Xiamen, China), and written informed consent
was obtained from the patients or their families.
Reagents and immunohistochemistry
(IHC)
Specimens were cut into 4-µm sections, fixed with
10% neutral-buffered formalin and embedded in paraffin. A
streptavidin-peroxidase (SP) IHC method was applied. The SP IHC
kit, 3,3′-diaminobenzidine substrate and hematoxylin were obtained
from Maxim Biotechnology Co., Ltd (Fuzhou, China). Hematoxylin was
used to stain the tissues. Mouse anti-human primary monoclonal
antibodies against AKAP95 (1:150; cat. no. sc-100643) and cyclin D3
(1:300; cat. no. sc-6283) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Mouse monoclonal anti-AKT
antibody (1:150; cat. no. 2920S) was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Phosphate-buffered saline was
used as a negative control for the antibody.
Criteria for analysis of the IHC
results
When the slides were observed through a microscope
(BA310Digital; Motic China Group Co., Ltd, Xiamen, China), positive
protein expression was indicated by a yellow-brown color, which was
caused by the protein immunoreaction. By contrast, protein
expression was considered to be negative when no yellow-brown color
was observed. For each slide, 10 fields of view were randomly
selected and observed, and in each field of view, 200 cancer cells
were counted and the protein expression was observed. The
percentage of positive cells was calculated and defined as shown in
Table I. In the statistical
analysis, - and +- were considered to indicate negative protein
expression, while +, ++ and +++ were considered to indicate
positive protein expression (7).
 | Table I.Criteria for the analysis of
immunohistochemical results. |
Table I.
Criteria for the analysis of
