Introduction
Myocardial hypertrophy, which is one of the most
important risk factors for myocardial infarction and
cardiac-related mortality, is a chronic condition mainly induced by
long-term pressure overload (1). It
has been widely accepted that cGMP-dependent protein kinase type I
(PKG 1) is closely associated with the physiological function of
myocardial cells. PKG 1 is an intracellular receptor that plays an
important role in the activation of the BKCa channel, protein
phosphorylation and the regulation of vascular smooth muscle cell
gene expression. PKG 1 has also been found to inhibit the signaling
pathways involved in myocardial hypertrophy (2).
MicroRNAs (miRs) are a class of non-coding 18–22
nucleotide RNA molecules, which have important roles as gene
regulators in eukaryotic organisms (3). miRs bind to the 3′-untranslated region
of target mRNAs and inhibit the initiation of translation.
Recently, it has been shown that miRs are widely associated with
pathophysiological changes in myocardial cells (4). The upregulation of miR-1 has been
reported in myocardial cells, modulating the expression of
myocardial connexin 43 and myocardial hypertrophy (5). Furthermore, the downregulation of
miR-133 has been shown to inhibit norepinephrine- and
endothelin-1-induced myocardial hypertrophy (6). In addition, the miR-181 family has
recently been shown to have an important role in the pathogenesis
of cancer and other diseases. The expression of miR-181b is
increased in myocardial infarction, suggesting its involvement in
disease progression (7). However,
the mechanism underlying the involvement of miR-181b in myocardial
impairment has yet to be elucidated, particularly regarding its
association with PKG 1 in disease progression.
In the present study the expression of miR-181b in
the peripheral blood of patients with myocardial hypertrophy was
explored and its association with PKG 1 and the related mechanism
was investigated in an in vitro cardiac hypertrophy
model.
Materials and methods
Patients
Forty-two patients with myocardial hypertrophy (38
males and 13 females) and a mean age of 68.9 years (range, 63–71
years), who had been admitted to the Zaozhuang Municipal Hospital
(Zaozhuang, China) between December 2011 and January 2014, were
enrolled in the study. According to the clinical classification,
these patients were diagnosed with stage II hypertension of a
>5-year duration. In addition, 20 healthy subjects were used as
controls. Prior written and informed consent was obtained from
every participant, and the study was approved by the Ethics Review
Board of the Zaozhuang Municipal Hospital.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol® reagent
(Invitrogen™ Life Technologies, Carlsbad, CA, USA), and the RT was
conducted using the Poly(A) tailing method. qPCR was performed with
the Takara SYBR® PrimeScript™ RT-PCR kit (Takara Bio, Inc., Shiga,
Japan), according to the manufacturer's instructions. The reaction
system contained 10 µl qPCR-Mix, 0.5 µl of each primer, 1 µl cDNA
and 8 µl double-distilled (dd)H2O. U6 was used as
internal control. The primers were synthesized by Sangon Biotech
(Shanghai, China), and the primer sequences were as follows:
miR-181b forward, 5′-AAC ATT CAT TGC TGT CGGT-3′ (3′ universal
primer, which was provided in the kit, was used as the reverse
primer); U6 forward, 5′-ATT GGA ACG ATA CAG AGA AGA TTA-3′ and U6
reverse, 5′-AAT ATG GAA CGC TTC ACG AAT-3′. The qPCR conditions
consisted of denaturation at 95°C for 10 min, followed by 40 cycles
of 95°C for 1 min and 60°C for 30 sec. The relative expression
levels of the target genes were calculated using the
2−ΔΔCt method (8).
Primary myocardial cell culture
Ventricular myocardium was separated and isolated
from 3-day-old neonatal rats (Dashuo Biotechnology Co., Ltd.,
Chengdu, China). The tissues were digested with 5 ml 0.5% trypsin
and 0.5% collagenase at 37°C for 5 min. The cell suspension was
collected into the centrifuge tube containing 2 µl calf serum
(Gibco-BRL, Grand Island, NY, USA). Following centrifugation at 200
× g for 5 min at room temperature, the supernatant was discarded,
and the cells were re-suspended with 5 µl HEPES-buffered Dulbecco's
modified Eagle's medium (H-DMEM; Gibco-BRL) containing 10% fetal
bovine serum (FBS; Gibco-BRL). The cells were cultured in a 37°C,
5% CO2 incubator. For the establishment of the
myocardial hypertrophy model, the primary myocardial cells were
subjected to treatment with 100 µm phenylephrine (PE; Sigma, St.
