Introduction
Hepatocellular carcinoma (HCC) is among the most
common cancer types worldwide, with a global incidence exceeding
600,000 new cases per year (1,2).
Resection and ablation remain the primary treatments for HCC;
however, recurrence is common after curative resection or ablation,
and is responsible for the majority of patient mortality.
Microvascular invasion and micrometastasis are frequently
responsible for the post-operative recurrence of HCC (3,4). Drugs
designed to limit HCC typically exhibit intrinsic hepatotoxicity
that may further compromise liver function. In addition, HCC cells
often express multi-drug resistant proteins, which reduce the
efficacy of chemotherapeutics and small molecule drugs for
preventing the recurrence of HCC (5).
Due to its favorable safety and efficacy profile,
immunotherapy has increasingly been investigated as an innovative
adjuvant treatment for the prevention of HCC recurrence. Four
randomized controlled trials of HCC immunotherapy following
treatments, including resection and transcatheter arterial
chemoembolization (TACE) combined with radiofrequency ablation
(RFA), have been conducted (6–9). These
trials report that immunotherapy was able to increase the rate of
recurrence free survival (RFS); however, immunotherapy did not
significantly improve overall survival (OS) rates (10).
However, a number of issues concerning HCC
immunotherapy remain unclear. The majority of published studies
describe an immunotherapy treatment using a single type of immune
cell (6–9). The combined effects of different types
of successively-administered immune cells, which is more
representative of the native immune response to tumor cells,
remains to be determined. In addition, the immune cell infusion
protocol may potentially be adjusted to complement the specific
patient immune status. A dynamic protocol, applied throughout the
recovery period, may achieve a more beneficial outcome compared
with previously-published fixed protocols applied over a relatively
short period of time (6–9,11).
Finally, studying the precise changes in immune and liver function
throughout the therapy may further clarify the impact of
immunotherapy.
The present authors have previously reported the
outcome of a phase I clinical study of combination therapy with
microwave ablation (MWA) and immunotherapy in patients with HCC
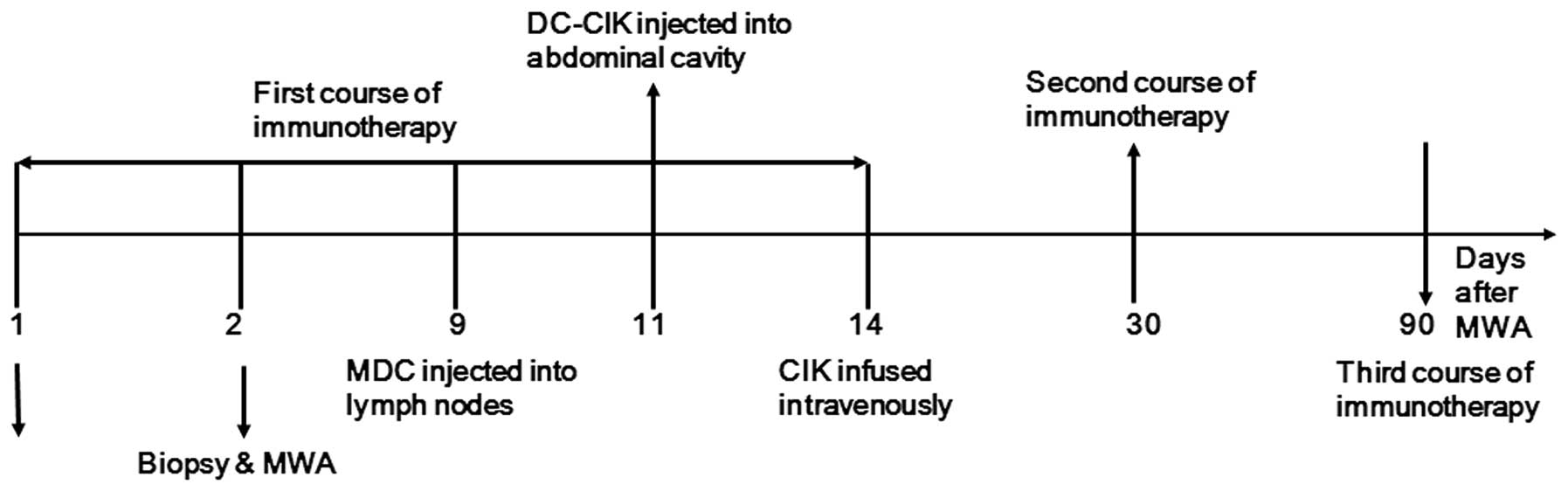
(12). Three courses of
immunotherapy were applied, including immature dendritic cells
(DCs), cytokine-induced killer (CIK) cells and cytotoxic T
lymphocytes (CTLs), within three months following radical MWA of
the HCC. The preliminary conclusion of the study was that this
adoptive immunotherapy was safe and successfully ameliorated the
loss of peripheral lymphocytes (12). Immature DCs were injected into the
marginal area of ablated tumors, myeloid dendritic cells (MDC) were
injected into groin lymph nodes, DC-CIK cells and CTLs were
injected into the abdominal cavity, and CIK cells were infused
