Introduction
Hemangioma is one of the most common types of
infantile benign tumor and is a congenital dysplasia that appears
in the process of embryonic blood vessel formation. The incidence
rate of all newborns is 1.1–2.6% (1), and the condition, which usually occurs
in the head and neck of infants, is more common in premature
infants, particularly females. According to the biological
characteristics of the endothelial cells in hemangioma and its
clinical manifestations, the natural course of the condition can be
divided into the proliferating, involuting and involuted phases
(2,3). Hemangiomas at the proliferative stage
exhibit rapid growth and may affect the normal development of the
body. The development of hemangioma in certain parts of the body
may affect the appearance of the patient, have an impact on the
normal function of body and even prove life-threatening; therefore,
such hemangiomas require aggressive treatment promptly (4,5).
At present, with the improvement in living
standards, noninvasive methods of treatment tend to be preferred by
patients; therefore, drug treatments are commonly selected. Drug
therapy includes topical drug therapy and oral medication. In 2008,
Léauté-Labrèze et al (6)
found that the β-receptor blocker propranolol was able to treat
infantile hemangioma; with the exception of individual cases of low
blood pressure, no serious adverse reactions were observed in the
other children throughout the treatment course. Furthermore,
clinical practice in recent years has shown that propranolol exerts
a rapid, curative effect in the treatment of hemangioma, with few
inter-patient differences and adverse reactions; however, the
underlying therapeutic mechanism is not clear. The aim of the
present study, therefore, was to assess the clinical efficacy and
safety of propranolol in the treatment of infantile hemangioma by
monitoring clinical and biochemical parameters. Furthermore, the
study aimed to investigate the mechanism underlying the effects of
propranolol by measuring the serum expression levels of vascular
endothelial growth factor (VEGF), basic fibroblast growth factor
(bFGF) and matrix metalloproteinase-9 (MMP-9) prior to and
following medication.
Patients and methods
Patient inclusion
Between September 2009 and December 2012, 129
patients with newly diagnosed cases of infantile hemangioma (both
in- and outpatients) were enrolled as the study subjects. For
inclusion in the study, the patients had to be aged <1 year and
exhibit the following characteristics of hemangioma onset: i)
Mosquito bite- or pin-like red spots at birth or 7–10 days later or
the appearance of patchy erythema at birth, with patchy expansion
or subcutaneous soft masses; ii) a rapid tumor growth history; and
iii) a strawberry or light blue tumor surface that fades or reduces
in color when pressed. Patients with certain diseases, including
sinus bradycardia, bronchial asthma, cardiogenic shock, heart block
(type II–III atrioventricular block) and severe or acute heart
failure, were excluded from the study.
Grouping
The infants were assigned to either the treatment
group or the observation group according to the approval of the
family of each patient. The observation group comprised 32 cases,
including 11 male patients and 21 female patients. The patients
were aged between 1 month, 10 days and 10 months, with an average
age of 105 days. The location of the hemangiomas was as follows:
Head and neck, 13 cases; limbs, 8 cases; trunk, 8 cases; and
perineum, 3 cases. The treatment group comprised 97 cases,
including 31 male patients and 66 female patients, with ages
ranging between 24 days and 11 months, 2 days (average, 105 days).
The patients' weights ranged between 3.8 and 10.0 kg, with an
average body weight of 6.07 kg. The weight for observation group
ranged between 3.8 and 9.2 kg, with an average body weight of 6.30
kg. The location of the hemangiomas was as follows: Head and neck,
59 cases; limbs, 14 cases; trunk, 12 cases; perineum, 5 cases; and
multiple sites, 7 cases.
Data collection
The patients of the observation group were
clinically followed-up and generally reviewed once every 2–4 weeks,
in order to measure the size of the tumor and observe the changes
in color and tension.
The patients of the treatment group were admitted
and the family of each patient was informed of the objectives of
the oral propranolol treatment, as well as the safety and
associated risks. Written consent was obtained prior to treatment.