Louis, MO, USA) for 72 h, prior to the subsequent experiments.
Immunohistochemistry
Cells were cultured on slides and then fixed with
cold acetone at 4°C for 1 h. Following three washes with
ddH2O, the slides were incubated in
H2O2 in the dark for 20 min, and then washed
twice with phosphate-buffered saline (PBS). Sheep serum (1:200;
Gibco-BRL) was added for blocking at 37°C for 1 h. Rabbit
anti-mouse anti-α-sarcomeric actinin antibody (1:1,000; cat. no.
ab137346; Abcam, Cambridge, MA, USA) was added to the cells, which
were incubated at 4°C overnight. Rabbit anti-mouse secondary
antibody (1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was then added for incubation at 37°C for 30 min. DAPI (Santa Cruz
Biotechnology, Inc.) was added for 5 min. Myocardial cells were
observed using a laser scanning confocal microscope (LSM710; Carl
Zeiss AG., Jena, Germany).
Western blot analysis
Cells were treated with a radioimmunoprecipitation
assay buffer, and the supernatant was extracted from the lysate.
Protein concentration was determined using the bicinchoninic acid
assay (Pierce Biotechnology, Inc., Rockford, IL, USA), according to
the manufacturer's instructions. After having been mixed with 2X
sodium dodecyl sulfate (SDS) loading buffer, the protein samples
were separated with SDS-polyacrylamide gel electrophoresis and then
transferred onto a polyvinylidene difluoride membrane. The membrane
was blocked using 50 g/l (w/v) non-fat milk at room temperature for
2 h and then incubated with rabbit anti-mouse anti-PKG 1 polyclonal
antibody (1:1,000; cat. no. sc-211; Santa Cruz Biotechnology, Inc.)
or mouse anti-GAPDH monoclonal antibody (1:5,000; cat. no.