immunohistochemical results.
| Range | Positivity |
|---|
| 0-<10% | – |
| ≥10%, <25% | +− |
| ≥25%, <50% | + |
| ≥50%, <75% | ++ |
| ≥75% | +++ |
Statistical analysis
Statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). The
constituent ratio of subjects was compared with the χ2 test, while
correlations were analyzed using Spearman's rank correlation
analysis. P<0.05 (α level) was considered to indicate a
statistically significant difference.
Results
Expression of AKAP95, cyclin D3 and
AKT in lung cancer and pericarcinoma tissues
Expression levels of AKAP95 in the lung cancer and
pericarcinoma tissues were reported as follows: The percentage of
AKAP95 positive samples was 82.35% in the cancer tissue and 33.33%
in the pericarcinoma tissue. The difference between them was
statistically significant (P<0.01), as shown in Table II.
 | Table II.AKAP95 expression in lung cancer and
pericarcinoma tissues. |
Table II.
AKAP95 expression in lung cancer and
pericarcinoma tissues.
| AKAP95 | Pericarcinoma | Lung cancer | χ2 | P-value |
|---|
| Positive | 5 | 42 | 13.59 | 0.001 |
| Negative | 10 | 9 |
|
|
Expression of cyclin D3 in lung cancer
and pericarcinoma tissues
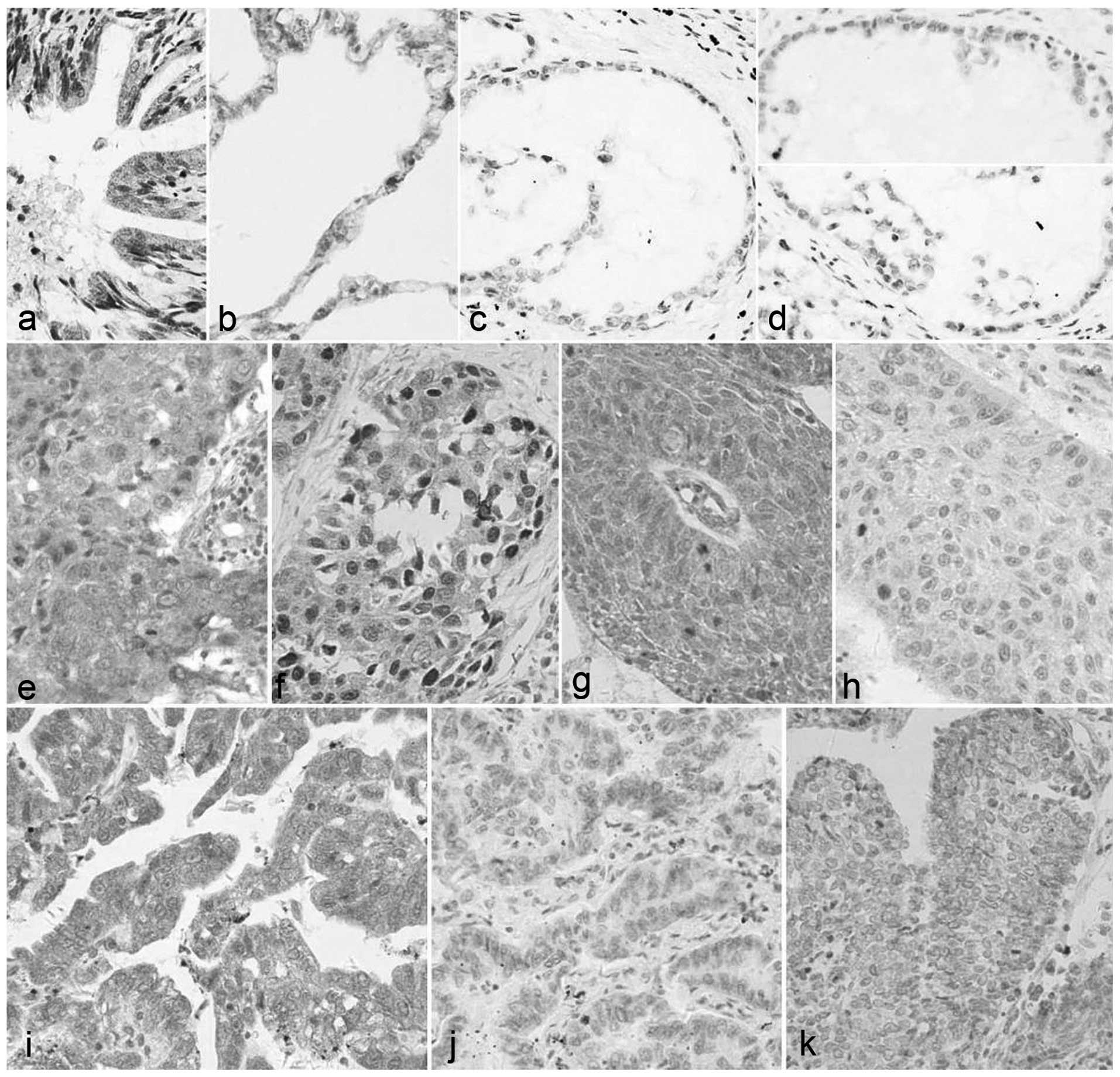
Positive expression of cyclin D3 was 68.63% (35/51)
in the cancer tissues and 28.57% (4/14) in the pericarcinoma
tissue. As shown in Table III, the
difference in the positive rate of cyclin D3 expression between the
cancer and pericarcinoma tissues was statistically significant
(P<0.01). The protein expression of cyclin D3 is shown in
Fig. 1.
 | Table III.Cyclin D3 expression in lung cancer
and pericarcinoma tissues. |
Table III.
Cyclin D3 expression in lung cancer
and pericarcinoma tissues.
