Introduction
The occurrence of surgical site infection (SSI)
extends the duration of hospitalization, raising the costs of
admission and potentially reducing the quality of life of the
patients (1). Since the publication
of the Guideline for the Prevention of Surgical Site Infection in
1999 by the Center for Disease Control and Prevention (2), there has been a declining trend in SSI.
Takesue et al (3) reported,
based on the results of a multi-center research project, that the
implementation of effective infection prevention practices can
maintain SSI incidence rates to <15%.
Probiotics that improve the intestinal microbial
balance in the host are considered to have beneficial effects on
human health (4). By comparing the
intestinal environment in patients with colorectal cancer (CRC) and
healthy individuals, Wang et al (5) found that there is an intestinal
microbial imbalance in patients with CRC, represented by a
reduction in the number of butyrate producers and an increase in
opportunistic pathogens. Disturbance of the intestinal microbiota
appears to be an important factor inducing perioperative SSI
(6). This disturbance is caused by
the stress of invasive surgery, the administration of antibacterial
drugs to prevent infection, the weakness of intestinal tract
peristalsis and the atrophy of the intestinal mucosa due to the
perioperative fasting and intestinal tract ischemia (6).
We hypothesized that the perioperative
administration of probiotics should reduce the incidence of SSIs
among the patients undergoing elective CRC surgery. In addition,
the study was designed to investigate the effect of the
perioperative administration of probiotics on the immune response,
intestinal microbiota and surgical outcome in the clinical
setting.
Materials and methods
Patient enrolment
The present study focused on 156 consecutive
surgeries carried out by the same team from among all the elective
CRC surgeries performed at Fukuoka University Hospital (Fukuoka,
Japan) between April 2009 and March 2013, following the exclusion
of inoperable patients and the provision of informed consent from
the patients. The patients involved in surgeries conducted between
April 2009 and October 2011 were placed in the non-probiotic group
(group A, 81 patients) and those involved in surgeries between
November 2011 and March 2013 were placed in the probiotic group
(group B, 75 patients). This study was approved by the Human
Research Review Committee of Fukuoka University Hospital
(12-3-08).
Treatment
All surgeries were performed by the same team, which
included three surgeons, and perioperative management was performed
under the same conditions for all patients (other than the
probiotic treatment). For the probiotic treatment, six tablets of
BIO-THREE® (Toa Pharmaceutical Co., Ltd., Tokyo, Japan) were
administered orally daily. Each BIO-THREE tablet contained 2 mg
Enterococcus faecalis T110, 10 mg Clostridium
butyricum TO-A and 10 mg Bacillus mesentericus TO-A. All
patients received a regular diet preoperatively. The administration
of the BIO-THREE (six tablets/day) was started three to 15 days
prior to the surgery, and then was restarted the same day the
patient started drinking water. All the patients underwent the same
intestinal preparation with magnesium citrate (Magcorol P®; Horii
Pharmaceutical Co., Ltd., Tokyo, Japan) without per oral
administration of antibiotics. Antibiotic prophylaxis was initiated
with the administration of 1 g cefmetazole sodium 30 min prior to
the surgery, with additional administration every 3 h during the
surgery. The intravenous administration of antibiotics continued
twice per day until the second postoperative day (POD). Fecal and
blood sampling was performed prior to surgery and at 1 week
following surgery. The fecal samples were suspended in 4 M
guanidinium thiocyanate, 100 mM Tris-HCl (pH 9.0) and 40 mM EDTA
following washing three times with sterile distilled water. The
mixture was then beaten with glass beads using a mini bead beater
(BioSpec Products Inc., Bartlesville, OK, USA).