intravenously. The results indicated that immunotherapy soon after
MWA is a safe procedure that is capable of reducing the viral load
in >50% of the patients. The percentage of
CD4+CD25+ regulatory T lymphocytes decreased
significantly, while the percentage of CD8+ effector T
cells was increased significantly in the peripheral blood following
therapy (12).
In the present study, an adjusted protocol was
employed, which involved a prolonged course of immunotherapy and an
expanded range of observational indices. The aim of this study was
to investigate the influence of immunotherapy on the immune and
liver function, rate of tumor recurrence and mortality in patients
with HCC.
Patients and methods
Study design and patients
This single-center, open-label phase II study of MWA
followed by adoptive immunotherapy in HCC patients was approved by
the Ethics Committee of Chinese PLA General Hospital (Beijing,
China).
A total of 29 patients with HCC that were treated at
the Department of Interventional Ultrasound in the Chinese PLA
General Hospital between February 2009 and December 2010 were
enrolled in this study. HCC was diagnosed and staged according to a
previously-reported schedule (5).
For each patient, a minimum of two contrast-enhanced ultrasound and
computed tomography (CT)/magnetic resonance imaging (MRI) images
were obtained and analyzed. The inclusion criteria were as follows:
i) Single nodular hepatic tumor with a maximum diameter of 5 cm;
ii) ≤3 nodular hepatic tumors with a maximum dimension of 3 cm;
iii) absence of portal vein thrombosis or extrahepatic metastases;
iv) Child-Pugh classification of A or B (13); v) tumor accessible via a percutaneous
approach (14); vi) cirrhosis with
chronic hepatitis B or C; and vii) >10 g/dl hemoglobin levels, a
white blood cell count of >2×109/l, a platelet count
of >75×109/l, a serum creatinine level of <110
µmol/l, <3 times the upper limit of aspartate aminotransferase
(AST), <2.5 times the upper limit of serum bilirubin, and a
prothrombin time of <19 sec. The exclusion criteria were as
follows: i) Pregnant or breast-feeding; ii) psychiatric problems,
addiction or any other disorder that prevented informed consent;
iii) active uncontrolled infection; iv) concurrent systemic
corticosteroid treatment; v) systemic autoimmune disease; vi)
clinically significant ischemic heart disease or cardiac failure;
vii) chemotherapy or radiotherapy within the preceding 6 months;
and viii) <3 courses of immunotherapy. Written informed consent
was obtained from all the subjects.
MVA treatment and grouping
All treatments were performed using sonographic
guidance. The instruments, technique and protocol of MWA used in
this study are the same as those previously reported (12). Among the 29 eligible patients, 14
were randomly selected to receive immunotherapy following MWA
(immunotherapy group) and 15 patients not to receive any
post-ablation adjuvant therapy (control group).
