Introduction
The incidence of diabetes mellitus is increasing at
an alarming rate in developed and developing countries (1,2). Diet,
exercise and medications remain the primary choice of type 2
diabetes mellitus (T2DM) therapy, but the long-term success rate of
lifestyle modifications can be disappointing. Despite the emergence
of new antidiabetic drugs, the glycemic control achieved is far
from perfect and some drugs may cause weight gain and increase the
risk of cardiovascular disease (3–5).
However, in cases where behavioral and pharmacological strategies
prove insufficient in the treatment of T2DM, several types of
gastrointestinal surgery can provide effective alternatives.
Roux-en-Y gastric bypass (RYGB) surgery is one of the most
effective and commonly performed procedures worldwide (6). Following RYGB surgery, up to 40–85%
patients achieve complete remission from T2DM, and lifelong T2DM
remission has been reported amongst those patients (7–9).
Regardless of the good therapeutic effect that bariatric surgery
has on T2DM, bariatric surgery can have complications and its total
mortality rate is 0.28% [95% confidence interval (CI), 0.22–0.34]
within 30 days after surgery, and 0.35% between 30 days and 2 years
(95% CI, 0.12–0.58) after surgery (10). The proportion of patients seeking to
have bariatric surgery is <1% (11). However, the implantation of a
recently invented duodenal-jejunal bypass sleeve (DJBS) in the
duodenum and proximal jejunum can mimic the biliopancreatic
diversion effect of RYGB surgery and has comparable ability to
induce weight loss and glycemic control with minimal invasion
(12).
The weightloss-inducing effect of DJBS implantation
was first verified by Milone et al (13) in a porcine model. Gersin et al
(14) placed the first DJBS with the
aid of gastroscope in a morbidly obese patient for 3 months. The
patient achieved a total weight loss of 9.09 kg without severe
complications. A series of clinical trials confirmed the excellent
therapeutic effect of DJBS on diabetes mellitus (15,16).
However, the specific mechanism by which DJBS placement induces
glycemic control in obese and diabetic patients is not well known.
Studies have found that gut hormone secreted from enteroendocrine
cells plays an important role in bariatric surgery- and
DJBS-mediated glycemic control (17,18), and
that glucagon-like peptide-1 (GLP-1) produced by intestinal L cells
is one of the most important gut hormones.
The gastrointestinal tract is important in metabolic
diseases, including diabetes mellitus (19). The plasma insulin response to
intravenous glucose is only 30–40% of that to oral glucose
(20), that is, orally ingested
glucose triggers nearly 2-3-fold more insulin secretion than the
same amount of glucose delivered intravenously. The involvement of
intestinal hormones in the ‘incretin effect’ has been described in
the classical concept of the ‘entero-insular axis’ (21,22). Two
hormones, glucose-dependent insulinotropic polypeptide (GIP) and
GLP-1, take important roles in this phenomenon. The combined action
of GLP-1 and GIP is believed to account for up to 70% of the total
insulin secretory response after a meal (23). GLP-1 is mainly expressed in mucosal L
cells located predominantly in the distal intestine (ileum and
colon). In humans, a large number of GLP-1-expressing cells are
distributed in the distal jejunum and ileum, the cell density
increases from the proximal to the distal colon and the highest
number is in the rectum, whereas in rat, the highest cell density
is in the ileum (24).
GLP-1 is secreted from L cells located in the
intestine and has a significant effect on glycemic control. In the
rat RYGB model, studies have found that duodenal jejunum bypass
increases the plasma GLP-1 level and augments the total intestinal
L-cell number (25); similar
increases in GLP-1 level have also been observed in diet-induced
obese rats (26) and diabetic
patients (16) after DJBS
implantation. However, it is not clear whether the increase in
circulating GLP-1 levels after DJBS implantation is caused by
enhanced GLP-1 synthesis, or an increase in the intestinal L-cell
number. Intestinal L-cell and mucosal hypertrophy following RYGB
surgery has been reported in diabetic rats (25). Since DJBS mimics most of the effects
of RYGB surgery, the present study aimed to observe the effects of
DJBS implantation on glycemic control, plasma GLP-1 levels and
L-cell numbers in diabetic rats, and thereby to explore the role of
intestinal L cells and their production in the DJBS
implantation-mediated remission of diabetes.
Materials and methods
Materials
Streptozotocin (STZ) was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and stored at −20°C in the dark.
STZ was dissolved in citric acid solution (pH 4.2–4.5) prior to
injection. Insulin was purchased from Tonghua Dongbao
Pharmaceutical Co., Ltd. (Jilin, China). Chloral hydrate was
purchased from Sigma-Aldrich and dissolved in 0.9% NaCl solution to
provide a final concentration of 10% prior to use. Dipeptidyl
peptidase-4 (DPPIV) inhibitor was purchased from Santa Cruz
Biotechnology (Dallas, TX, USA) and dissolved in DMSO to provide a
final concentration of 100 mM.
Animals and diet
In total, 40 Sprague-Dawley male rats weighing
200±10 g were obtained from Qinglongshan Experimental Animal Center
(Nanjing, China; permission no: SOXKLSU 2009-0001). The rats were
housed in a temperature- (22±2°C) and humidity (55±5%)-controlled
room and kept on a 12:12 light-dark cycle (light on at 06:00 a.m.).
Rats were kept in standard polypropylene cages (two rats/cage);
food and water were provided ad libitum unless otherwise
indicated. The study was also approved by the Animal Use and Care
Committee of Nanjing Medical University (Nanjing, China).
Development of type 2 diabetes in rats
with a high-fat diet and low-dose of STZ
After acclimating for 7 days, 8 rats were randomly
selected from the 40 rats and allocated to the normal diet control
group (Ncontrol, n=8) to receive standard rat chow (Qinglongshan
Experimental Animal Center) throughout the study. The other 32 rats
had access to a high-fat diet (containing 60% energy from fat;
Trophic Animal Feed High-Tech Co., Ltd., Nantong, China) ad
libitum to induce insulin resistance. All rats had free access
to the assigned diet and tap water for 8 weeks continuously. Rat
body weight was measured every 2 weeks, and fasting glucose was
tested every 4 weeks using a hand glucometer (One Touch Ultra;
Lifescan, Johnson & Johnson, Chesterbrook, PA, USA). At week 8,
all rats received an oral glucose tolerance test (OGTT) and insulin
tolerance test (ITT); rats with impaired OGTT and ITT were selected
to receive a low-dose intraperitoneal injection of STZ (35 mg/kg)
after overnight fasting. Citrate buffer (vehicle) alone was
injected into control rats; normal diet-fed rats also received same
dose of STZ. One week after the STZ injection, rats with
non-fasting plasma glucose ≥16.7 mmol/l were considered diabetic
(27) and randomized into three
groups according to the plasma glucose level: Diabetes mellitus
control (DMcontrol, n=8); diabetes mellitus sham surgery (DMsham,
n=8), which underwent a sham surgery; and diabetes mellitus with
DJBS implantation group (DMdjbs, n=8). Following the DJBS placement
surgery, all diabetic rats were fed with normal standard rat chow
until the end of the experiment. At 12 weeks after DJBS
implantation, all rats were sacrificed with 10% chloral hydrate
(Nanjing Shenbeijia Biotechnology Co, Ltd., Nanjing, China) (2
ml/100 g body weight) and intestinal tissues were collected and
fixed in 4% formaldehyde for paraffin embedding or tissue
homogenation.