Fecal terminal-restriction fragment
length polymorphism assay
Twenty-four patients (n=10, group A; n=14, group B)
were included in the evaluation. Amplification of the 16S rDNA, the
digestion of restriction enzymes, size fractionation of T-RFs and
analysis of TRFLP data were performed according to the protocol
described by Nagashima et al (7). Briefly, polymerase chain reaction (PCR)
was performed using the total fecal DNA (10 ng/μL) and primers of
516f (5′-TGCCAGCAGCCGCGGTA-3′; Escherichia coli positions,
516–532) and 1510r (5′-GGTTACCTTGTTACGACTT-3′; E. coli
positions, 1510-1492). The 5′-ends of the forward primers were
labeled with 6′-carboxyfluorescein, which was synthesized by
Applied Biosystems (Tokyo, Japan). The amplified 16S rDNA genes
were purified using a GFX PCR DNA and Gel Band Purification kit (GE
Healthcare Bio-Sciences, Tokyo, Japan) and redissolved in 30 µl
distilled water. The purified PCR products (2 µl) were digested
with 10 units of BslI at 55°C for 3 h. The length of the T-RF was
determined using an ABI PRISM 3130×1 genetic analyzer (Applied
Biosystems) in GeneScan mode. Standard size markers were used
(MapMarker X-Rhodamine Labeled; 50–1,000 bp; BioVentures, TN, USA).
The fragment sizes were estimated using the local southern method
of the GeneMapper software (Applied Biosystems). The T-RFs were
divided into 30 operational taxonomic units (OTUs), according to
the protocol described by Nagashima et al (7) The OTUs were quantified as the
percentage of values of an individual OTU per total OTU area,
expressed as the peak percentage area under the curve (%AUC). The
cluster analyses were performed using GeneMaths software (Applied
Maths, Belgium), based on the BslI T-RFLP patterns. Pearson
similarity coefficient analysis and the unweighted pair-group
method with arithmetic means were used to establish the type of
dendrogram.
Cylex® ImmuKnow® assay
Peripheral venous blood samples were taken
preoperatively and on the first, fourth and eight PODs. Samples
from 20 patients (n=10, group A; n=10, group B) were evaluated by
the Cylex ImmuKnow assay (Cylex, Columbia, MD, USA). Whole blood
samples, which were subjected to sodium heparin anticoagulation
treatment, were collected at Fukuoka University Hospital and tested
in the laboratory. The immune response was measured using the Food
and Drug Administration (FDA)-approved Cylex ImmuKnow assay in
accordance with the manufacturer's instructions (Cyclex) (8). Cluster of differentiation
(CD4)-(ImmuKnow) T cells were positively selected within the
microwells using magnetic particles coated with anti-human CD4
monoclonal antibodies and a strong magnet. The release of adenosine
triphosphate (ATP) was measured using luciferin/luciferase and a
luminometer (Berthold Technologies, LLC, Knoxville, TN, USA). The
level of immune response was assessed based on the amount of ATP,
expressed in ng/ml.
Recording of infectious
complications
Detailed daily records of the postoperative course
were kept for each patient. The infectious complications included
SSI (superficial incisional, deep incisional and space/organ),
postoperative pneumonia, urinary tract infections and enteritis.
These were recorded for up to 30 days after surgery. An SSI was
defined as spontaneous or surgically released purulent discharge
with positive culture results.
Statistical analysis
The statistical analysis was performed using the
χ2 and t-tests to compare the two groups, and a logistic
regression analysis for the multivariable analysis. Significant
differences were concluded from results using a value of P<0.05
in all cases. The statistical analysis software program used was
SPSS for Windows (version 11.5; SPSS, Tokyo, Japan).
Results
Demographic characteristics of study
participants
A total of 156 patients were assigned to one of the
two treatment arms. The demographic characteristics of the study
patients, site of the tumor, use of open/laparoscopic surgery,
creation of an ostomy, cancer stage and intraoperative
characteristics are shown in Table
I. A significant difference was noted in the preoperative body
mass index (BMI) and whether or not the surgical procedure was
performed by an open or laparoscopic method. With regard to the
intraoperative characteristics, no significant difference was
noted. With regard to the postoperative course, the length of time
prior to the passage of gas and meal intake in group B was
significantly shorter than that in group A (Table II).
 | Table I.Baseline characteristics of the
patients with colorectal cancer (n=156). |
Table I.