sc-365062; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The
membrane was washed three times with PBS-Tween 20, and the
horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G
(IgG) (1:3,000; Santa Cruz Biotechnology, Inc.) and goat
anti-rabbit IgG (1:1,000; Santa Cruz Biotechnology, Inc.) secondary
antibodies were then incubated with the membrane, respectively, at
room temperature for 2 h.
Transfection
Transfection was performed using Lipofectamine® 2000
(Invitrogen Life Technologies). Primary myocardial cells were
cultured in antibiotic-free H-DMEM (Gibco-BRL) containing 10% FBS
(Gibco-BRL). Twenty-four hours later, 1.5 µl 20 pmol/µl miR-181b
inhibitor (RiboBio Co., Ltd., Guangzhou, China) and 1 µl
Lipofectamine 2000 were separately mixed with 50 µl Opti-MEM®
(Gibco-BRL). The media were then mixed and used to incubate the
cells. Six hours later, the transfection medium was replaced by the
H-DMEM containing 10% FBS. Random sequence was used as a
transfection control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 11.0 software (SPSS Inc., Chicago, IL, USA) was used for the
statistical analysis. Comparisons between the groups were conducted
using the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
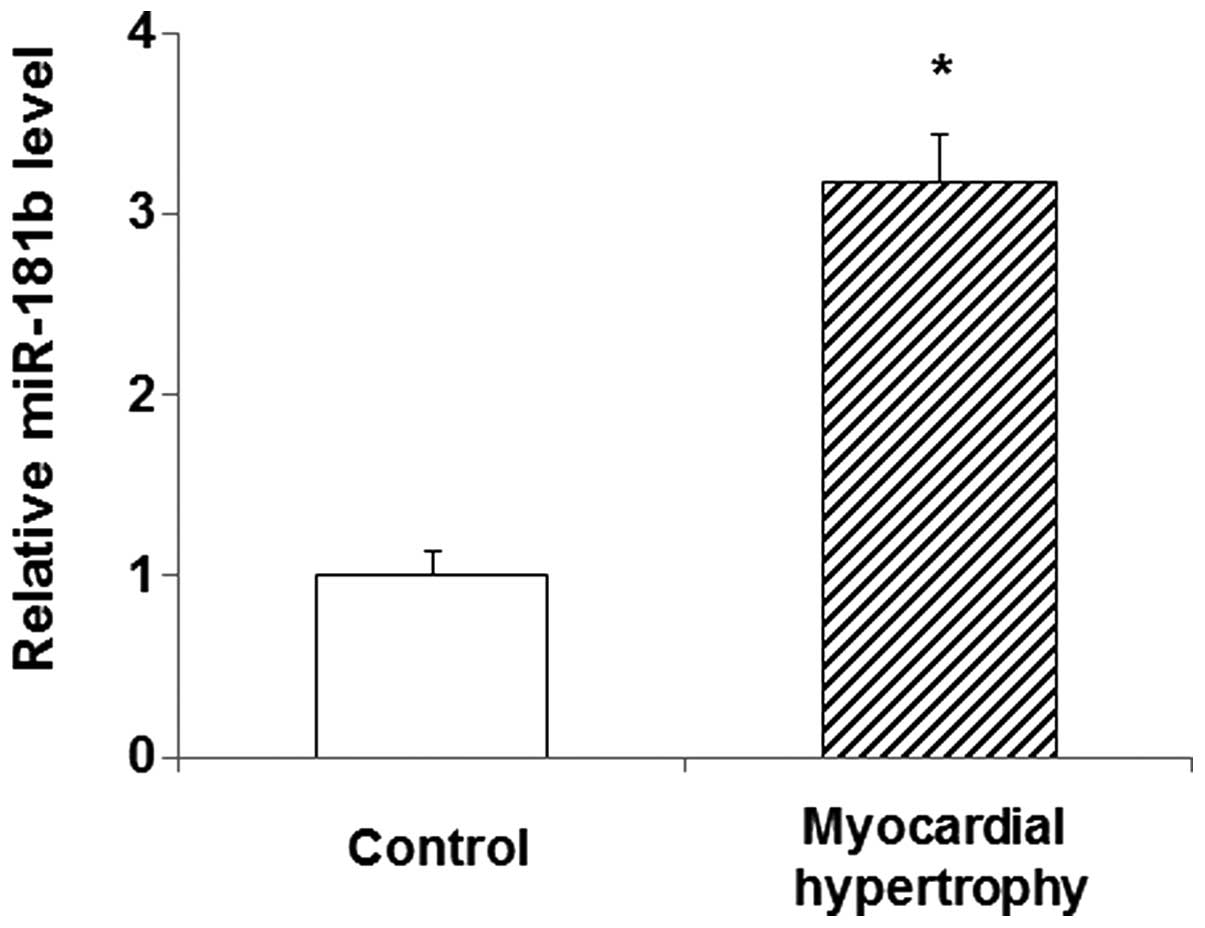
Levels of miR-181b in the peripheral
blood of patients with myocardial hypertrophy
In order to explore the role of miR-181b in
myocardial hypertrophy, its expression level was detected in the
peripheral blood of patients diagnosed with the disease using qPCR.
The results showed that the miR-181b expression in the peripheral
blood of the patients was significantly increased compared with
that in the blood of the normal control subjects (P<0.05)