DJBS implantation and sham
surgery
The DJBS was made by Garson Medical Stent Apparatus
Co., Ltd. (Changzhou, China) with reference to the tubes used by
Aguirre et al (26). The DJBS
is a nutrient-impermeable, flexible tube with a self-expandable
metal anchor crown at the proximal end. The length and diameter of
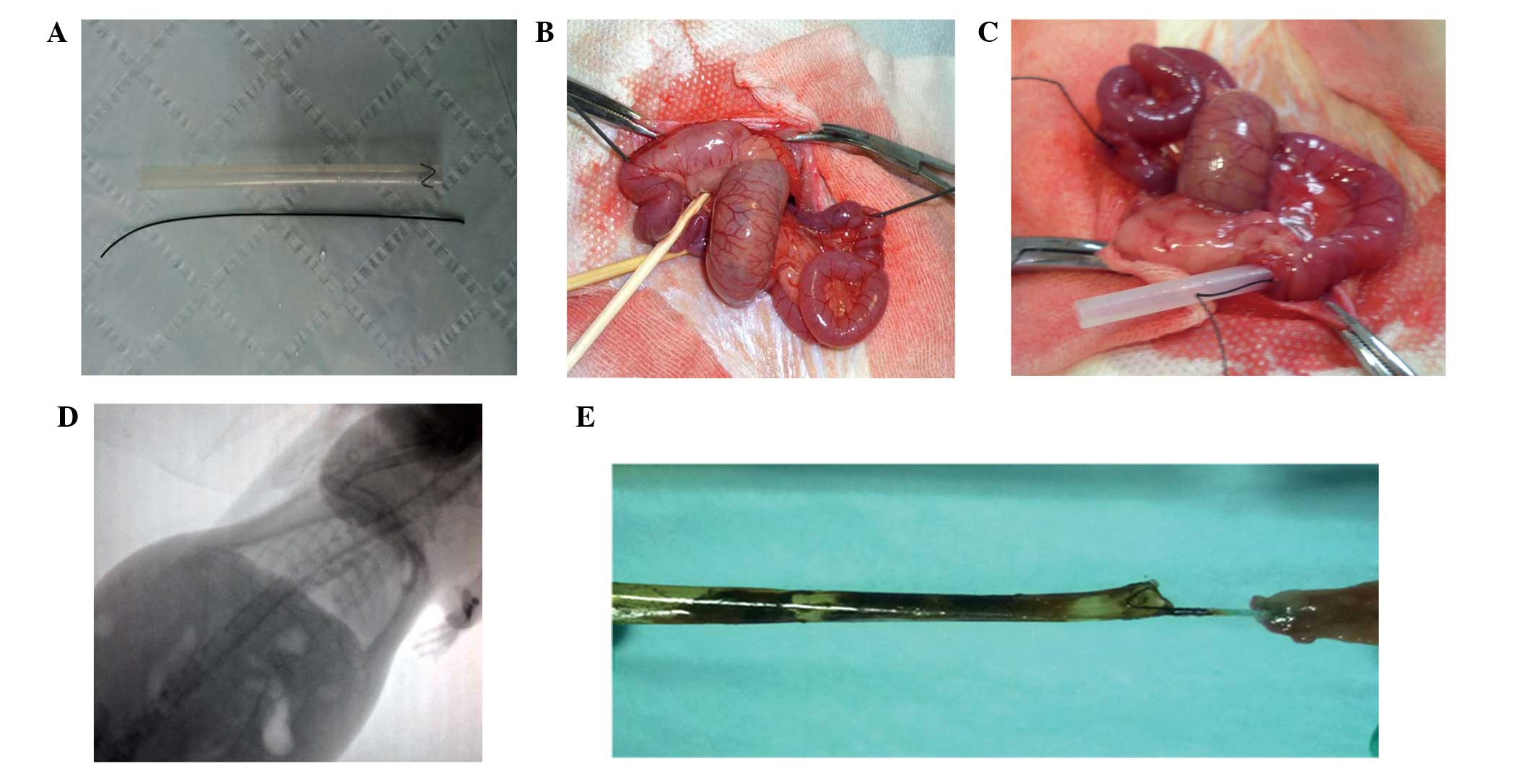
the tube are 10 cm and 5.5 mm, respectively (Fig. 1A). After release, the metal crown of
the tube anchors to the rat duodenal bulb and the soft sleeve
unfolds completely to the proximal jejunum below the Trietz
ligament. Thus chyme from the stomach can be delivered directly to
the proximal jejunum without contacting the mucosa of the proximal
intestine, and the mixing of chyme with bile and pancreatic juice
is also delayed until the proximal jejunum.
After overnight fasting, rats were anesthetized by
the administration of 10% chloral hydrate at a dose of 3 ml/kg body
weight. A midline laparotomy was made and the proximal intestine
was released from the ligament of Trietz. A 5-mm-long incision was
made in the front wall of the gastric antrum, and then a 0.035-inch
hydrophilic guide-wire with a soft tip was smoothly inserted from
the incision without injuring the enteron until it was 2 cm below
the Trietz ligament. When the tip of the guide-wire was 2 cm below
the Trietz ligament, the jejunum was punctured by a needle just
opposite to the tip of the guide-wire, and the guide-wire was
pulled from the puncture hole (Fig.
1B). The distal end of the DJBS was then sutured to the other
tip of the guide-wire by a silk thread and the DJBS was pulled into
the intestinal lumen by withdrawal of the guide-wire and the
thread. When the sleeve was completely unfolded, the silk thread
was cut near to the sleeve without pulling the sleeve out of the
intestinal wall (Fig. 1C). The two
incisions were then repaired, and the crown of the DJBS was moved
to the duodenum bulb and sutured to the gastric antrum wall with a
nylon thread. Finally, all incisions were closed by thread suture.
The sham surgery consisted of laparotomy with release of the
proximal intestine from the ligament of Trietz, and also gastric
antrum incision, guide-wire insertion and jejunum puncture, but did
not include the DJBS placement. The duration of anesthesia was
standardized to 1 h for both surgical groups. After surgery, rats
were fasted for 1 day and then fed a liquid diet for 2 days before
being transferred to normal chow. Starting during post-operative
week 2 (pow2), animals were evaluated by fluoroscopy weekly to
ensure that the DJBS remained in place (Fig. 1D). The position of the DJBS and that
the sleeve film remained intact were eventually verified by
necropsy (Fig. 1E) after the rats
were sacrificed. The non-fasting blood glucose levels and body
weights of the rats were tested every 2 weeks after surgery.