Baseline characteristics of the
patients with colorectal cancer (n=156).
| Characteristic | Group A | Group B | P-value |
|---|
| Agea, years | 69.1±11.3 | 68.0±13.8 | 0.58 |
| Gender, M/F | 44/37 | 47/28 | 0.34 |
| BMIb, kg/cm2 | 23.3±3.8 | 21.7±2.7 | 0.0034 |
| PNIa | 48.8±7.5 | 48.0±7.4 | 0.47 |
| Diabetes mellitus, n
(%) | 14 (17.3) | 12 (16.0) | 0.83 |
| Smoking, n (%) | 18 (22.2) | 14 (18.7) | 0.73 |
| Chronic renal
failure, n (%) | 9 (11.1) | 5 (6.7) | 0.33 |
| ASA score, n |
|
| 0.08 |
| 1 | 29 | 38 |
|
| 2 | 46 | 29 |
|
| 3 | 6 | 8 |
|
| Immunosuppression, n
(%) | 2 (2.6) | 2 (2.7) | 0.94 |
| Open/laparoscopic,
n | 52/29 | 35/40 | 0.041 |
| Site of tumor, n |
|
| 0.86 |
| C | 3 | 5 |
|
| A | 8 | 14 |
|
| T | 6 | 5 |
|
| D | 3 | 0 |
|
| S | 25 | 16 |
|
| R | 22 | 8 |
|
| Creation of an
ostomy, n (%) | 16 (19.6) | 14 (18.7) | 0.86 |
| Cancer stage,
n |
|
| 0.34 |
| I | 29 | 31 |
|
| II | 32 | 16 |
|
|
IIIA | 11 | 10 |
|
|
IIIB | 3 | 3 |
|
| IV | 6 | 8 |
|
| Surgery
timec, min | 209 (100–630) | 240 (115–555) | 0.09 |
| Blood
lossc, ml | 120 (0–2060) | 100 (0–1190) | 0.08 |
| Intraoperative
hypotension, n (%) | 19 (23.5) | 25 (33.3) | 0.26 |
| Intraoperative
hypothermia, n (%) | 2 (2.5) | 2 (2.7) | 0.62 |
 | Table II.Postoperative characteristics. |
Table II.
Postoperative characteristics.
|
| Group A, n=81 | Group B, n=75 | P-value |
|---|
| Time of flatus
(days) | 2.8±2.0 | 2.0±1.1 | 0.001 |
| Time of meal intake
(days) | 4.7±1.8 | 3.9±1.5 | 0.002 |
An SSI was observed in 27 (17.3%) of the 156
patients. A breakdown of the infectious complications showed that
21 (13.4%) patients had a superficial incisional SSI, 16 of who
were in group A (19.8%) and five of who were in group B (6.7%)
(P=0.016). All superficial incisional SSIs were below grade II in
the Clavien-Dindo classification. Among the six (3.8%) patients
with deep and organ/space SSI, four were in group A (4.9%) and two
were in group B (2.7%) (Table
III). In group A, two of the patients were grade II and two
were grade IIIB in the Clavien-Dindo classification. In group B,
the two patients were grade IIIB. Pneumonia, urinary tract
infection and enteritis were below grade II.
 | Table III.Infectious complications. |
Table III.
Infectious complications.
| Complications | n | Group A, n (%) | Group B, n (%) | P-value |
|---|
| Surgical site
infection | 27 |
|
|
|
|
Superficial incisional | 21 | 16
(19.7) | 5
(6.7) |
0.016 |
| Deep,
organ/space | 6 | 4
(4.9) | 2
(2.7) | 0.40 |
| Pneumonia | 1 | 0 (0) | 1
(1.3) | – |
| Urinary tract
infection | 1 | 0 (0) | 1
(1.3) | – |
| Enteritis | 8 | 3
(3.7) | 5
(6.7) | 0.40 |
| Total | 37 | 23
(28.3) | 14
(18.7) | 0.15 |
The rate of SSI incidence by age, gender, BMI,
prognostic nutrition index (9),
history of diabetes mellitus, smoking, chronic renal failure,
American Society of Anesthesiologists (ASA) score, state of
immunosuppression, open/laparoscopic procedure, length of the
surgery, volume of blood loss, intraoperative hypotension,
hypothermia and oral administration of probiotics was investigated
in the univariate analysis. It was found that the patients with SSI
had a higher frequency of undergoing an ostomy creation and more
blood loss. Probiotic administration was higher in the group of
patients without SSI (68, vs. 7; Table
IV. In the multivariate analysis, only the oral administration
of probiotics was identified as an independent risk factors for SSI
(Table V).
 | Table IV.SSI (univariate analysis). |
Table IV.