Once the relevant examinations had been performed to confirm that
no contraindications to propranolol treatment existed, the
treatment was started at the dose of 1.5 mg/kg per day
(Shijiazhuang Pharmaceutical Group Co., Ltd., Hebei, China). The
propranolol was administered once a day in the form of a draught in
the morning. During the treatment period, the heart rate of each
patient was measured prior to and 1, 3 and 6 h after medication for
a total of 3 days. In addition, the blood glucose levels prior to
and 2 h after medication were monitored, again for a total of 3
days. If the infants had exhibited no obvious abnormal reaction
after 3 days, they were discharged from hospital to continue the
medication until tumor regression was observed. During this period,
the liver and renal function and the thyroid function were assessed
4 and 8 weeks after the start of medication, respectively, the
tumor size was measured and changes in the color and tension of the
hemangioma were observed. Any changes in the mental status,
appetite, stool appearance or weight of the patients during
medication were additionally noted during the follow-up.
Sample collection and testing
Following the provision of informed consent by the
family members, the blood samples (2 ml, without anticoagulant) of
40 infants in the treatment group were collected prior to and 8
weeks after medication. The samples were placed in the test tube,
kept static at room temperature for 30 min and then subjected to
centrifugation at 658.44 × g to isolate the serum. The serum was
placed into an Eppendorf (EP) tube and preserved at −80°C for
subsequent testing. Following the collection of the samples, the
serum concentrations of VEGF, bFGF and MMP-9 were detected using
the ELISA method.
Experimental reagents and
consumables
The standard equipment used in the study included a
centrifuge, balance, EP tubes, freezer (−80°C), enzyme mark
instrument (450 nm), high-precision pipette and gun head, constant
temperature box (37°C), disposable tubes and absorbent paper. The
human VEGF, bFGF and MMP-9 ELISA kits were obtained from Beijing
Dingguochangsheng Biotechnology Co., Ltd. (Beijing, China).
Clinical efficacy criteria
The grading criteria proposed by Achauer et al
(7) were used to analyze the effect
of the treatment/observation on the hemangiomas. The results were
obtained after 8 weeks of treatment or observation. The four grades
were as follows: Grade I (poor), reduction in tumor size of
<25%; Grade II (medium), reduction in tumor size and skin lesion
color of 26–50%; Grade III (good), reduction in tumor size and skin
lesion color of 51–75%; Grade IV (excellent), reduction in tumor
size and skin lesion color of >75% (disappearance of the tumor
and restoration or near-restoration of the skin lesion color to
that of normal skin). Grades II–IV (medium, good and excellent)
were considered to represent an effective response, while Grade I
(poor) was an invalid response.
Statistical analysis
The data were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Count data were compared
using the χ2 test, and measurement data were compared
using a paired-samples t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical efficacy observation