OGTT
After overnight fasting, the OGTT test was
performed. A 50% glucose solution gavage (1 g/kg) was administered
and glucose levels in a blood sample taken from the tail vein were
measured using a glucometer (One Touch Ultra) at 0, 30, 60, 120 and
180 min after glucose administration. The area under the curve
(AUC) of glucose was calculated using the trapezoidal method.
ITT
After an overnight fasting, a dose of 0.4 IU/kg
human insulin (Tonghua Dongbao Pharmaceutical Co., Ltd., China) was
injected intraperitoneally into conscious rats. Blood glucose
levels were measured using a glucometer (One Touch Ultra) at
baseline and 30, 60, 90 and 120 min after insulin injection.
Plasma GLP-1 analysis
Prior to DJBS implantation (pow0) and at 12 weeks
after surgery (pow12), after an overnight fasting, all rats
received a glucose gavage (2 g/kg). At 20 min after glucose
administration, blood samples were collected from the tail vein of
all rats into an EDTA tube containing DPPIV inhibitor IV, K579
(cat. no. sc-202583; Santa Cruz Biotechnology). Blood samples were
centrifuged at 1,640 × g for 10 min and isolated plasma was stored
at −80°C for further detection. Plasma total GLP-1 levels were
tested with enzyme-linked immunosorbent assay (ELISA) kits specific
to rat GLP-1 (Santa Cruz Biotechnology) according to the
manufacturer's instructions.
Intestinal GLP-1 analysis
At 12 weeks after DJBS implantation, rat intestinal
tissue (proximal jejunum, distal ileum, middle colon and rectum)
were sampled under anesthesia for tissue homogenization. The
intestinal tissue supernatant was produced as described by Liu
et al (28). The GLP-1 level
of the intestinal tissue supernatant was tested by the same GLP-1
enzyme-linked immunosorbent assay kits as were used for plasma
analysis, according to the manufacturer's instructions.
Immunohistochemical staining and
GLP-1-positive cell count in intestinal tissues
Tissues from the proximal jejunum, distal ileum,
middle colon and rectum tissues were collected at 12 weeks after
the surgery. The tissues were fixed in 4% formalin for 24 h at 4°C
and then embedded in paraffin blocks. Serial sections (5 µm) were
cut from the paraffinized tissues. After dewaxing and rehydration,
antigen retrieval was conducted at 95°C with 1X phosphate-buffered
saline (PBS) buffer (pH 7.4) for 10 min using a microwave (Galanz,
Guangdong, China). Immunohistochemical staining was performed using
mouse monoclonal anti-GLP-1 (cat. no. sc-57166; Santa Cruz
Biotechnology: 1:50). The UltraVision Quanto Detection System HRP
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used
according to the manufacturer's instructions. After
immunohistochemical staining, sections were dehydrated through
graded ethanol and covered with a glass coverslip prior to
vitrification with xylene.
The number of GLP-1-positive cells was counted in
three non-serial cross-sections of proximal jejunum, distal ileum,
middle colon and rectum from each rat. Specific immunoreactive
cells were observed under a conventional light microscope[CX31;
Olympus (China) Co., Ltd., Beijing, China] and photomicrographs
were taken with a digital camera (Olympus Corporation, Tokyo,
Japan; final magnification, x200). The number of GLP-1-positive
cells was counted in 10 randomly chosen x200 fields in each section
for all animals, Only cells with definite nuclei were counted. All
values were presented as the mean ± standard deviation (SD). The
GLP-1 positive cell number of each section was expressed as the
number of GLP-1 positive cells per high-powered field (HP).
Statistical analysis
Data are presented as means ± SD. Data were analyzed
by repeated measures, analysis of variance (ANOVA) or multivariate
ANOVA (MANOVA), as appropriate. Glucose and insulin tolerance tests
were evaluated by AUC analysis. Pairwise comparisons between
different groups at different time points were analyzed by least
significant difference. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
conducted using the commercially available SPSS software package,
version 18.0 (SPSS, Inc., Chicago, IL, USA). Graphs were drawn
using Prism (GraphPad, San Diego, CA, USA).
Results
Effect of a high-fat diet on the
glucose and insulin tolerance of rats
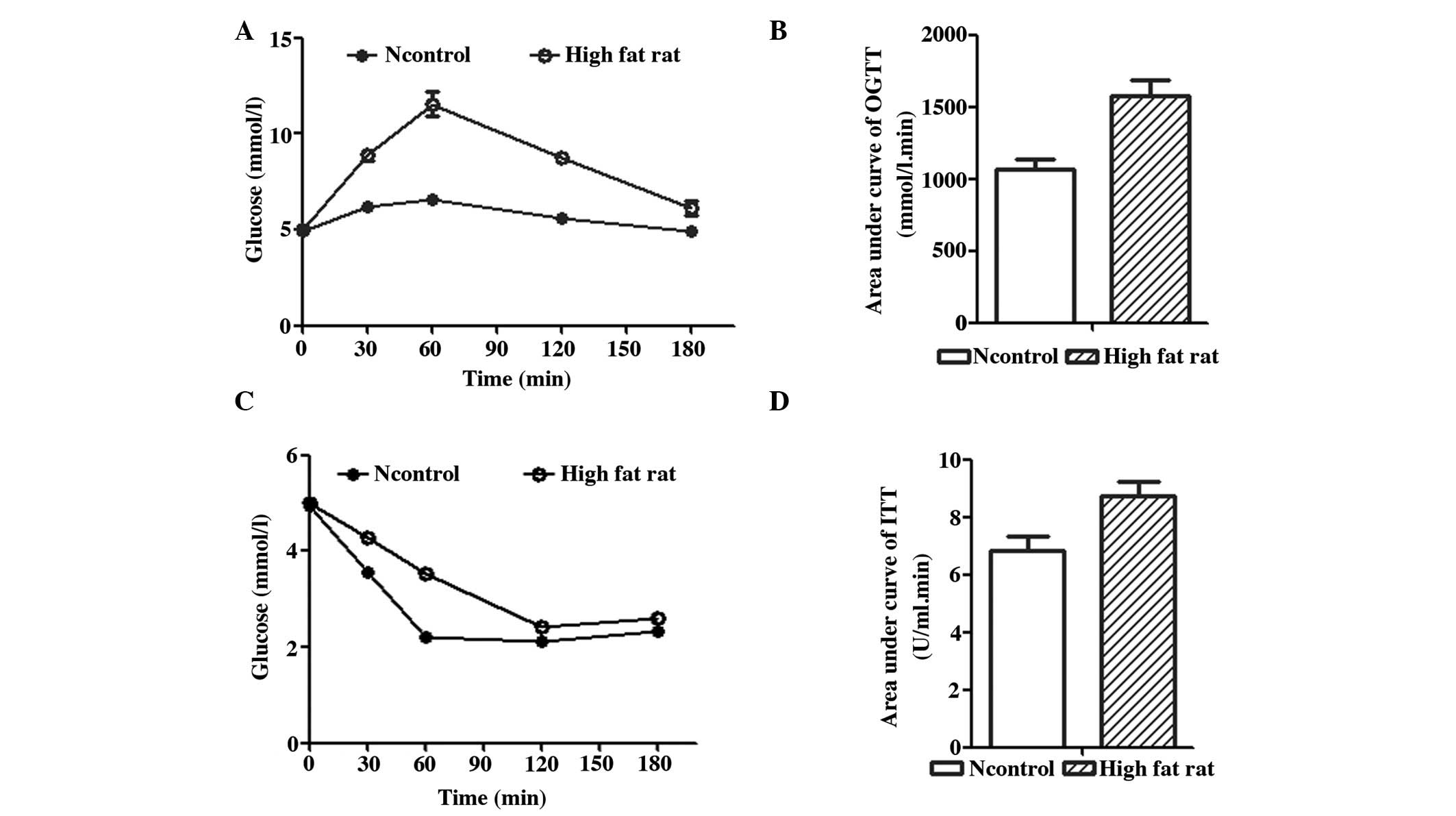
As shown in Fig. 2,
after being fed a high-fat diet for 8 weeks, rats exhibited insulin
resistance as evidenced by impaired oral glucose tolerance and
insulin tolerance (Fig. 2A and C).