Immunotherapy protocol
In order to reduce the liver injury resulting from
puncture that was reported in the previous study (12), the protocol was modified as follows:
Immunocytes were isolated from 45 ml peripheral blood on the
morning of the day of ablation. Tumor tissue was obtained by a
sonography-guided biopsy (18G, BARD, Bard International Inc.,
Covington, GA, USA) using an 18-gauge needle prior to MWA for
pathological diagnosis and lysate preparation. After 9 days, the
MDCs were resuspended in 1 ml saline and injected into the
bilateral groin lymph nodes (0.5 ml each) under sonographic
guidance. After 12 days, DC-CIK cells were resuspended in 25 ml
saline and injected into the right abdominal cavity under
sonographic guidance. After 15 days CIK cells were resuspended in
50 ml saline and infused intravenously. This protocol was repeated
after 1 month, and again after 3 months (Fig. 1). Thereafter, an additional course of
immunotherapy was administered after 6, 9, 12, 18 and 24 months in
the case that the results of blood tests did not meet the following
conditions: i) Absolute lymphocyte count, >1.2×109/l; ii)
CD3+/CD4+, CD3+/CD8+,
CD8+/CD28+, CD8+/CD28−
and CD3+/CD16+/CD56+ T lymphocyte
subset counts within the normal range. These subsets were selected
as they are associated with the immune state, and influence the
prognostic of patients with HCC. The normal ranges were adopted
based on our previous survey study on healthy volunteers (15–18).
Preparation of MDCs and effector cells
from peripheral blood
Immunocytes were prepared by Beijing Yongtai Immune
Application Technology Co., Ltd. (Beijing, China) in a
manufacturing practice-compliant facility. MDCs, DC-CIK cells and
CIK cells were isolated in accordance with previously reported
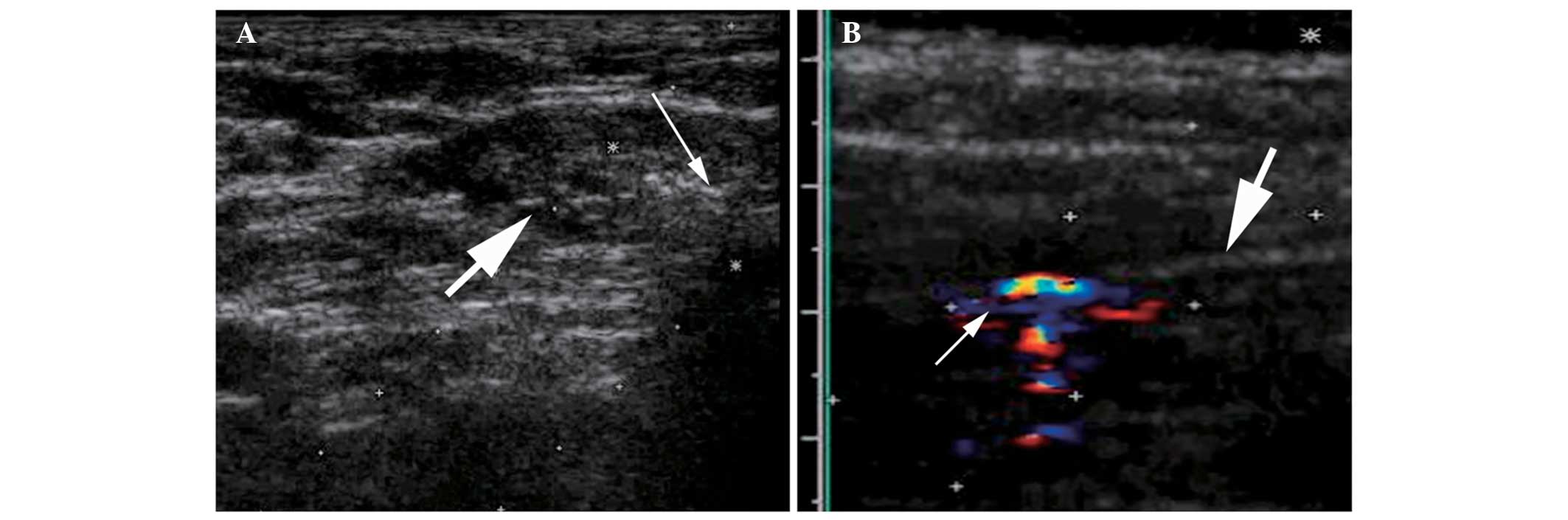
methods (12). MDCs and DC-CIK cell
infusions were guided by ultrasound (Fig. 2). Ultrasound and contrast-enhanced
ultrasound were performed using a Sequoia US system (Siemens
Healthcare, Mountain View, CA, USA) with a 3.5–5.0-MHz linear
multi-frequency transducer. The ultrasound contrast agent used was
SonoVue (Bracco Imaging, Milan, Italy).