SSI (univariate analysis).
|
Characteristics | No superficial
incisional SSI | Superficial
incisional SSI | P-value |
|---|
| Agea, years | 67.9±12.8 | 71.7±11.0 | 0.149 |
| Gender, M/F | 75/60 | 18/9 | 0.164 |
| BMIb, kg/cm2 | 22.5±3.1 | 22.8±4.5 | 0.701 |
| PNIa | 48.7±7.6 | 47.1±6.7 | 0.297 |
| Diabetes mellitus,
n | 21 | 5 | 0.777 |
| Smoking, n | 28 | 4 | 0.422 |
| Chronic renal
failure, n | 8 | 4 | 0.244 |
| ASA score, n |
|
| 0.147 |
| 1 | 67 | 8 |
|
| 2 | 75 | 16 |
|
| 3 | 14 | 3 |
|
| Immunosuppression,
n | 3 | 1 | 0.681 |
| Site of tumor,
n |
|
| 0.095 |
| C | 8 | 3 |
|
| A | 28 | 4 |
|
| T | 15 | 1 |
|
| D | 49 | 9 |
|
| S | 35 | 10 |
|
| R |
|
|
|
| Creation of an
ostomy, n | 18 | 12 | 0.030 |
| Open/laparoscopic,
n | 68/61 | 19/8 | 0.094 |
| Surgery
timea, min | 247.9±103.9 | 265.3±93.9 | 0.425 |
| Estimated blood
lossa, ml | 157.8±233.6 | 437.5±423.7 | 0.001 |
| Intraoperative
hypotension, n | 2 | 2 | 0.396 |
| Intraoperative
hypothermia, n | 44 | 4 | 0.179 |
| Probiotics, n | 68 | 7 | 0.003 |
 | Table V.SSI (multivariate analysis). |
Table V.
SSI (multivariate analysis).
| Factor | SSI/total (n) | SSI rate (%) | Risk ratio | 95% CI | P-value |
|---|
| Creation of an
ostomy |
|
|