In the OGTT, the plasma glucose level of the rats fed a high-fat
diet increased quickly and then decreased slowly (Fig. 1A). The AUC in the OGTT of rats fed a
high-fat diet (Fig. 2B) was
significantly higher than that of rats fed a normal diet
(1,576.50±106.24 mmol/L.min vs. 1,066.75±72.98 mmol/L.min; F=62.56;
P<0.01). In the ITT, the AUC of rats fed a high-fat diet
(Fig. 2D) also increased
significantly compared with that of rats fed a normal diet
(8.74±0.49 vs. 6.83±0.52 U/ml.min; F=28.68; P<0.01). The
impairment observed in the OGTT and ITT indicated that the rats fed
a high-fat diet were insulin resistant.
Characteristics of the rats with
diabetes induced by a high-fat diet and low-dose STZ injection
On the basis of insulin resistance, rats readily
developed overt diabetes mellitus following the injection of a low
dose of STZ (35 mg/kg). However, the same dose of STZ did not
influence the plasma glucose level of rats fed a normal diet (data
not shown). This rat model of diabetes is similar to T2DM in
humans. Diabetic rats presented the clinical characteristics of
polyphagia, polydipsia (Fig. 3C) and
polyuria as compared with the rats fed with a normal diet. The body
weights of the diabetic rats continued to decreased throughout the
experiment.
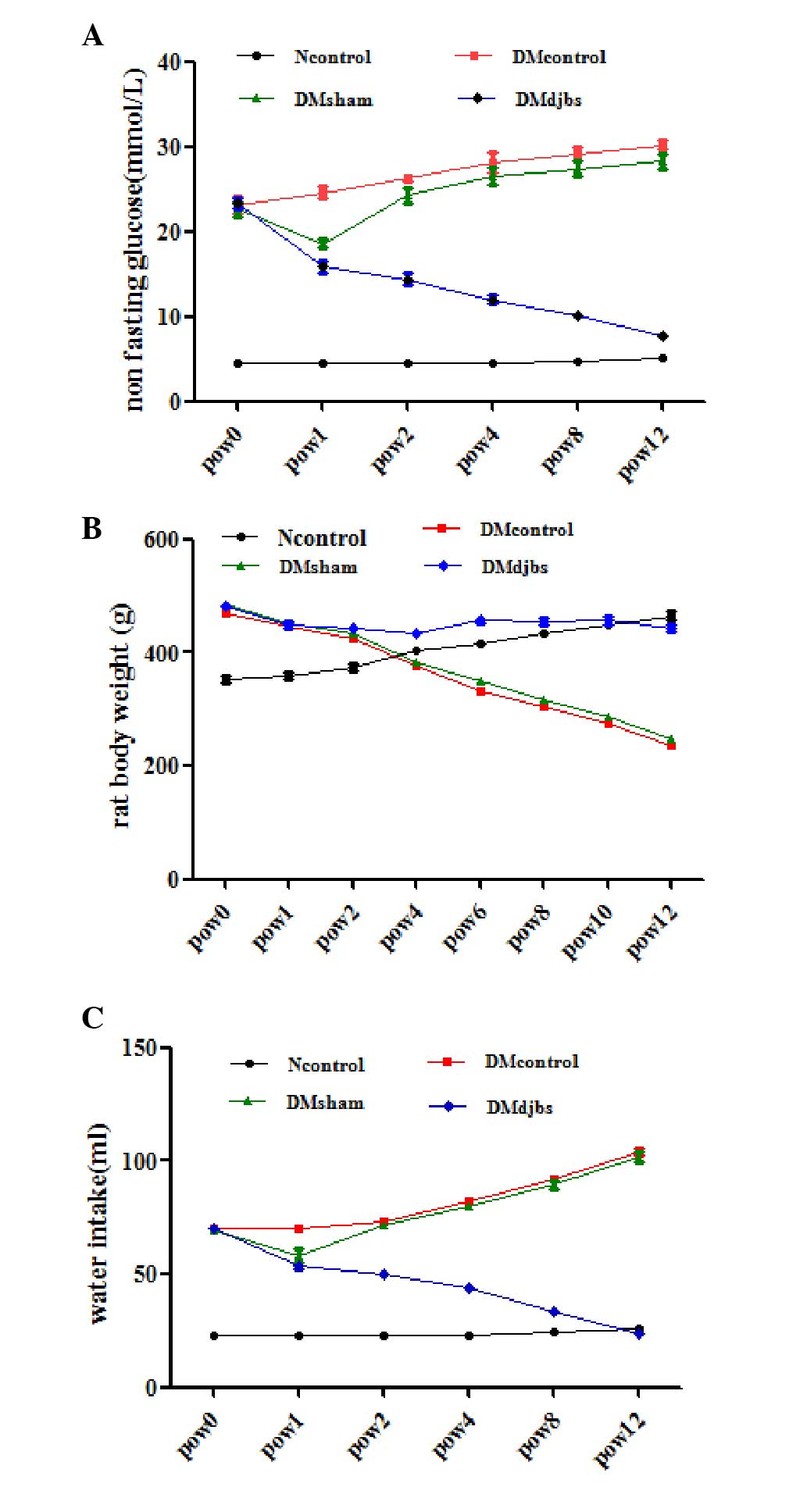 | Figure 3.(A) Non-fasting glucose levels of
rats in different groups. After DJBS placement for 12 weeks, the
glucose level of the DJBS-implanted rats decreased significantly
compared with that of the sham surgery and diabetic control rats
(F=70.697; P<0.01). (B) Body weights of DJBS-implanted rats
decreased slightly, while the body weights of the sham surgery and
diabetic control rats decreased significantly compared with those
of the DJBS-implanted rats and rats fed a normal diet (F=402.968,
P<0.01). (C) Water intake of the DJBS-implanted rats decreased
significantly compared with that of the sham surgery and control
rats (F=117.19, P<0.01). DJBS, duodenal-jejunal bypass sleeve;
Ncontrol, normal control; DMcontrol, diabetes mellitus control;
DMsham, diabetes mellitus sham surgery; DMdjbs, diabetes mellitus
with DJBS; pow, post-operative week. |
Effect of DJBS implantation on the
remission of diabetes mellitus in rats
At 12 weeks after DJBS implantation, the DJBS was
found to have remained intact and in position (Fig. 1D and E). Prior to DJBS implantation,
no difference in plasma glucose level was observed among the
DJBS-implanted, sham surgery and control diabetic rats (Fig. 3A; 23.3±1.6 vs. 22.7±2.2 vs. 23.1±2.4
mmol/l; P>0.05). At 12 weeks after DJBS implantation, the
non-fasting glucose level of the DJBS-implanted rats decreased
significantly from 23.3±1.6 to 7.7±0.8 mmol/l, but that of the rats
in the sham surgery and diabetic control groups continued to rise
(Fig. 3A). The glucose levels of
rats in the four groups were significantly different (Fig. 3A; F=70.697; P<0.01) 12 weeks after
surgery. The plasma glucose level of the DJBS-implanted rats was
significantly lower than that of the sham surgery and diabetic
control rats (Fig. 3A; 7.7±0.8 vs.