Clinical outcomes and follow-up
All the patients were diagnosed using histological
analysis. Routine blood panel, tumor markers (including
α-fetoprotein, carcinoembryonic antigen and carbohydrate antigen
19-9), hepatitis B/C viral load and hepatic and renal function,
sonography, contrast-enhanced sonography, contrast-enhanced CT
and/or contrast-enhanced MRI were performed prior to immunotherapy
and at 1, 3, 6, 9, 12, 18 and 24 months after MWA. All CT studies
were conducted using a multi-detector row CT (Lightspeed 16; GE
Medical Systems, Inc., Milwaukee, WI, USA) and contrast medium
(iopromide, Ultravist 300; Schering AG, Berlin, Germany). All MRI
studies were conducted using a 1.5-T unit (Signa Echo-Speed; GE
Medical Systems), and Magnevist contrast medium (0.1 mmol/kg body
weight; Bayer HealthCare Pharmaceuticals, Leverkusan, Germany).
The disease free survival was defined as the period
following immunotherapy when no disease was detectable within 16
months of MWA.
Statistical analysis
Data was analyzed using SAS statistical software,
version 8.2 for Windows (SAS Institute Inc., Cary, NC, USA). The
continuous data were expressed as mean ± standard deviation.
Multiple groups were compared using analysis of variance or rank
sum test. Paired groups were compared by independent-samples
t-test, rank sum test or χ2 test. Patient survival time
was compared between groups using a Kaplan-Meier survival curve and
log-rank tests. Two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics of
patients
A total of 29 patients with HCC were recruited in
the present study from the Department of Interventional Ultrasound
in the Chinese PLA General Hospital between February 2009 and
December 2010. A group of 14 patients opted to receive
immunotherapy after MWA (immunotherapy group), and 15 patients
opted not to receive any post-ablation adjuvant therapy (control
group). The baseline characteristics of patients in the two groups
did not differ significantly (P>0.05; Table I). By December 2010, 64 courses of
immunotherapy had been performed in 14 patients, including 3
courses of immunotherapy completed in 6 patients, 4 courses in 2
patients, 5 courses in 1 patient, 6 courses in 3 patients, 7
courses in 1 patient and 8 courses in 1 patient. One patient
experienced severe abdominal pain 30 min after the sixth DC-CIK
infusion into the right abdominal cavity; however, the symptom
disappeared rapidly with anti-allergy treatment. No grade III/IV
severe adverse events occurred as a result of the other 63 courses
of immunotherapy (19,20). However, a fever of <39°C occurred
in 28 courses (43.1%), in 6 patients (42.9%), but all fevers were
resolved within 4–24 h without intervention.
 | Table I.Baseline clinical characteristics of
patients. |
Table I.
Baseline clinical characteristics of
patients.
| Characteristic | Control group
(n=15) | Immunotherapy group
(n=14) | P-value |
|---|
| Gender
(male/female) | 14/1 | 14/0 | 0.368 |
| Age (years, mean ±
SD) | 54.0±7.5 | 51.5±9.3 | 0.154 |
| Maximum diameter of
tumor (mm, mean ± SD) | 25.14±7.87 | 23.79±7.30 | 0.845 |
| Hepatitis B/C | 14/1 | 14/0 | 0.368 |
| Child-Pugh
classification (A/B) | 15/0 | 13/1 | 0.261 |
| Pathology
(Well/moderately/poorly-differentiated) | 5/6/4 | 5/7/2 | 0.236 |
| ALT (µ/l) | 41.3±7.8 | 42.8±7.5 | 0.598 |
| AST (µ/l) | 46.1±7.2 | 46.4±7.9 | 0.252 |
| Follow-up time
(months) | 16 | 19 | 0.663 |
Immunotherapy improves the
immunostatus and liver function of patients with HCC
The absolute lymphocyte counts of patients in the
control and immunotherapy groups did not differ significantly prior
to ablation (P﹥0.05). However, after 6 months and following 3
courses of immunotherapy, the mean absolute lymphocyte count in the
immunotherapy group (2.10±0.93.109/l) significantly exceeded that
in the control group (1.36±0.70.109/l; P﹤0.05; Table II).