In the treatment group, the longest period of
propranolol administration was 1 year; however, for the convenience
of the study and statistical analysis, the results were taken after
8 weeks of medication. Following treatment or observation for 8
weeks, the results showed the curative effect of the treatment
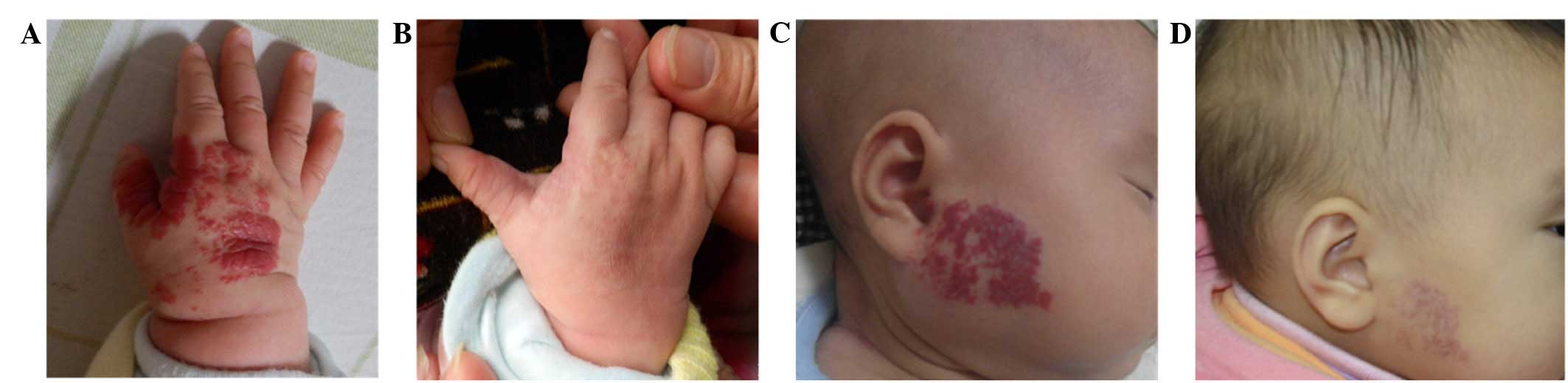
group to be excellent in 27 cases (Fig.
1), good in 34 cases, medium in 35 cases and poor in 1 case,
with a total efficiency of 98.97%. By comparison, the curative
effect of the observation group was excellent in 0 cases, good in 0
cases, medium in 10 cases and poor in 22 cases, with the total
efficiency of 31.25%. The efficacy of the treatment group was
significantly higher than that of the observation group (P<0.05)
(Table I).
 | Table I.Comparison of the curative effects
between the treatment group and the observation group. |
Table I.
Comparison of the curative effects
between the treatment group and the observation group.
| Group | Total cases (n) | Excellent (n) | Good (n) | Medium (n) | Poor (n) | Total efficacy
(%) |
|---|
| Treatment | 97 | 27 | 34 | 35 | 1 | 98.97a |
| Observation | 32 | 0 | 0 | 10 | 22 | 31.25 |
Changes in the serum concentrations of
VEGF, bFGF and MMP-9 prior to and following treatment
The serum concentrations of VEGF, bFGF and MMP-9 in
the children after 8 weeks of therapy were decreased significantly
compared with those prior to treatment. The concentration changes
were statistically significant (P<0.05) (Table II).
 | Table II.Changes in the serum concentrations of
VEGF, bFGF and MMP-9 in the treatment group before and 8 weeks
after medication. |
Table II.
Changes in the serum concentrations of
VEGF, bFGF and MMP-9 in the treatment group before and 8 weeks
after medication.
| Protein | Before
medication | 8 weeks after
medication |
|---|
| VEGF (pg/ml) |
264.1825±15.32993 |
142.6950±18.83824a |
| bFGF (pg/ml) |
59.4100±4.02030 |
38.8450±5.83790a |
| MMP-9 (ng/ml) |
411.2080±28.35919 |
279.9350±29.79854a |
Changes in heart rate and biochemical
parameters prior to and following treatment
During the 3 days of hospitalization, the heart
rates of the patients in the treatment group before and 1, 3 and 6
h after medication exhibited significant differences; however, the
clinical observation revealed no bradycardia or other symptoms. In
addition, the blood glucose levels and liver and renal function
prior to and following medication exhibited no significant changes.
With regard to the thyroid function, the comparison of the free
triiodothyronine (FT3) levels prior to and following treatment did
not show a significant difference, but the changes in the
concentrations of free thyroxine (FT4) and sensitive
thyroid-stimulating hormone (sTSH) did have statistical
significance: The serum concentration of FT4 following medication
was higher than that prior to medication, but mostly remained
within the normal range, and the serum concentration of sTSH
following medication was lower than that prior to medication, but
again mostly remained within the normal range. No clinical symptoms
developed during the medication period.