| 0.087–1.200 | 0.090 |
| + | 12/30 | 40.0 | 1.00 |
| − | 15/126 | 11.9 | 0.32 |
|
|
| Estimated blood
loss |
|
| 1.00 | 1.000–1.004 | 0.140 |
| Probiotics |
|
|
| 1.340–12.200 | 0.013 |
| + | 7/75 | 9.3 | 1.00 |
| − | 20/81 | 24.7 | 4.10 |
|
|
ImmuKnow ATP assay
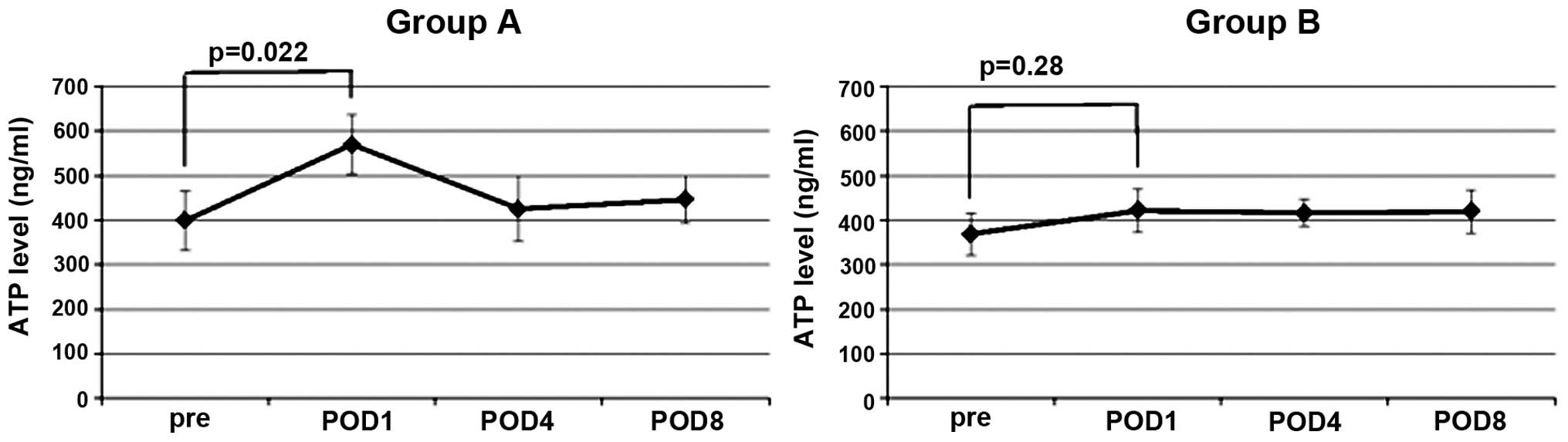
In the two groups of patients with CRC, the ImmuKnow
ATP values were observed to peak on the first POD. In group A, the
ImmuKnow value of the first POD was increased significantly
compared with the preoperative value (P=0.022). By contrast, the
ImmuKnow value on the first POD in group B was not significantly
higher than the preoperative value (P=0.28) (Fig. 1). On the fourth and eighth PODs, the
ImmuKnow ATP values were observed to exhibit a decreasing trend,
but no significant differences were noted in the two groups.
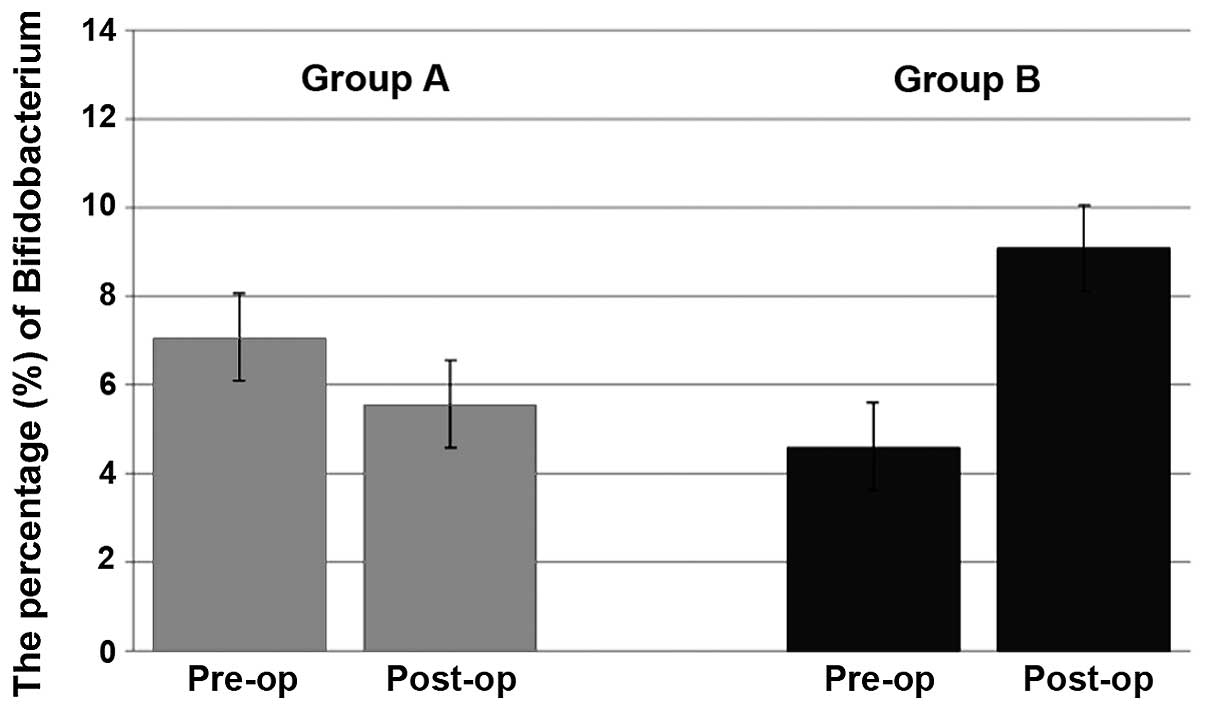
Changes in fecal microbiota
The changes in the fecal microbiota resulting from
the surgery and subsequent to probiotic administration were next
investigated. Almost no changes in the number of beneficial
bacteria, such as Bifidobacterium, were observed in group A;
however, the mean proportion of Bifidobacterium increased in
group B between 4.6 and 9.1% (Fig.