30.2±1.1 vs. 29.4±1.7 mmol/l; F=862.99; P<0.01). Pairwise
comparison showed no difference in plasma glucose level between the
diabetic control and sham surgery rats (30.2±1.1 vs. 29.4±1.7
mmol/l; P>0.05). In parallel with the reduction in glucose
levels, the symptoms of polyphagia, polydipsia (Fig. 3C) and polyuria were all relieved
completely 12 weeks after DJBS placement; however, the symptoms of
the sham surgery and diabetic control rats continued to deteriorate
(Fig. 3A and C).
Following DJBS implantation, the body weights of the
DJBS-implanted diabetic rats decreased only slightly, while the
rats in the diabetic control and sham surgery groups continued to
lose weight at a greater rate. At pow12, the body weights of the
rats in the sham surgery and diabetic control groups were
significantly lower than those of the DJBS-implanted and
normal-diet-fed control groups (Fig.
3B; 246.5±6.6 vs. 237±12.9 vs. 442.8±14.3 vs. 465±18.3 g;
F=402.968; P<0.01.).
Effect of DJBS implantation on the
plasma and intestinal GLP-1 levels of rats
Prior to DJBS placement, the plasma GLP-1 levels of
the diabetic rats declined markedly compared with those of the
normal-diet-fed rats following glucose administration. However, at
12 weeks after DJBS placement, the plasma GLP-1 levels of the
DJBS-implanted rats were significantly higher than those of the
rats in the sham surgery and diabetic control groups (Fig. 4A; 4.87±1.06 vs. 2.17±0.11 vs.
2.19±0.10 pmol/l; F=106.37; P<0.01,). The GLP-1 concentration in
the distal ileum of the DJBS-implanted rats was also higher
compared with that of the rats in the control and sham surgery
groups (Fig. 4B; 88.4±4.9 vs.
60.8±4.4 vs. 59.4±3.5 pmol/g; F=55.423; P<0.01.). However, no
significant differences in GLP-1 level were found in tissue from
the proximal jejunum, middle colon and rectum. DJBS placement
increased the GLP-1 levels of diabetic rats in the plasma and
distal ileum tissue.
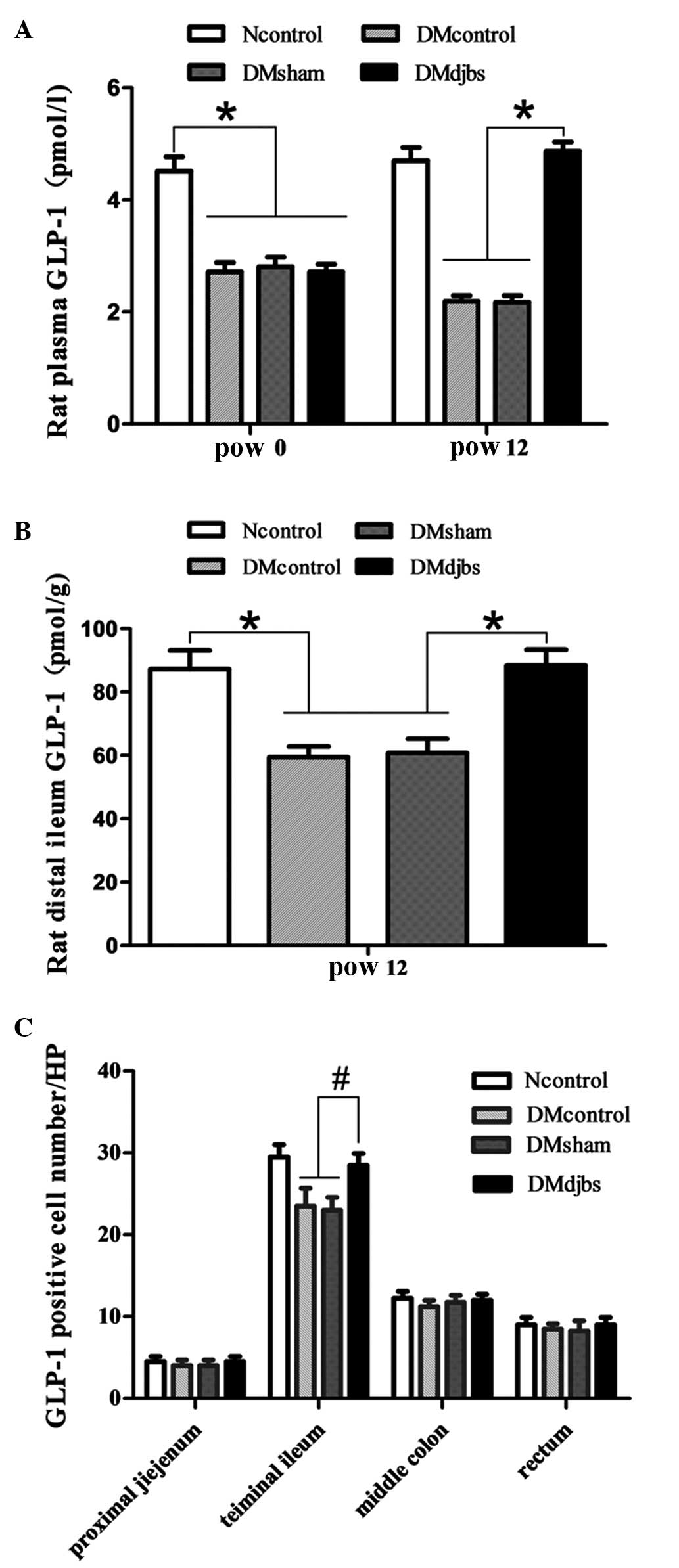 | Figure 4.(A) Plasma GLP-1 levels of diabetic
rats were lower than those of rats fed a normal diet at pow0
(F=106.37; *P<0.01). After DJBS placement for 12 weeks, the
plasma GLP-1 levels of DJBS-implanted rats increased significantly
compared with those of diabetic control and sham surgery rats
(F=423.237; P<0.01); the GLP-1 levels of the sham surgery and
diabetic control rats continued to decrease. (B) The GLP-1
concentration in the distal ileum tissue of DJBS-implanted rats was
significantly higher than that of diabetic control and sham surgery
rats (F=55.423; *P<0.01). (C) The GLP-1-positive cell number in
the distal ileum tissue differed significantly among the four
groups (F=5.826; #P<0.05). No significant difference
was found in the proximal jejunum (F=0.08; P>0.05), middle colon
(F=2.56; P>0,05) or rectum (F=0.56; P>0.05). GLP,