Side effects
During medication, infants of the treatment groups
showed good spirits. Seven patients exhibited a slight loss of
appetite, diarrhea and other symptoms, which required no special
treatment and were self-healing, and no other adverse reactions
were observed.
Discussion
Hemangiomas are a type of common, benign tumor
associated with the abnormal proliferation of vascular endothelial
cells. Hemangiomas are caused by uncontrolled vascular generation
or an imbalance of angiogenic cytokines and angiogenesis
inhibitors. VEGF, bFGF and MMP-9 play an important role in the
formation of hemangiomas (4,8–10): VEGF
is a type of glycosylated secreted polypeptide factor that can
induce endothelial cell proliferation and migration, enhance the
permeability of blood vessels and promote the growth of new vessels
and stromal cells (8,11); bFGF is a type of strong endothelial
cell and wall connective tissue growth factor, which can promote
the migration of endothelial cells into the extracellular matrix to
form capillary tissues and induce the secretion of VEGF (11); MMPs are a class of zinc and
calcium-dependent extracellular proteolytic enzymes, which not only
can promote the release of the angiogenic factors VEGF and bFGF to
induce angiogenesis, but also can degrade the extracellular matrix
and promote the migration of endothelial cells and the formation of
the tube structure (4).
The association between serum VEGF concentration and
endothelial cell proliferation in hemangioma has been demonstrated
previously (8,9), with the serum VEGF concentration of
proliferative hemangiomas found to be significantly higher than
that of involuting hemangiomas, vascular malformation and the
normal control group. In the present study, the serum
concentrations of VEGF, bFGF and MMP-9 8 weeks after medication
were significantly reduced compared with those prior to treatment,
and the results of this study were consistent with those of
previous studies (4,10). The reduction in the concentration of
these three proteins is associated with the termination or
regression of the growth of the hemangioma, which indicates that
one of the mechanisms of propranolol in the treatment of
proliferative hemangiomas may involve the downregulation of VEGF,
bFGF and MMP-9; however, the exact mechanism remains to be
elucidated. It has previously been found that VEGF expression is
regulated by the extracellular signal-regulated kinase
(ERK)/mitogen-activated protein kinase (MAPK) pathway (12), and protein kinase A (PKA) is a type
of cyclic adenosine-3′,5′-monophosphate (cAMP)-dependent protein
kinase (13) that can promote VEGF
secretion through the activation of the ERK/MAPK pathway. cAMP is
the product of ATP following the action of cell adenylate cyclase,
which is the earliest determined intracellular second messenger.
cAMP is involved in a variety of the metabolic processes of the
body enzymes, which can not only regulate the division,
differentiation and development of cells, but also can regulate the
expression of genes and promote the transcription of mRNA (14). cAMP can activate cAMP-dependent PKA,
and PKA enables intracellular protein phosphorylation, regulating
the activity of the proteins and promoting their reactions; thus, a
model of a cAMP/PKA signal transduction pathway is formed. A
previous study (5) suggested that,
through the inhibition of the cAMP/PKA signaling pathway on the
activation of the ERK/MAPK signaling pathway, propranolol could
inhibit the secretion of VEGF in proliferative hemangioma. In a
different study (15), it was
suggested that, by downregulating the expression of the Raf/MAPK
pathway, propranolol could decrease the expression of VEGF and bFGF
in the late period of hemangioma treatment, so as to promote the
further regression of the hemangioma; however, further study is
required to confirm these theories.
The results of the present study revealed a
significant difference between the curative effect of the treatment
group and that of the observation group, which confirmed the
validity of propranolol in the treatment of proliferative
hemangioma. The comparison of the heart rate of 97 infants with
hemangioma prior to propranolol treatment and 1, 3 and 6 h after
medication also revealed statistically significant differences;
however, no bradycardia or other symptoms were observed. No
significant changes were found between the blood glucose levels,
and liver and renal function prior to and following medication;
with regard to the thyroid function, the concentration of FT3
exhibited no significant changes prior to and following treatment,
but the changes in the concentrations of FT4 and sTSH were
significant. We consider that oral propranolol does not affect the
secretion of thyroid hormones, but can inhibit the conversion of T4
into T3 in peripheral tissues, resulting in an increase in the
serum concentration of FT4 following oral propranolol
administration. Furthermore, with the increase in the concentration
of FT4, the negative feedback from serum FT4 can inhibit the
secretion of sTSH, leading to a decrease in the sTSH concentration.