(Fig. 1). This elevated expression
of miR-181b in myocardial hypertrophy suggests that miR-181b is
involved in the pathology and progression of the disease. In the
following experiments, the role of miR-181b, and its association
with PKG 1 in particular, was investigated in the primary
myocardial cell culture treated with PE.
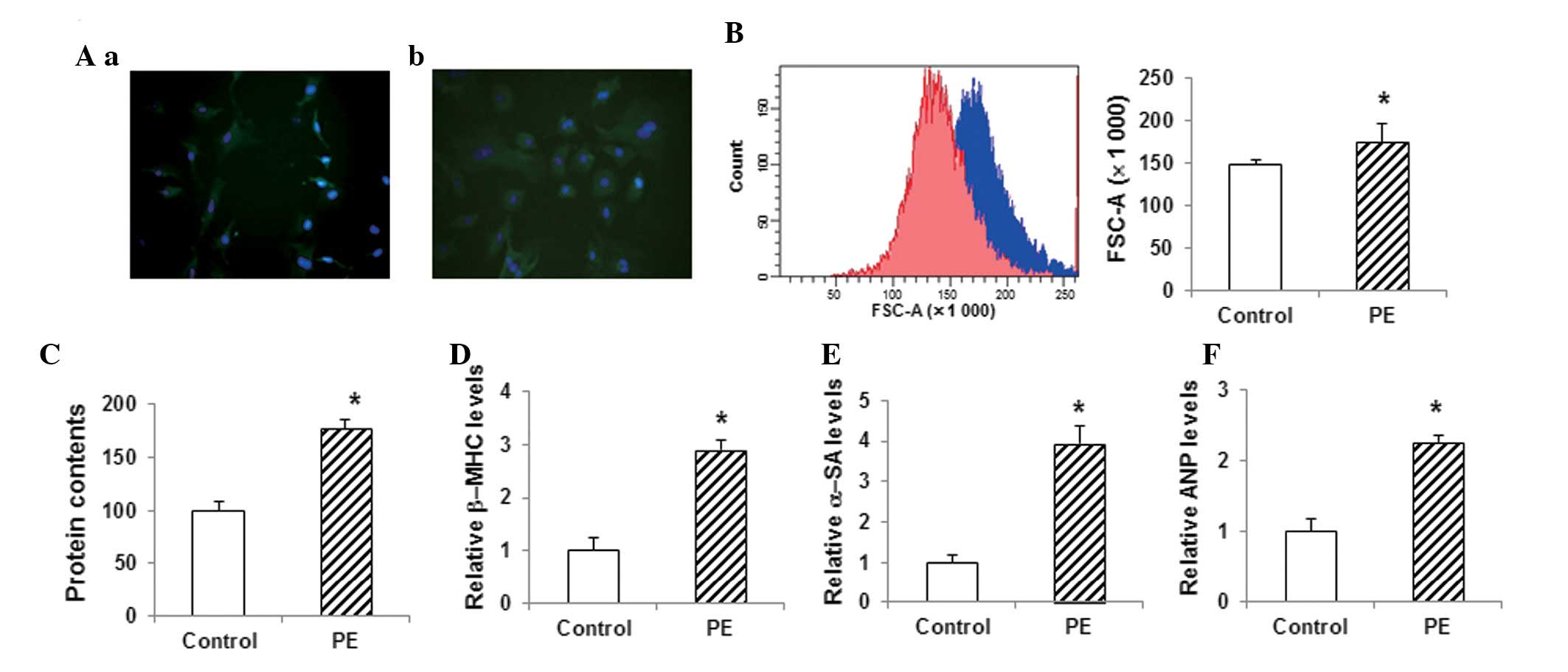
Construction and identification of the
in vitro model of myocardial hypertrophy
The cell model of myocardial hypertrophy was
established with primary myocardial cells obtained from 3-day-old
rats. When stained with anti-α-SA antibody and DAPI, the primary
myocardial cells exhibited a fusiform morphology (Fig. 2Aa). These primary cells were
subjected to PE treatment to induce hypertrophy. Following the
treatment of the myocardial cells with 100 µM PE for 72 h, the
immunohistochemistry results showed significant hypertrophy
compared with the control group (Fig.
2Ab). Furthermore, the flow cytometric analysis showed that the
size of the cardiac myocytes in the PE-treated group was
significantly increased (P<0.05) (Fig. 2B). The total protein content in the
PE-treated group was also significantly increased compared with
that in the control group (P<0.05) (Fig. 2C). qPCR assays showed that the
myocardial expression levels of the hypertrophy-related genes
β-myosin heavy chain (β-MHC), α-SA and atrial natriuretic peptide
(ANP) were significantly increased in the PE-treated group compared
with those in the control group (P<0.05) (Fig. 2D–F). These results suggested that the
in vitro myocardial hypertrophy model had been successfully
constructed and was therefore suitable for the following
analyses.