| Cyclin D3 | Pericarcinoma | Lung cancer | χ2 | P-value |
|---|
| Positive | 4 | 35 | 7.344 | 0.007 |
| Negative | 10 | 16 |
|
|
Expression of AKT in lung cancer and
pericarcinoma tissues
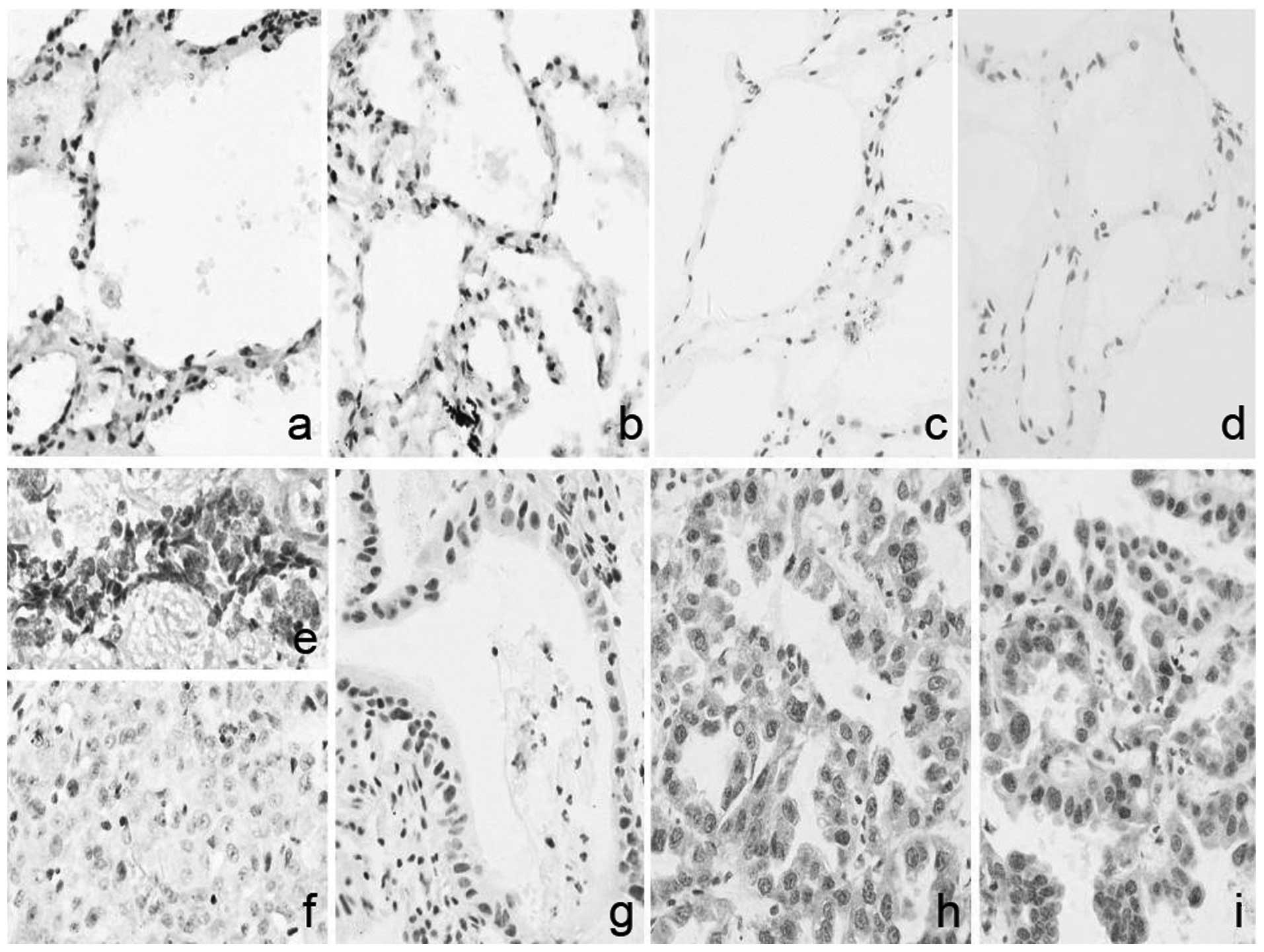
Positive expression of AKT, as shown in Fig. 2, in the cancer and pericarcinoma
tissues was 76.47% (39/51) and 38.46% (5/13), respectively. As
shown in Table IV, the difference
in the positive ratio of AKT expression between the cancer tissue
and pericarcinoma tissue was statistically significant
(P<0.05).
 | Table IV.AKT expression in lung cancer and
pericarcinoma tissues. |
Table IV.