Despite the observed changes in this study, however, the
concentrations of FT4 and sTSH following medication remained within
the normal range, and the children did not exhibit any clinical
symptoms.
During medication, a few children in the present
study exhibited loss of appetite, diarrhea, slow weight gain and
certain other phenomena, but these conditions all alleviated
spontaneously, without apparent adverse reactions, such as severe
hypoglycemia, hypotension or bradycardia. This indicates that
propranolol is highly safe; however, the long-term effect of the
drug is not clear, and a long-term follow-up is required. In
addition, there is no uniform standard regarding when to terminate
propranolol administration in the treatment of hemangioma, and
there have been numerous reports of propranolol withdrawal leading
to the recurrence of the hemangioma (16–19),
which necessitated the renewal of the medication administration. An
associated study (20) showed that
the changes in hemangioma tend to be larger in the first 5 months
of treatment and that further treatment effects are less obvious.
Zhao et al (10) found that
propranolol has no significant effect on involuting hemangiomas;
however, the experience of the author led to the recommendation
that the administration of propranolol should be slowly stopped
following the subsidence of the hemangioma, as this can reduce the
vascular tumor recurrence rate. Combining the results of this study
and the relevant literature, we suggest that propranolol is a safe
and effective option for the treatment of infantile hemangioma and
should be considered as a first-line therapy.
Current clinic practice mainly involves the use of
oral propranolol tablets for treatment; however, the tablet dose is
not easy to control and the tablets are not easy for infants to
take. Furthermore, since the gastrointestinal tract absorbs
propranolol for liver metabolism and the renal excretion process,
the drug exhibits low bioavailability and can easily interact with
other medications. In addition, propranolol has a short half-life,
and large fluctuations in the blood drug concentration can be
observed (21). We therefore suggest
that propranolol should be made into an injection or external
preparation for local endovascular aneurysm injection or local
surface use, which would not only lead to a high local
concentration, but also to a reduction in the systemic adverse
reactions.
The occurrence and development of numerous types of
cancer, in addition to hemangioma, are closely associated with
angiogenesis (22). Inflammatory
factors and hypoxia can induce the expression of VEGF, and the
combination of VEGF and the VEGF receptor 1/2/3 can activate the
phosphoinositide 3-kinase/Akt, p38MAPK and FAK signaling pathway
(22). The use of VEGF and its
receptor as a target is an area of particular focus in the
development of anticancer drugs, and further studies are required
to determine whether propranolol can be developed as an adjuvant
cancer treatment.
In conclusion, oral propranolol has a good curative
effect in the treatment of proliferative hemangioma, with few side
effects and a high level of safety. The mechanism underlying the
effects of propranolol may be associated with the downregulation of
VEGF, bFGF and MMP-9 expression.
Acknowledgements
This study was supported by the Class B Science and
Technology Project of the Education Department of Fujian Province
(no. JB11055), the Scientific Research Fund project of the National
Health and Family Planning Committee-Fujian Province Joint Research
Project of Health Education (no. WKJ-FJ-03) and the National Key
Clinical Specialty Discipline Construction Program.
References
|
1
|
Schwartz RA, Sidor MI, Musumeci ML, et al:
Infantile hemangiomas: A challenge in pediatric dermatology. J Eur
Acad Dermatol Venereol. 24:631–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jinnin M, Ishihara T, Boye E, et al:
Recent progress in studies of infantile hemangioma. J Dermatol.