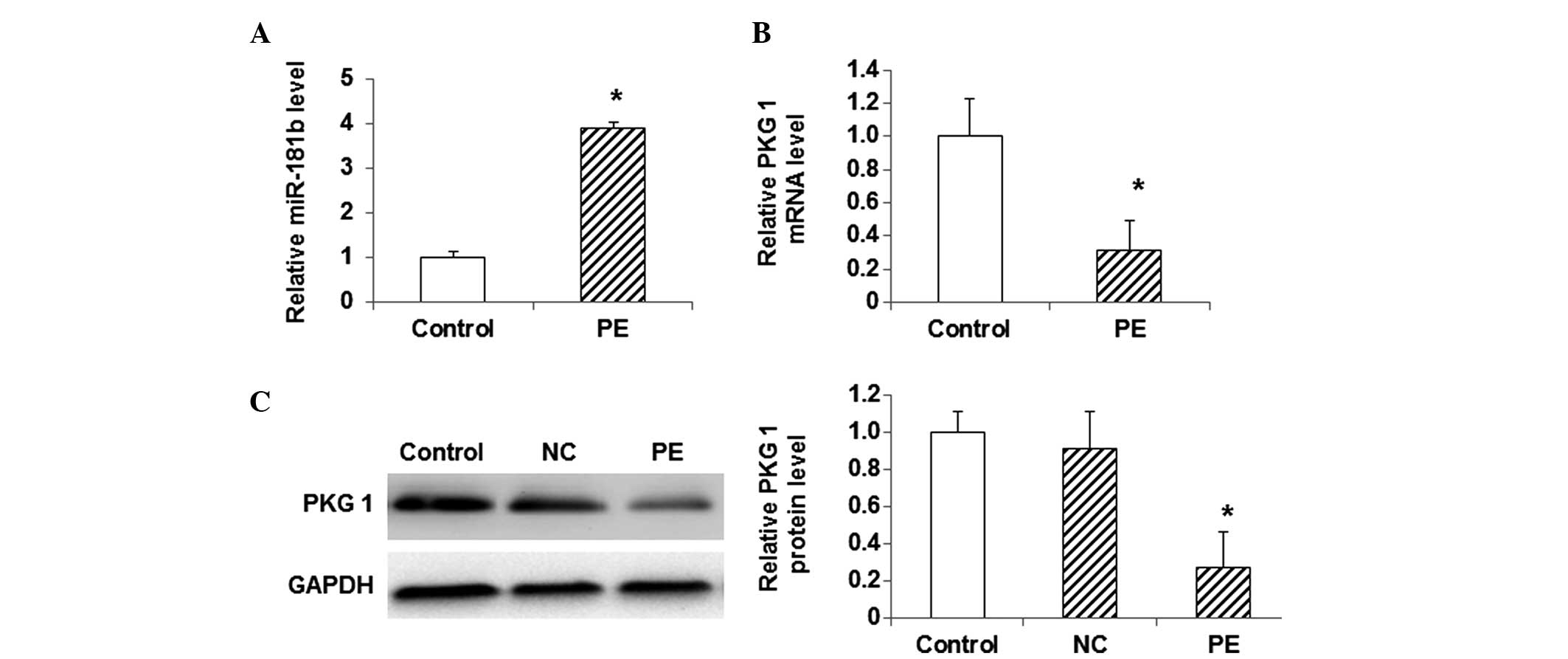
Expression levels of miR-181b and PKG
1 in myocardial hypertrophy cells
To investigate the association between miR-181b and
PKG 1 in the model of myocardial hypertrophy, the expression levels
of miR-181b, as well as the mRNA and protein expression levels of
PKG 1, were detected using qPCR and western blot analysis. The
results from the qPCR analysis showed that, compared with the
control cells, the miR-181b expression was significantly elevated
in the PE-treated cells (P<0.05) (Fig. 3A). By contrast, the mRNA level of PKG
1 was significantly decreased in the PE-treated group compared with
that in the control group (P<0.05) (Fig. 3B). Similarly, the western blot
analysis indicated that the protein expression level of PKG 1 was
significantly lower in the PE-treated group than that in the
control group (P<0.05) (Fig. 3C).
These results showed that the expression level of miR-181b was
elevated in the myocardial hypertrophy cells, while the mRNA and
protein expression levels of PKG 1 were decreased.
Effects of miR-181b inhibition on PKG
1 expression and hypertrophy in PE-treated myocardial cells
In order to determine whether or not the changes in
the expression of miR-181b and PKG 1 were associated with one
another, an inhibitor of miR-181b was used to treat these
myocardial hypertrophy cells and the expression level of PKG 1 was
detected. The PE-treated myocardial cells were transfected with
miR-181b inhibitor, and the inhibition of miR-181b following
transfection was confirmed by the results of the qPCR analysis
(Fig. 4A). qPCR also showed that the
mRNA expression level of PKG 1 was slightly increased following the
miR-181b inhibitor transfection in PE-treated cells, compared with
the control group (Fig. 4B).
Furthermore, western blotting indicated that the protein expression
of PKG 1 was significantly increased in the PE-treated cells
following miR-181b inhibitor transfection, compared with the
control cells (P<0.05) (Fig. 4C).
Flow cytometric analysis showed a notable decrease in the
myocardial cell size, a decreased total protein content and reduced
mRNA expression levels of β-MHC, α-SA and ANP in the PE-treated
myocardial cells following miR-181b inhibitor transfection
(P<0.05) (Fig. 4D–H). These
findings demonstrate the ability of miR-181b inhibition to restore
the reduced PKG 1 expression in PE-treated myocardial cells and
alleviate myocardial hypertrophy, as indicated by the reduced
cellular sizes and decreased expression levels of myocardial
hypertrophy-related genes.