AKT expression in lung cancer and
pericarcinoma tissues.
| AKT | Pericarcinoma | Lung cancer | χ2 | P-value |
|---|
| Positive | 5 | 39 | 5.309 | 0.021 |
| Negative | 8 | 12 |
|
|
Correlations between the expression of
AKAP95, cyclin D3 and AKT with pathology indicators in lung cancer
tissue
Correlations between the expression of AKAP95,
cyclin D3 and AKT with pathology indicators are shown in Table V. AKAP95 expression was shown to
correlate with the grade of cancer tissue differentiation and
histological type, but not with lymph node metastasis. No
statistically significant differences were observed in the cyclin
D3 expression levels in cancer tissues with different grades of
differentiation, tissues with different histological type or
tissues with or without lymph node metastasis (P>0.05),
indicating that cyclin D3 expression was not correlated with
differentiation grade, histological type or lymph node metastasis
(Table V). With regard to AKT
expression, a statistically significant difference was observed
between tissues with different grades of differentiation
(P<0.05); however, no statistically significant differences were
observed in between the tissues with a different histological type
or in tissues with or without lymph node metastasis (P>0.05),
which indicated that AKT expression was correlated with the grade
of differentiation, but not with the histological type or lymph
node metastasis.
 | Table V.Associations between the protein
expression of AKAP95, cyclin D3 and AKT with the pathology index in
lung cancer tissues. |
Table V.
Associations between the protein
expression of AKAP95, cyclin D3 and AKT with the pathology index in
lung cancer tissues.
| A, AKAP95 |
|
|
|
|
|
|---|
|
|---|
| Item | Cases (n) | Positive (n) | Negative (n) | χ2 | P-value |
|---|
| Grade of
differentiation |
|
|
|
| 0.029 |
|
Well-differentiated | 4 | 2 | 2 | 6.49 |
|
|
Moderately differentiated | 25 | 24 | 1 |
|
|
| Poorly
differentiated | 19 | 15 | 4 |
|
|
| Histological
type |
|
|
|
| 0.018 |
|
Small-cell lung cancer | 9 | 7 | 2 | 9.00 |
|
| Squamous
cell lung carcinoma | 22 | 20 | 2 |
|
|
| Lung
adenocarcinoma | 15 | 14 | 1 |
|
|
| Alveolar
carcinoma | 4 | 1 | 3 |
|
|
| Lymph node |
|
|
|
| 0.714 |
|
Positive | 29 | 23 | 6 | 0.43 |
|
|
Negative | 22 | 19 | 3 |
|
|
|
| B, Cyclin D3 |
|
| Item | Cases (n) | Positive (n) | Negative (n) | χ2 | P-value |
|
| Grade of
differentiation |
|
|
|
| 0.076 |
|
Well-differentiated | 4 | 3 | 1 | 5.208 |
|
|
Moderately differentiated | 25 | 22 | 3 |
|
|
| Poorly
differentiated | 19 | 11 | 8 |
|
|
| Histological
type |
|
|
|
| 0.063 |
|
Small-cell lung cancer | 9 | 3 | 6 | 6.715 |
|
|
Squamous cell lung
carcinoma | 22 | 18 | 4 |
|
|
| Lung
adenocarcinoma | 15 | 11 | 4 |
|
|
|
Alveolar carcinoma | 4 | 3 | 1 |
|
|
| Lymph node |
|
|
|
| 0.583 |
|
Positive | 29 | 19 | 10 | 0.302 |
|
|
Negative | 22 | 16 | 6 |
|
|
|
| C, AKT |
|
| Item | Cases (n) | Positive (n) | Negative (n) | χ2 | P-value |
|
| Grade of
differentiation |
|
|
|
| 0.046 |
|
Well-differentiated | 4 | 1 | 3 | 5.542 |
|
|
Moderately differentiated | 25 | 21 | 4 |
|
|
| Poorly
differentiated | 19 | 14 | 5 |
|
|
| Histological
type |
|
|
|
| 0.723 |
|
Small-cell lung cancer | 9 | 7 | 2 | 4.459 |
|
|
Squamous cell lung
carcinoma | 22 | 14 | 8 |
|
|
| Lung
adenocarcinoma | 15 | 14 | 1 |
|
|
|
Alveolar carcinoma | 4 | 3 | 1 |
|
|
| Lymph node |
|
|
|
| 0.906 |
|
Positive | 29 | 22 | 7 | 0.014 |
|
|
Negative | 22 | 17 | 5 |
|
|
Correlations among AKAP95, cyclin D3
and AKT expression levels in lung cancer tissue
As shown in Tables
VI and VII, AKAP95 expression
was found to significantly correlate with cyclin D3 and AKT
expression (P<0.05). However, no statistically significant
correlation was observed between cyclin D3 and AKT expression
(P>0.05; Table VIII).
 | Table VI.Correlation analysis between AKAP95
and cyclin D3 expression. |
Table VI.