37:283–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boye E and Olsen BR: Signaling mechanisms
in infantile hemangioma. Curr Opin Hematol. 16:2022009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao ZF, Lü RR, Huo R, Fu HB and Xu GQ:
The change of serum vascular endothelial growth factor and matrix
metalloproteinases-9 in proliferative hemangioma treated with
propranolol. Zhong Hua Zheng Xing Wai Ke Za Zhi. 27:359–361.
2011.
|
|
5
|
Liu D: Propranolol treatment of infantile
proliferation hemangiomas experimental study (unpublished PhD
thesis). Luzhou Medical College. 2012.
|
|
6
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, et al: Propranolol for severe hemangiomas of infancy. N
Engl J Med. 358:2649–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Achauer BM, Chang CJ and Vander Kam VM:
Management of hemangioma of infancy: Review of 245 patients. Plast
Reconstr Surg. 99:1301–1308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Q, Lin X, Shang Q, et al: The
determination and significance of VEGF in the serum of hemangioma
patients. Zhong Hua Zheng Xing Wai Ke Za Zhi. 18:98–100. 2002.
|
|
9
|
Zhang L, Lin X, Wang W, et al: Clinical
implications of serum VEGF concentration in hemangioma. Zhong Hua
Xiao Er Wai Ke Za Zhi. 25:11–12. 2004.
|
|
10
|
Zhao Z, Lv R, Huo R, et al: Change of bFGF
in patients with hemangioma treated with oral Propranolol. Zhong
Guo Mei Rong Zheng Xing Wai Ke Za Zhi. 22:504–507. 2011.
|
|
11
|
Folkman J: Clinical application of
research on angiogenesis. N Engl J Med. 333:1757–1763. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fredriksson JM, Lindquist JM, Bronnikov GE
and Nedergaard J: Norepinephrine induces vascular endothelial
growth factor gene expression in brown adipocytes through a
beta-adrenoreceptor/cAMP/protein kinase A pathway involving Src but
independently of Erk1/2. J Biol Chem. 275:13802–13811. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su Y, Huang X, Raskovalova T, et al:
Cooperation of adenosine and prostaglandin E2 (PGE2) in
amplification of cAMP-PKA signaling and immunosuppression. Cancer
Immunol Immunother. 57:1611–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roesler WJ, Vandenbark GR and Hanson RW:
Cyclic AMP and induction of eukaryotic gene transcription. J Biol
Chem. 263:9063–9066. 1998.
|
|
15
|
Gelmetti C, Frasin A and Restano L:
Innovative therapeutics in pediatric dermatology. Dermatol Clin.
28:619–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sans V, Roque ED, Berge J, et al:
Propranolol for severe infantile hemangiomas: Follow-up report.
Pediatrics. 124:e423–e431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schupp CJ, Kleber JB, Günther P and
Holland-Cunz S: Propranolol therapy in 55 infants with IH: Dosage,
duration, adverse effects and outcome. Pediatr Dermatol.
28:640–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Georgountzou A, Karavitakis E,
Klimentopoulou A, et al: Propranolol treatment for severe infantile
hemangiomas: A single-centre 3-year experience. Acta Paediatr.
101:e469–e474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang L, Ma G, Jin Y, et al: Recurrence of
infantile hemangioma after termination of propranolol treatment.
Ann Plast Surg. 72:173–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bagazgoitia L, Torrelo A, Gutiérrez JC, et
al: Propranolol for infantile hemangiomas. Pediatr Dermatol.
28:108–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He SY, Zhang J and Ding JS: The
application research progress and mechanism of Propranolol treaing
on Hemangioma. Zhong Nan Yao Xue. 9:274–276. 2011.
|
|
22
|
Xiong B and Yi C: Vascular endothelial
growth factor family and gene therapy. Zhong Hua Yi Xue Mei Xue Mei
Rong Za Zhi. 12:328–330. 2000.
|