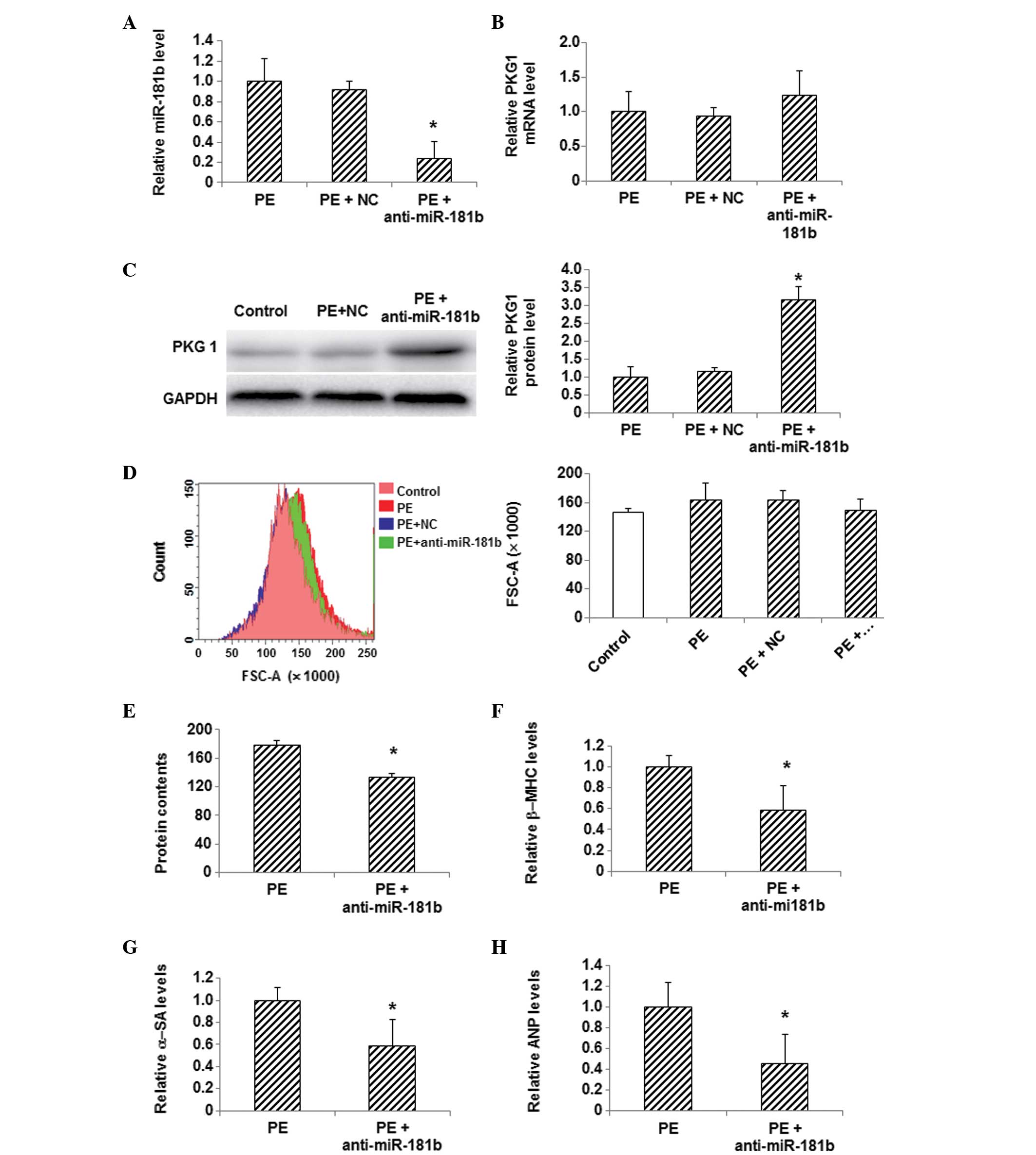 | Figure 4.Effects of miR-181b inhibition on PKG
1 expression and hypertrophy in PE-treated myocardial cells. (A)
The expression level of miR-181b was detected via qPCR in
myocardial cells following the transfection of miR-181b inhibitor.