2).
Discussion
The development of various perioperative management
techniques has contributed to a decrease in the incidence of SSIs;
however, the rate of superficial incisional SSI incidence in
elective CRC surgery remains between 2.5 and 20.5% (10). In the present study, the rate of SSI
was 17.3%.
Previous studies have reported that incisional SSIs
are caused by the imbalance of infectious bacteria, surgical
technique and the patient's condition (3,4,6). The factors associated with infectious
bacteria are the use of preoperative, non-absorbable, oral
antibiotics and prophylactic antibiotic use (11). The factors associated with the
surgical technique are the preoperative skin preparation, the
length of the surgery, the use of open versus laparoscopic surgery,
the creation or closure of an ostomy, the suture material used for
fascial closure and the type of skin closure (12). It has been reported that the relevant
factors associated with the patient's condition are the gender,
BMI, ASA score, immunosuppression, smoking, wound classification,
requirement for a blood transfusion, subcutaneous fat thickness and
postoperative hyperglycemia (13).
It has recently been reported that perioperative
probiotic and synbiotic treatment can reduce infectious
complications, such as incisional SSI, in esophageal cancer,
biliary cancer and CRC surgery (14–16);
however, the evidence in those reports was relatively weak, and
neither perioperative probiotic treatment nor synbiotic treatment
were found to be independently associated as a risk factor of
incisional SSI.
It has been demonstrated that probiotics can improve
the intestinal microbial environment and activate host immune
function, leading to the prevention of infectious complications
(17–19). In the present study, BIO-THREE
tablets were selected as the probiotics, as one BIO-THREE tablet
contains 2 mg Enterococcus faecalis T110, 10 mg
Clostridium butyricum TO-A and 10 mg Bacillus
mesentericus TO-A, all of which are well-documented beneficial
bacteria, and these tablets can be effectively absorbed to increase
the ratio of beneficial bacteria in the body (20). The intestinal microbiota plays an
important part in the host immune system (21). The intestinal microbiota includes
beneficial, opportunistic and harmful bacteria. Beneficial bacteria
in the intestinal microbiota protect the intestinal tract against
the invasion of harmful bacteria, while harmful bacteria exploit
decreases in the host resistance to exhibit pathogenicity (22). In the present study, the ratio of
beneficial bacteria tended to be increased with the perioperative
administration of probiotics. Beneficial bacteria increase the
concentration of short-chain fatty acids (SCFAs), such as acetic,
propionic and butyric acids. These SCFAs have numerous important
roles in the colon, including maintaining the acidity of the
intestinal environment, stimulating the proliferation of epithelial
cells and intestinal motility, enhancing the secretion of mucin by
the epithelium and acting as metabolites for energy metabolism in
epithelial cells (23). In the
present study, intestinal peristalsis was improved earlier in group
B than group A.
The clinical validity of the Immune Cell Function
Assay (Cylex ImmuKnow assay) as an objective tool for assessing
immune function has been validated previously, when the assay was
used to tailor immunosuppression to optimize the treatment of
rejection and infections, as well as in immunosuppression weaning
protocols (24). Prior to its
introduction, there had been no available tool for the direct
assessment of the immune function of a patient. The FDA approved
the use of the Immune Cell Function Assay as a means of quantifying
cell-mediated immunity by measuring the concentration of ATP from
CD4+ T cells in 2002. The Cylex ImmuKnow assay has since
been shown to be clinically useful in the assessment of the
relative risk of infection and rejection (24). To the best of our knowledge, this is
the first report that has evaluated the perioperative immune
function of patients undergoing CRC surgery with the Cylex ImmuKnow
assay.