Correlation analysis between AKAP95
and cyclin D3 expression.
|
| Cyclin D3 |
|
|
|---|
|
|
|
|
|
|---|
| AKAP95 | – | +− | + | ++ | +++ | rs | P-value |
|---|
| – | 1 | 1 | 1 | 0 | 0 |
|
|
| +− | 0 | 2 | 1 | 0 | 1 |
|
|
| + | 1 | 1 | 3 | 1 | 0 | 0.346 | 0.013 |
| ++ | 3 | 5 | 9 | 5 | 2 |
|
|
| +++ | 0 | 2 | 4 | 5 | 3 |
|
|
 | Table VII.Correlation analysis between AKAP95
and AKT expression. |
Table VII.
Correlation analysis between AKAP95
and AKT expression.
|
| AKT |
|
|
|---|
|
|
|
|
|
|---|
| AKAP95 | – | +− | + | ++ | +++ | rs | P-value |
|---|
| – | 2 | 0 | 1 | 0 | 0 |
|
|
| +− | 1 | 1 | 1 | 1 | 0 |
|
|
| + | 0 | 0 | 4 | 1 | 1 | 0.287 | 0.041 |
| ++ | 2 | 4 | 7 | 9 | 2 |
|
|
| +++ | 0 | 2 | 4 | 6 | 2 |
|
|
 | Table VIII.Correlation analysis between cyclin
D3 and AKT expression. |
Table VIII.
Correlation analysis between cyclin
D3 and AKT expression.
|
| AKT |
|
|
|---|
|
|
|
|
|
|---|
| Cyclin D3 | – | +− | + | ++ | +++ | rs | P-value |
|---|
| – | 1 | 1 | 1 | 2 | 0 |
|
|
| +− | 1 | 2 | 3 | 2 | 3 |
|
|
| + | 3 | 3 | 8 | 4 | 0 | 0.215 | 0.130 |
| ++ | 0 | 0 | 4 | 7 | 0 |
|
|
| +++ | 0 | 1 | 1 | 2 | 2 |
|
|
Discussion
With the rapid development of society and the
increasing rate of industrialization, individuals are exposed to
more chemical carcinogens. Investigating the molecular mechanisms
underlying lung cancer development and progression is vital. AKAP95
is a protein belonging to the AKAP family that is located in the
nucleus. As an anchoring protein to PKA, AKAP95 binds to PKA
through a PKA RII subunit, and is subsequently involved in target
protein phosphorylation by PKA and cAMP signal transduction
(1). AKAP95 also plays an important
role in chromosome condensation in mitosis, DNA replication and
cell apoptosis (8–11), and is able to regulate cyclin D3-CDK4
activity. Phosphoinositide 3-kinase (PI3K), an activator of AKT
protein (12), has a synergistic
effect with cyclin D3 in humans and mice, on promoting Burkitt
lymphoma (13). AKT phosphorylates
the Ser9 of glycogen synthase kinase (GSK)3β and further
upregulates cyclin D1 and cyclin D2 expression, but not cyclin D3
expression (6). Thus, the present
study investigated the expression of AKAP95, cyclin D3 and AKT and
the correlations among these three proteins.