(B) mRNA and (C) protein expression levels of PKG 1 were detected
in myocardial cells using qPCR and western blotting, respectively,
following miR-181b inhibitor transfection. (D) Sizes of cardiac
myocytes in the control (pink), PE (red), PE + NC (green) and PE +
anti-miR-181b (transfected with miR-181b inhibitor) (blue) groups
were detected with flow cytometry. (E) Total cellular protein
levels were determined using the bicinchoninic acid assay. (F-H)
The mRNA expression levels of the hypertrophy-related genes (F)
β-MHC, (G) α-SA and (H) ANP in myocardial cells following
transfection were detected using qPCR. Compared with the PE-treated
group, *P<0.05. miR, microRNA; PE, phenylephrine; NC, random
sequence; PKG 1, cGMP-dependent protein kinase type I; β-MHC,
β-myosin heavy chain; α-SA, α-sarcomeric actinin; ANP, atrial
natriuretic peptide; FSC, forward scatter; qPCR, quantitative
polymerase chain reaction. |
Discussion
Myocardial hypertrophy has been closely linked with
other chronic diseases, including hypertension, diabetes and
chronic obstructive pulmonary disease (9). Long-term pressure overload is
considered to be a triggering factor for myocardial hypertrophy,
which can change the gene expression and signaling pathway
functions as the disease progresses. When pressure overload
persists, hypertrophic changes occur in myocardial cells,
disturbing gene expression and protein synthesis and impairing
mitochondria and endoplasmic reticulum (10,11). As
compensatory stress responses, protein and adenosine triphosphate
synthesis and ion channel activity in these cells are subsequently
enhanced (12–14), maintaining the myocardial systolic
function and protecting against pressure overload; however,
long-term hypertrophy could eventually result in impaired cellular
structure and function. These pathological changes would in turn
lead to cell death and interstitial fibrosis, eventually resulting
in heart failure. The present study investigated the molecular
mechanism of myocardial hypertrophy, in in order to search for
novel biological indicators, which could contribute to the
diagnosis and treatment of the disease.
PKG 1 has been closely associated with the
physiological functions of myocardial cells, and is also involved
in the modulation of Ca2+ concentration and smooth
muscle tension in vessels (15). A
recent study indicated that PKG 1 can inhibit myocardial cell
hyperplasia and hypertrophy. Through cGMP regulation, PKG 1 can
phosphorylate and activate troponin T. In addition, PKG 1 can
inhibit the calcium-dependent calcineurin/nuclear factor of
activated T cells signaling pathway and modulate cell hypertrophy
by regulating the L-type calcium channel (16); however, further studies are required
to determine whether there are other mechanisms underlying the
functions of PKG 1 in myocardial hypertrophy.
The role of miRNA in the pathogenesis of myocardial
hypertrophy has attracted a great deal of attention in recent
years. It has been reported that miR-98 and miR-9 are closely
associated with the development of myocardial hypertrophy (17). The newly discovered miRNA family
miR-181 has been found to promote tumor proliferation, invasion and
migration (18). In this study, the
role of miR-181b in the progression of myocardial hypertrophy was
investigated. The results indicated that the expression level of
miR-181b was significantly increased in the peripheral blood of
patients with myocardial hypertrophy. To further confirm the role
of miR-181b in myocardial hypertrophy, an in vitro
cardiomyocyte hypertrophy model was constructed using PE induction.
Following 72 h of PE treatment, the size and morphology of the
primary myocardial cells were observed. Furthermore, the total
protein content was analyzed and the expression levels of the
myocardial hypertrophy-related genes β-MHC, α-SA and ANP were
detected. The myocardial hypertrophy model was characterized and
identified according to these assessments. The results indicated
that the expression level of miR-181b was significantly increased,
while the mRNA and protein expression levels of PKG 1 were
decreased in the PE-treated cells, suggesting that miR-181b is
negatively correlated with PKG 1 expression. When the PE-treated
primary myocardial cells were transfected with an miR-181b
inhibitor, the miR-181b expression was significantly downregulated,
while the mRNA and protein expression levels of PKG 1 were markedly
increased. In addition, flow cytometry showed that the size of the
myocardial cells was reduced following the inhibition of miR-181b.
The total cellular protein content and the expression levels of
hypertrophy-related genes were significantly decreased in the
PE-treated cells transfected with miR-181b inhibitor, indicating
alleviated myocardial hypertrophy in these cells.
In conclusion, according to the findings of the
present study, miR-181b could play an important role in the
pathogenesis of myocardial hypertrophy by regulating the expression
of PKG 1. The expression level of miR-181b was significantly
increased in the peripheral blood of patients with myocardial
hypertrophy. In the myocardial hypertrophy model cells induced by
PE treatment, the expression level of miR-181b was elevated, while
the mRNA and protein expression levels of PKG 1 were decreased.