glucagon-like peptide; DJBS, duodenal-jejunal bypass sleeve;
Ncontrol, normal control; DMcontrol, diabetes mellitus control;
DMsham, diabetes mellitus sham surgery; DMdjbs, diabetes mellitus
with DJBS; pow, post-operative week. |
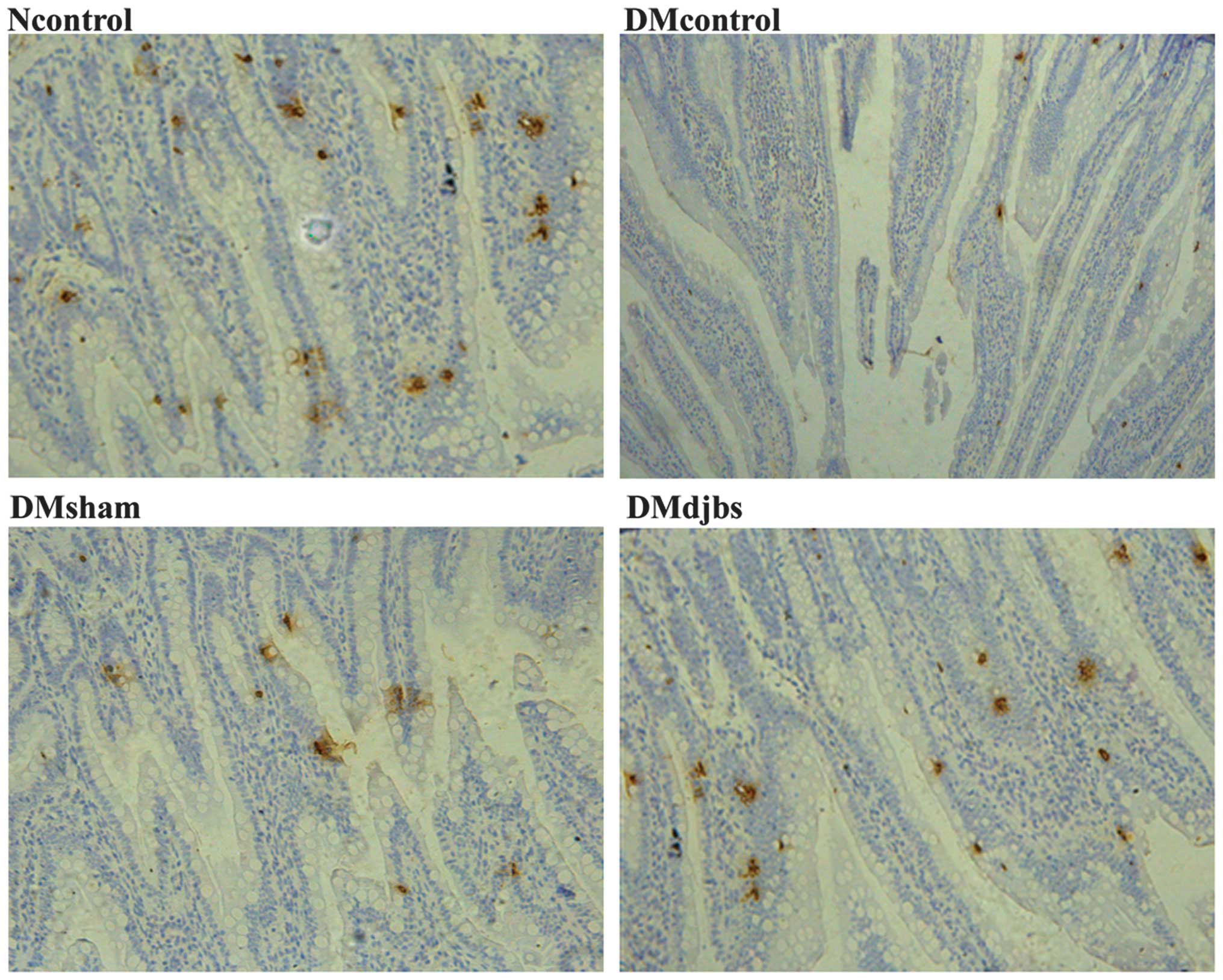
Number of GLP-1-positive cells
As shown in Fig. 5,
GLP-1 was mainly expressed by the intestinal glands. The number of
the GLP-1-positive cells increased from the proximal to the distal
intestine. When the DJBS implant had been in place for 12 weeks,
the GLP-1 positive cell number increased significantly in the
distal ileum of the DJBS-implanted diabetic rats (F=5.826;
P<0.05). The total number of GLP-1-positive cells in the distal
ileum of the Ncontrol, DMcontrol, DMsham and DMdjbs groups was
29.5±3.1, 23.5±4.4, 23.0±3.2 and 31.0±2.6/HP, respectively
(Fig. 4C). However, no difference in
GLP-1-positive cell number among the groups was found in the tissue
from the proximal jejunum, colon and rectum.
Discussion
GLP-1 is an important gut hormone involved in
diabetes. The physiological effects of GLP-1 include stimulation of
insulin biosynthesis and secretion, promotion of β-cell
proliferation, reduction of food intake and inhibition of glucagon
secretion (29,30). Circulating GIP and GLP-1 levels are
very low in the fasting state and rapidly increase following food
ingestion. In patients with T2DM or impaired glucose tolerance, the
plasma GLP-1 level is reduced compared with that in normal
individuals (31,32). However, following bypass of the
duodenum and jejunum by surgery (33) or luminal sleeve (16), GLP-1 secretion increases again in
diabetic patients. This indicates that proximal small intestine
bypass can restore impaired GLP-1 secretion in diabetic patients.
In the present study, it was also found at that at 12 weeks after
DJBS implantation, the glucose-stimulated GLP-1 secretion was
greater in the rats with DJBS placement than in diabetic control
and sham surgery rats (4.87±1.06 vs. 2.17±0.11 vs. 2.19±0.10
pmol/l; F=106.37; P<0.01; Fig.
3A). The GLP-1 level of the DJBS-implanted diabetic rats
increased from 2.72±0.14 prior to surgery to 4.87±0.17 pmol/l,
which was comparable with the GLP-1 level in normal rats; however,
the rats in the diabetic control and sham surgery groups did not
exhibit any improvement. In addition to the increase in plasma
GLP-1 levels, the GLP-1 concentration in the distal ileum was also
significantly higher than that in the rats of the diabetic control
and sham surgery groups (Fig. 3B).
Although increases of anorexigenic gut hormones such as GLP-1 have
been observed in diabetic patients and obese rats following DJBS
implantation (16,26), it is not clear whether increases of
circulating GLP-1 levels are associated with enhanced stimulation
or proliferation of the enteroendocrine cells or a combination of
these factors.