Abnormal cell cycle regulation during the G1/S phase
is critical in tumor development. Type D cyclins contain three
members, namely cyclins D1, D2 and D3. Cyclins D1 and D2 have been
confirmed to be overexpressed in multiple types of tumor,
indicating their potential as oncogenes (14,15). Out
of 198 cases of non-Hodgkin lymphoma, 43 cases exhibited cyclin D3
overexpression (21.72%), while out of 170 cases of primary invasive
breast cancer 38 cases exhibited cyclin D3 overexpression (22.36%)
(4,5). A previous study reported that cyclin D3
expression was positive in 23/33 cases of primary lung cancer
(69.70%) (16). The current study
demonstrated that the positivity ratio of cyclin D3 expression in
lung cancer tissue was 68.63%, which was significantly higher
compared with that in pericarcinoma tissue (28.57%). In addition,
the positivity ratio of cyclin D3 expression in lung cancer was
higher compared with that in non-Hodgkin lymphoma and primary
invasive breast cancer, which may be associated with the
histological type of the tumor. Cyclin D3 expression in non-Hodgkin
lymphoma was found to correlate with the clinical groupings
(4). In the present study, cyclin D3
expression was not shown to correlate with the grade of cancer
tissue differentiation, histological type or lymph node metastasis,
while cyclin D1 expression has been previously correlated with
histological type, but not with the grade of cancer tissue
differentiation or lymph node metastasis (17). Therefore, cyclin D3 may play a
different role in different tumors, and the function of cyclin D1
and cyclin D3 may not be the same in the development of lung
cancer, which is consistent with the different roles of cyclin D1
and cyclin D3 observed in gastric cancer development (18).
Overexpression of phosphorylated AKT is an
independent prognostic factor of non-small-cell lung cancer
(19). The present study revealed
that the positivity ratio of AKT expression was 76.47% in lung
cancer tissue, which was significantly higher compared with that in
the pericarcinoma tissue (38.46%). AKT expression was also shown to
correlate with the grade of cancer tissue differentiation,
indicating the involvement of overexpression of AKT in lung cancer
development. In an additional study by Mayadagli et al (12), the positivity ratio of AKT expression
in squamous cell lung carcinoma was higher compared with that in
non-squamous non-small cell lung cancer. Furthermore, in the
current study, the positivity ratio of AKT expression in the
different histological types was 93.3% (14/15) in lung
adenocarcinoma, 77.8% (7/9) in small cell lung cancer, 75% (3/4) in
alveolar carcinoma and 63.64% (14/22) in squamous cell lung
carcinoma. Thus, there is inconsistency with the study by Mayadagli
et al, since the positivity ratio of AKT expression in squamous
cell lung carcinoma was the lowest.
In contrast to the previously reported correlation
with cyclin D1 expression in lung cancer (17), the present study demonstrated that
AKAP95 expression was also correlated with cyclin D3 expression and
AKT expression, indicating that AKAP95 may exert a synergistic
effect on promoting tumor growth with AKT and cyclin D3. Abnormal
activation of the oncogenic Ras protein has been shown to result in
a strong activation of the PI3K/AKT pathway, as PI3K can activate
AKT protein (12). This pathway is
activated in the majority of cancer cells and plays critical roles
in cell proliferation and apoptosis (20). Dominguez-Sola and Dalla-Favera
(13) reported that PI3K and cyclin
D3 exerted a synergistic effect on promoting Burkitt lymphoma,
which possibly explains the synergistic effect of AKT with cyclin
D3; however, AKT expression exhibited no correlation with cyclin D3
expression in the current study. AKT may upregulate cyclin D1 and
cyclin D2 expression through phosphorylating the Ser9 of GSK3β;
however, is unable to upregulate cyclin D3 expression, which was
consistent with the study by Fatrai et al (6).
In conclusion, the present study demonstrated that
AKAP95 correlated with the expression of cyclin D3 and AKT in lung
cancer tissues. In addition, AKT expression exhibited a strong
correlation with the grade of cancer tissue differentiation;
however, no correlation was observed with cyclin D3. The mechanism
underlying the regulation of cyclin D proteins by AKT warrants
further research. The present study identified a correlation
between AKAP95, and both cyclin D3 and AKT. These results suggest
that AKAP95 has an effect on the cell cycle through two different
pathways.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation (no. 81071927), the Fujian Innovation
Project (no. 2012-CXB-25) and the Xiamen University Innovation
Program (no. CXB2013024).