When miR-181b was inhibited in these cells, the expression of PKG 1
was restored and myocardial hypertrophy was alleviated. The present
results suggest that miR-181b can be used as a novel target for the
clinical diagnosis and treatment of myocardial hypertrophy.
Acknowledgements
The authors would like to thank Professor Shujian
Sui from the Second Hospital of Shandong University and Professor
Tongbao Liu from the Shandong Provincial Hospital for their advice
and assistance with the study design, sample and data collection,
statistical analysis and manuscript preparation.
References
|
1
|
Oka T, Akazawa H, Naito AT and Komuro I:
Angiogenesis and cardiac hypertrophy: Maintenance of cardiac
function and causative roles in heart failure. Circ Res.
114:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hofmann F and Wegener JW: cGMP-dependent
protein kinases (cGK). Methods Mol Biol. 1020:17–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen KC, Sung PL, Yen MS, Chuang CM, Liou
WS and Wang PH: MicroRNAs regulate several functions of normal
tissues and malignancies. Taiwan J Obstet Gynecol. 52:465–469.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hodgkinson CP, Kang MH, Dal-Pra S,
Mirotsou M and Dzau VJ: MicroRNAs and Cardiac Regeneration. Circ
Res. 116:1700–1711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Ma XJ, Wang HJ, et al: Expression of
Cx43-related microRNAs in patients with tetralogy of Fallot. World
J Pediatr. 10:138–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katta A, Thulluri S, Manne ND, et al:
Overload induced heat shock proteins (HSPs), MAPK and miRNA (miR-1
and miR133a) response in insulin-resistant skeletal muscle. Cell
Physiol Biochem. 31:219–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Domigan CK and Iruela-Arispe ML: Recent
advances in vascular development. Curr Opin Hematol. 19:176–183.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bubner B and Baldwin IT: Use of real-time
PCR for determining copy number and zygosity in transgenic plants.
Plant Cell Rep. 23:263–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lyon RC, Zanella F, Omens JH and Sheikh F:
Mechanotransduction in cardiac hypertrophy and failure. Circ Res.
116:1462–1476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novoyatleva T, Sajjad A and Engel FB:
TWEAK-Fn14 cytokine-receptor axis: A new player of myocardial
remodeling and cardiac failure. Front Immunol. 5:502014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan HS, Shangguan HJ, Shang Z, Yang L,
Meng XM and Qiao SB: Endoplasmic reticulum stress caused by left
ventricular hypertrophy in rats: Effects of telmisartan. Am J Med
Sci. 342:318–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soares JB, Rocha-Sousa A, Castro-Chaves P,
Henriques-Coelho T and Leite-Moreira AF: Inotropic and lusitropic
effects of ghrelin and their modulation by the endocardial
endothelium, NO, prostaglandins, GHS-R1a and KCa channels.
Peptides. 27:1616–1623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tagashira H, Bhuiyan MS, Shioda N and
Fukunaga K: Fluvoxamine rescues mitochondrial Ca2+
transport and ATP production through σ(1)-receptor in hypertrophic
cardiomyocytes. Life Sci. 95:89–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghule AE, Kulkarni CP, Bodhankar SL and
Pandit VA: Effect of pretreatment with coenzyme Q10 on
isoproterenol-induced cardiotoxicity and cardiac hypertrophy in
rats. Curr Ther Res Clin Exp. 70:460–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang HM, Kim BK, Kim JY, et al: PPARγ
modulates vascular smooth muscle cell phenotype via a protein
kinase G-dependent pathway and reduces neointimal hyperplasia after
vascular injury. Exp Mol Med. 45:e652013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandael DH, Mahapatra S, Calorio C,
Marcantoni A and Carbone E: Cav1.3 and Cav1.2 channels of adrenal
chromaffin cells: Emerging views on cAMP/cGMP-mediated
phosphorylation and role in pacemaking. Biochim Biophys Acta.
1828:1608–1618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harada M, Luo X, Murohara T, Yang B,
Dobrev D and Nattel S: MicroRNA regulation and cardiac calcium
signaling: Role in cardiac disease and therapeutic potential. Circ
Res. 114:689–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong SJ, Liu J, Wang X and Qu LX:
microRNA-181 promotes prostate cancer cell proliferation by
regulating DAX-1 expression. Exp Ther Med. 8:1296–1300.
2014.PubMed/NCBI
|