In order to investigate whether the increase in
GLP-1 levels after DJBS implantation was associated with an
increase in the number of intestinal L cells, changes in the
numbers of intestinal GLP-1-positive cells were also observed, and
an increase in the number of GLP-1-positive cells in the rat distal
ileum mucosa was observed 12 weeks after DJBS placement. An
increase in the number of L cells has previously been observed in
rats following RYGB surgery (25),
since DJBS mimics certain effects of RYGB surgery, and the results
of the present study are similar those previously observed in
RYGB-implanted rats (25). However,
in the present study, an increase in the number of L cells was only
found in the distal ileum. This may be because the L-cell density
is the highest in the rat distal ileum, and the observation time
was not long enough.
The secretion of GLP-1 is influenced greatly by the
rate (34) and load (35) of nutrient entry into the small
intestine, the flow patterns within it (36), and also the length of small intestine
exposed to nutrition (37); GLP-1
secretion is dependent upon >60 cm of the intestine being
exposed to glucose (37), and GLP-1
release is prolonged when sucrose reaches the colon (38). Furthermore, the secretion of GLP-1 in
response to sucrose is increased when malabsorption is induced by
the α-glucosidase inhibitor acarbose (39), which presumably allows stimulation of
a greater length or more distal region of the gut by ingested
sugar.
Following DJBS implantation, the duodenum and
proximal jejunum mucosa are isolated from partially ingested
nutrients delivered from the stomach; therefore, the mucosa of the
proximal jejunum is directly exposed to partially digested
nutrients. The mixing of chyme with bile and pancreatic juice is
also delayed until the proximal jejunum. Thus, a direct stimulatory
effect of chyme on the duodenal mucosa is absent, but the
stimulatory effect of partially digested chyme on the mucosa of the
proximal jejunum is improved. The enteroendocrine cells distributed
in the digestive tract may detect the improved stimulatory effect
of the partially digested alimentary flow and boost hormone
secretion. In the present study, it was found that the levels of
GLP-1 in the plasma and intestinal tissue increased 12 weeks after
DJBS implantation. The increased stimulatory effect of partially
digested nutrients on intestinal L cells might lead to the increase
in GLP-1 levels in diabetic rats after DJBS implantation.
Following the placement of a DJBS in the duodenum
and proximal jejunum, interaction between chyme, bile and
pancreatic juice is avoided. Also the absorption of nutrients is
delayed to the jejunum below the end of the DJBS; thus, partially
digested nutrients will arrive at the small intestine at greater
concentrations and contact the intestinal mucosa for a longer
distance. The intestinal mucosa will, therefore, interact with
greater quantities of incompletely digested nutrients, and the
increased stimulatory effect on L cells may lead to an increase in
GLP-1 secretion. Since L cells produce both GLP-1 and GLP-2, and
GLP-2 can promote growth of the intestinal mucosa, it is possible
that the more strongly stimulated L cells also release more GLP-2,
which will stimulate the growth of the intestinal mucosa. Thus,
hypertrophy of the intestinal mucosa will increase the total number
of L cells. Hypertrophy-dependent doubling of L cells has been
observed in rats following Roux-en-Y gastric bypass surgery
(25); however, in the present
study, the GLP-1-positive cell number was found to increase only in
the rat distal ileum after DJBS placement for 12 weeks. This is
probably due to the observation time not being long enough and the
L-cell density being highest in the rat distal ileum. Whether the
increased number of GLP-1 positive cells is associated with
hypertrophy of the intestinal mucosa or L-cell proliferation
requires further investigation.
In the present study, only the change in the number
of L cells and the association with plasma GLP-1 level were
investigated; the expression of GLP-1 at the genetic level was not
tested. However, the GLP-1 level was detected by ELISA in
intestinal tissues and blood. An ELISA is able to determine GLP-1
concentration quantitatively. The increased blood GLP-1 level might
be attributed to the increased L-cell number in the distal
ileum.
In summary, DJBS placement can effectively induce
glycemic control in rats with diabetes induced by a high-fat diet
and low-dose STZ injection; 12 weeks after DJBS implantation, the
diabetes mellitus was relieved completely. Concurrently, the GLP-1
level and intestinal L-cell number also increased markedly compared
with those in the sham surgery and control groups. DJBS
implantation-induced changes to alimentary nutritional digestion
and absorption may enhance the stimulation of L cells, thereby
boosting GLP-1 secretion and contributing to the DJBS-induced
remission of diabetes in rats. However, the specific molecular
entities that stimulate the L cells and the exact pathway involved
in boosting the GLP-1 release require further investigation.
Acknowledgements
This study was funded by Jiangsu Province Social
Development Program (BL2012031) and the State Key Laboratory of
Fluid Power Transmission and Control open fund (GZKF-201022). The
authors thank Professor Fan for his direction of the experiments,
and Ying Zhang for assistance in completing the difficult DJBS
implantation surgery.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Prac. 87:4–14. 2010. View Article : Google Scholar
|
|
2
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nissen SE and Wolski K: Rosiglitazone
revisited: An updated meta-analysis of risk for myocardial
infarction and cardiovascular mortality. Arch Intern Med.
170:1191–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loke YK, Kwok CS and Singh S: Comparative
cardiovascular effects of thiazolidinediones: Systematic review and
meta-analysis of observational studies. BMJ. 342:d13092011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
UK Prospective Diabetes Study (UKPDS)
Group, . Intensive blood-glucose control with sulphonylureas or
insulin compared with conventional treatment and risk of
complications in patients with type 2 diabetes (UKPDS 33). Lancet.
352:837–853. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buchwald H and Oien DM:
Metabolic/bariatric surgery worldwide 2008. Obes Surg.
19:1605–1611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buchwald H, Estok R, Fahrbach K, Banel D,
Jensen MD, Pories WJ, Bantle JP and Sledge I: Weight and type 2
diabetes after bariatric surgery: Systematic review and
meta-analysis. Am J Med. 122:248–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mingrone G, Panunzi S, De Gaetano A,
Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M,
Ghirlanda G and Rubino F: Bariatric surgery versus conventional
medical therapy for type 2 diabetes. N Engl J Med. 366:1577–1585.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dogan K, Betzel B, Homan J, Aarts EO,
Ploeger N, de Boer H, Aufenacker TJ, van Laarhoven CJ, Janssen IM
and Berends FJ: Long-term effects of laparoscopic Roux-en-Y gastric
bypass on diabetes mellitus, hypertension and dyslipidaemia in
morbidly obese patients. Obes Surg. 24:1835–1842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams TD, Gress RE, Smith SC, Halverson
RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM and Hunt SC:
Long-term mortality after gastric bypass surgery. N Engl J Med.
357:753–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Encinosa WE, Bernard DM, Steiner CA and
Chen CC: Use and costs of bariatric surgery and prescription
weight-loss medications. Health Aff (Millwood). 24:1039–1046. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodriguez L, Reyes E, Fagalde P, Oltra MS,
Saba J, Aylwin CG, Prieto C, Ramos A, Galvao M, Gersin KS and Sorli
C: Pilot clinical study of an endoscopic, removable
duodenal-jejunal bypass liner for the treatment of type 2 diabetes.