In this study, the extent of systemic inflammation
was evaluated using the Cylex ImmuKnow assay. The activation of the
cytokine cascade can predict infectious conditions. The ImmuKnow
assay is an acute-phase reactant that increases in response to
cytokine stimulation, thereby serving as a marker of the magnitude
of inflammation (25). During the
perioperative treatment of the patients in the present study with
probiotics, the inflammation was estimated using the Cylex ImmuKnow
assay preoperatively and on the first, fourth and eighth PODs. In
group A, the ImmuKnow value of the first POD was increased
significantly compared with the preoperative value. By contrast,
the value of the first POD in group B did not increase
significantly. The administration of probiotics may have reduced
the inflammation associated with the surgical stress following the
CRC surgery. Perioperative administration of probiotics can
reinforce the immune function of the host and increase the
resistance to infections. As a result, it could reduce the
incidence of superficial incisional SSI (26). Furthermore, since the perioperative
administration of probiotics reduced the postoperative inflammatory
response, it could lead to a decrease in the length of the hospital
stay. The present results suggest that systemic inflammatory
responses can be favorably modified by probiotics. The microbial
imbalance induced by CRC and surgical stress may be rapidly
improved by perioperative probiotic treatment. Increasing the ratio
of beneficial bacteria aids in the maintenance of the host defense,
and colonic SCFAs may have beneficial effects on the epithelial
cell integrity and participate in the local defense of the
colon.
In the present study, the administration of
probiotics induced a decrease in superficial incisional SSI
incidence and an increase in CD4+ ATP activity.
Probiotic administration therefore appears to result in the
perioperative enhancement of the host immune function. In
conclusion, consecutive preoperative and postoperative probiotic
treatment could reduce the incidence of superficial incisional SSI,
and could increase the ratio of beneficial bacterial in the feces.
The ImmuKnow assay indicated that the oral administration of
probiotics induced ATP activity in the CD4+ lymphocytes.
This reduction in the incidence of superficial incisional SSI may
involves the enhancement of immune function through the activity of
ATP in CD4+ cells by probiotics. The oral administration
of probiotics as a food supplement is simple and safe. We therefore
recommend the perioperative use of probiotics in all patients
undergoing surgical treatment.
Acknowledgements
The study was partially supported by Toa
Pharmaceutical Co., Ltd. (Tokyo, Japan).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
SSIs
|
surgical site infections
|
|
T-RFLP
|
terminal-restriction fragment length
polymorphism
|
|
BMI
|
body mass index
|
|
POD
|
postoperative day
|
|
SCFA
|
short-chain fatty acid
|
References
|
1
|
Kusachi S, Kashimura N, Konishi T, et al:
Length of stay and cost for surgical site infection after abdominal
and cardiac surgery in Japanese hospitals: multi-center
surveillance. Surg Infect (Larchmt). 13:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mangram AJ, Horan TC, Pearson ML, et al:
Guideline for prevention of surgical site infection, 1999. Hospital
Infection Control Practices Advisory Committee. Infect Control Hosp
Epidemiol. 20:250–280. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takesue Y, Kusunoki M, Kobayashi M, et al:
The prevalence of surgical site infection (SSI) by the introduction
of the best practice in the perioperative management and operative
manipulation; multi-center prospective study of 1601 colorectal
patients. Nihon Geka Kansenshō Gakkai Zasshi. 3:471–476. 2006.
|
|
4
|
Rafter J: The effects of probiotics on
colon cancer development. Nutr Res Rev. 17:277–284. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang T, Cai G, Qiu Y, et al: Structural
segregation of gut microbiota between colorectal cancer patients
and healthy volunteers. ISME J. 6:320–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eguchi S, Takatsuki M, Hidaka M, et al:
Perioperative synbiotic treatment to prevent infectious
complications in patients after elective living donor liver
transplantation: a prospective randomized study. Am J Surg.