References
|
1
|
Diviani D, Dodge-Kafka KL, Li J and
Kapiloff MS: A-kinase anchoring proteins: scaffolding proteins in
the heart. Am J Physiol Heart Circ Physiol. 301:H1742–H1753. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arsenijevic T, Degraef C, Dumont JE, Roger
PP and Pirson I: A novel partner for D-type cyclins: protein kinase
A-anchoring protein AKAP95. Biochem J. 378:673–679. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arsenijevic T, Degraef C, Dumont JE, Roger
PP and Pirson I: G1/S Cyclins interact with regulatory subunit of
PKA via A-kinase anchoring protein, AKAP95. Cell Cycle.
5:1217–1222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Møller MB, Nielsen O and Pedersen NT:
Cyclin D3 expression in non-Hodgkin lymphoma. Correlation with
other cell cycle regulators and clinical features. Am J Clin
Pathol. 115:404–412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bukholm IR, Bukholm G and Nesland JM:
Over-expression of cyclin A is highly associated with early relapse
and reduced survival in patients with primary breast carcinomas.
Int J Cancer. 93:283–287. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatrai S, Elghazi L, Balcazar N, et al:
Akt induces beta-cell proliferation by regulating cyclin D1, cyclin
D2 and p21 levels and cyclin-dependent kinase-4 activity. Diabetes.
55:318–325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiscott PS, Grierson I and McLeod D:
Retinal pigment epithelial cells in epiretinal membranes: an
immunohistochemical study. Br J Ophthalmol. 68:708–715. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collas P, Le Guellec K and Taskén K: The
A-kinase-anchoring protein AKAP95 is a multivalent protein with a
key role in chromatin condensation at mitosis. J Cell Biol.
147:1167–1180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steen RL, Cubizolles F, Le Guellec K and
Collas P: A kinase-anchoring protein (AKAP)95 recruits human
chromosome-associated protein (hCAP)-D2/Eg7 for chromosome
condensation in mitotic extract. J Cell Biol. 149:531–536. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eide T, Carlson C, Taskén KA, Hirano T,
Taskén K and Collas P: Distinct but overlapping domains of AKAP95
are implicated in chromosome condensation and condensin targeting.
EMBO Rep. 3:426–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamada S, Kikkawa U, Tsujimoto Y and
Hunter T: A-kinase-anchoring protein 95 functions as a potential
carrier for the nuclear translocation of active caspase 3 through
an enzyme-substrate-like association. Mol Cell Biol. 25:9469–9477.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mayadagli A, Karabulut Gul S, Bilici A, et
al: Prognostic significance of protein kinase B/Akt pathway in
patients with non-small cell lung cancer. J BUON. 19:157–163.
2014.PubMed/NCBI
|
|
13
|
Dominguez-Sola D and Dalla-Favera R:
Burkitt lymphoma: much more than MYC. Cancer Cell. 22:141–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flørenes VA, Faye RS, Maelandsmo GM,
Nesland JM and Holm R: Levels of cyclin D1 and D3 in malignant
melanoma: deregulated cyclin D3 expression is associated with poor
clinical outcome in superficial melanoma. Clin Cancer Res.
6:3614–3620. 2000.PubMed/NCBI
|
|
15
|
Wolowiec D, Ciszak L, Kosmaczewska A, et
al: Cell cycle regulatory proteins and apoptosis in B-cell chronic
lymphocytic leukemia. Haematologica. 86:1296–1304. 2001.PubMed/NCBI
|
|
16
|
Papay J, Krenacs T, Moldvay J, et al:
Immunophenotypic profiling of nonsmall cell lung cancer progression
using the tissue microarray approach. Appl Immunohistochem Mol
Morphol. 15:19–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu SX, Kong XY, Yuan YY, et al:
Relationship between AKAP95, cyclin E1, cyclin D1 and
clinicopathological parameters in lung cancer tissue. Zhonghua Lao
Dong Wei Sheng Zhi Ye Bing Za Zhi. 31:890–894. 2013.PubMed/NCBI
|
|
18
|
Yu J, Miehlke S, Ebert MP, et al:
Expression of cyclin genes in human gastric cancer and in first
degree relatives. Chin Med J (Engl). 115:710–715. 2002.PubMed/NCBI
|
|
19
|
David O, Jett J, LeBeau H, et al:
Phospho-Akt overexpression in non-small cell lung cancer confers
significant stage-independent survival disadvantage. Clin Cancer
Res. 10:6865–6871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|