Diabetes Technol Ther. 11:725–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milone L, Gagner M, Ueda K, Bardaro SJ and
Ki-Young Y: Effect of a polyethylene endoluminal duodeno-jejunal
tube (EDJT) on weight gain: A feasibility study in a porcine model.
Obes Surg. 16:620–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gersin KS, Keller JE, Stefanidis D, Simms
CS, Abraham DD, Deal SE, Kuwada TS and Heniford BT: Duodenal-
jejunal bypass sleeve: A totally endoscopic device for the
treatment of morbid obesity. Surg Innov. 14:275–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schouten R, Rijs CS, Bouvy ND, Hameeteman
W, Koek GH, Janssen IM and Greve JW: A multicenter, randomized
efficacy study of the EndoBarrier gastrointestinal liner for
presurgical weight loss prior to bariatric surgery. Ann Surg.
251:236–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Jonge C, Rensen SS, Verdam FJ, Vincent
RP, Bloom SR, Buurman WA, le Roux CW, Schaper NC, Bouvy ND and
Greve JW: Endoscopic duodenal-jejunal bypass liner rapidly improves
type 2 diabetes. Obes Surg. 23:1354–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mumphrey MB, Patterson LM, Zheng H and
Berthoud HR: Roux-en-Y gastric bypass surgery increases number but
not density of CCK-, GLP-1-, 5-HT- and neurotensin-expressing
enteroendocrine cells in rats. Neurogastroenterol Motil.
25:e70–e79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muñoz R, Carmody JS, Stylopoulos N, Davis
P and Kaplan LM: Isolated duodenal exclusion increases energy
expenditure and improves glucose homeostasis in diet-induced obese
rats. Am J Physiol Regul Integr Comp Physiol. 303:R985–R993. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holst JJ: Enteroendocrine secretion of gut
hormones in diabetes, obesity and after bariatric surgery. Curr
Opin Pharmacol. 13:983–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holst JJ and Gromada J: Role of incretin
hormones in the regulation of insulin secretion in diabetic and
nondiabetic humans. Am J Physiol Endocrinol Metab. 287:E199–E206.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perley MJ and Kipnis DM: Plasma insulin
responses to oral and intravenous glucose: Studies in normal and
diabetic sujbjects. J Clin Invest. 46:1954–1962. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McIntyre N, Holdsworth CD and Turner DS:
New interpretation of oral glucose tolerance. Lancet. 4:20–21.
1964. View Article : Google Scholar
|
|
23
|
Morgan LM: The role of the entero-insular
axis in insulin secretion. Biochem Soc Trans. 18:101–102. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eissele R, Göke R, Willemer S, Harthus HP,
Vermeer H, Arnold R and Göke B: Glucagon-like peptide-1 cells in
the gastrointestinal-tract and pancreas of rat, pig and man. Eur J
Clin Invest. 22:283–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hansen CF, Bueter M, Theis N, Lutz T,
Paulsen S, Dalbøge LS, Vrang N and Jelsing J: Hypertrophy dependent
doubling of L-cells in Roux-en-Y gastric bypass operated rats. PLoS
One. 8:656962013. View Article : Google Scholar
|
|
26
|
Aguirre V, Stylopoulos N, Grinbaum R and
Kaplan LM: An endoluminal sleeve induces substantial weight loss
and normalizes glucose homeostasis in rats with diet-induced
obesity. Obesity (Silver Spring). 16:2585–2592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu SZ, Deng YX, Chen B, Zhang XJ, Shi QZ
and Qiu XM: Antihyperglycemic effect of the traditional Chinese
scutellaria-coptis herb couple and its main components in
streptozotocin-induced diabetic rats. J Ethnopharmacol.
145:490–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tolhurst G, Reimann F and Gribble FM:
Nutritional regulation of glucagon-like peptide-1 secretion. J
Physiol. 587:Pt 127–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gautier JF, Choukem SP and Girard J:
Physiology of incretins (GIP and GLP-1) and abnormalities in type 2
diabetes. Diabetes Metab. 34 (Suppl 2):S65–S72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vilsbøll T, Krarup T, Deacon CF, Madsbad S
and Holst JJ: Reduced postprandial concentrations of intact
biologically active glucagon like peptide 1 in type 2 diabetes
patients. Diabetes. 50:609–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
ToftNielsen MB, Damholt MB, Madsbad S,
Hilsted LM, Hughes TE, Michelsen BK and Holst JJ: Determinants of
the impaired secretion of glucagon-like peptide-1 in type 2
diabetes patients. J Clin Endocrinol Metab. 86:3717–3723. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dar MS, Chapman WH III, Pender JR, Drake
AJ III, O'Brien K, Tanenberg RJ, Dohm GL and Pories WJ: GLP-1
response to a mixed meal: What happens 10 years after Roux-en-Y
gastric bypass (RYGB)? Obes Surg. 22:1077–1083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chaikomin R, Doran S, Jones KL,
FeinleBisset C, O'Donovan D, Rayner CK and Horowitz M: Initially
more rapid small intestinal glucose delivery increases plasma
insulin, GIP and GLP-1 but does not improve overall glycemia in
healthy subjects. Am J Physiol Endocrinol Metab. 289:E504–E507.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pilichiewicz AN, Chaikomin R, Brennan IM,
Wishart JM, Rayner CK, Jones KL, Smout AJ, Horowitz M and
Feinle-Bisset C: Load-dependent effects of duodenal glucose on
glycemia, gastrointestinal hormones, antropyloroduodenal motility
and energy intake in healthy men. Am J Physiol Endocrinol Metab.
293:E743–E753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chaikomin R, Wu KL, Doran S, Jones KL,
Smout AJ, Renooij W, Holloway RH, Meyer JH, Horowitz M and Rayner
CK: Concurrent duodenal manometric and impedance recording to
evaluate the effects of hyoscine on motility and flow events,
glucose absorption and incretin release. Am J Physiol Gastrointest
Liver Physiol. 292:G1099–G1104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Little TJ, Doran S, Meyer JH, Smout AJ,
O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M and
Feinle-Bisset C: The release of GLP-1 and ghrelin, but not GIP and
CCK, by glucose is dependent upon the length of small intestine
exposed. Am J Physiol Endocrinol Metab. 291:E647–E655. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gentilcore D, Bryant B, Wishart JM, Morris
HA, Horowitz M and Jones KL: Acarbose attenuates the hypotensive
response to sucrose and slows gastric emptying in the elderly. Am J
Med. 118:12892005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qualmann C, Nauck MA, Holst JJ, Orskov C
and Creutzfeldt W: Glucagon-like peptide 1 (7–36 amide) secretion
in response to luminal sucrose from the upper and lower gut. A
study using alpha-glucosidase inhibition (acarbose). Scand J
Gastroenterol. 30:892–896. 1995. View Article : Google Scholar : PubMed/NCBI
|