201:498–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagashima K, Hisada T, Sato M and
Mochizuki J: Application of new primer-enzyme combinations to
terminal restriction fragment length polymorphism profiling of
bacterial populations in human feces. Appl Environ Microbiol.
69:1251–1262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kowalski R, Post D, Schneider MC, et al:
Immune cell function testing: an adjunct to therapeutic drug
monitoring in transplant patient management. Clin Transplant.
17:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.PubMed/NCBI
|
|
10
|
Nakamura T, Kashimura N, Noji T, et al:
Triclosan-coated sutures reduce the incidence of wound infections
and the costs after colorectal surgery: a randomized controlled
trial. Surgery. 153:576–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itani KM, Wilson SE, Awad SS, et al:
Ertapenem versus cefotetan prophylaxis in elective colorectal
surgery. N Engl J Med. 355:2640–2651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serra-Aracil X, García-Domingo MI, Parés
D, et al: Surgical site infection in elective operations for
colorectal cancer after the application of preventive measures.
Arch Surg. 146:606–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Young H, Knepper B, Moore EE, et al:
Surgical site infection after colon surgery: National Healthcare
Safety Network risk factors and modeled rates compared with
published risk factors and rates. J Am Coll Surg. 214:852–859.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka K, Yano M, Motoori M, et al: Impact
of perioperative administration of synbiotics in patients with
esophageal cancer undergoing esophagectomy: a prospective
randomized controlled trial. Surgery. 152:832–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugawara G, Nagino M, Nishio H, et al:
Perioperative synbiotic treatment to prevent postoperative
infectious complications in biliary cancer surgery: a randomized
controlled trial. Ann Surg. 244:706–714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JW, Du P, Gao J, et al: Preoperative
probiotics decrease postoperative infectious complications of
colorectal cancer. Am J Med Sci. 343:199–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanazawa H, Nagino M, Kamiya S, et al:
Synbiotics reduce postoperative infectious complications: a
randomized controlled trial in biliary cancer patients undergoing
hepatectomy. Langenbecks Arch Surg. 390:104–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheih YH, Chiang BL, Wang LH, et al:
Systemic immunity-enhancing effects in healthy subjects following
dietary consumption of the lactic acid bacterium Lactobacillus
rhamnosus HN001. J Am Coll Nutr. 20(2 Suppl): 149–156. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzaki T and Chin J: Modulating immune
responses with probiotic bacteria. Immunol Cell Biol. 78:67–73.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang YF, Liu PY, Chen YY, et al:
Three-combination probiotics therapy in children with salmonella
and rotavirus gastroenteritis. J Clin Gastroenterol. 48:37–42.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartosch S, Woodmansey EJ, Paterson JC, et
al: Microbiological effects of consuming a synbiotic containing
Bifidobacterium bifidumBifidobacterium lactis, and
oligofructose in elderly persons, determined by real-time
polymerase chain reaction and counting of viable bacteria. Clin
Infect Dis. 40:28–37. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitsuoka T: Significance of dietary
modulation of intestinal flora and intestinal environment. Biosci
Microflora. 19:15–25. 2000. View Article : Google Scholar
|
|
23
|
Willemsen LE, Koetsier MA, van Deventer SJ
and van Tol EA: Short chain fatty acids stimulate epithelial mucin
2 expression through differential effects on prostaglandin E(1) and
E(2) production by intestinal myofibroblasts. Gut. 52:1442–1447.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kowalski RJ, Post DR, Mannon RB, et al:
Assessing relative risks of infection and rejection: a
meta-analysis using an immune function assay. Transplantation.
82:663–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheeran P and Hall GM: Cytokines in
anaesthesia. Brit J Anaesth. 78:201–219. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duc le H, Hong HA, Barbosa TM, et al:
Characterization of Bacillus probiotics available for human use.
Appl Environ Microbiol. 70:2161–2171. 2004. View Article : Google Scholar : PubMed/NCBI
|