 | Table II.Absolute T lymphocyte count pre- and
post-immunotherapy. |
Table II.
Absolute T lymphocyte count pre- and
post-immunotherapy.
| T lymphocyte
count | Control group
(109/l; n=15) | Immunotherapy group
(109/l; n=14) | P-value |
|---|
|
Pre-immunotherapy | 1.24±0.85 | 1.82±0.86 | 0.082 |
| Six months
post-immunotherapy | 1.36±0.70 | 2.10±0.93 | 0.038 |
The lymphocyte subset distribution of patients in
the control and immunotherapy groups did not differ significantly
prior to ablation (P>0.05); however, certain cytotoxic subsets
(CD3+/CD8+, CD8+CD28+
and CD3+CD16+CD56+) were
over-represented and negative regulatory or helper subsets
(CD4+CD8+, CD4+ and
CD4+CD25+) were under-represented in the
immunotherapy group between 1 and 12 months after immunotherapy
(P<0.05; Table III).
 | Table III.Comparison of T-lymphocyte subgroup
distribution prior to and following immunotherapy. |
Table III.
Comparison of T-lymphocyte subgroup
distribution prior to and following immunotherapy.
|
|
| Time
post-immunotherapy (months) |
|---|
|
|
|
|
|---|
| Lymphocyte
subset |
Pre-immunotherapy | 1 | 3 | 6 | 9 | 12 |
|---|
|
CD3+ | 0.711 | 0.000a | 0.371 | 0.432 | 0.436 | 0.462 |
|
CD8+ | 0.843 | 0.115 | 0.026a | 0.092 | 0.115 | 0.774 |
|
CD8+CD28+ | 0.323 | 0.004a | 0.036a | 0.873 | 0.022a | 0.607 |
| NKT | 0.793 | 0.038a | 0.771 | 0.160 | 0.038a | 0.004a |
|
CD8+CD28− | 0.585 | 0.432 | 0.777 | 0.326 | 0.471 | 0.948 |
|
CD4+CD25+ | 0.915 | 0.544 | 0.024b | 0.025b | 0.795 | 0.629 |
|
CD4+CD8+ | 0.743 | 0.585 | 0.008b | 0.065 | 0.359 | 0.879 |
|
CD4+ | 0.674 | 0.053 | 0.027b | 0.278 | 0.480 | 0.311 |
|
CD3+CD16+CD56+
(NK) | 0.647 | 0.201 | 0.694 | 0.875 | 0.750 | 0.642 |
|
CD19+ | 0.763 | 0.057 | 0.817 | 0.529 | 0.289 | 0.706 |
The alanine aminotransferase (ALT), AST, albumin and
cholinesterase (ChE) levels of patients in the control and
immunotherapy groups did not differ significantly prior to
ablation. However, after 6 months and 3 courses of immunotherapy,
the mean albumin level in the immunotherapy group (44.39±3.87 g/l)
exceeded that in the control group (38.43±5.98 g/l; P<0.05;
Table IV). By contrast, the mean
ALT, AST and ChE levels did not differ significantly between the
control and immunotherapy groups after 6 months (Table IV).
 | Table IV.Comparison of liver function between
immunotherapy and control groups. |
Table IV.
Comparison of liver function between
immunotherapy and control groups.
| Serum
parameter | Control group
(n=15) | Immunotherapy group
(n=14) | P-value |
|---|
| ALT (µ/l) |
|
|
|
|
Pre-immunotherapy | 41.3±7.8 | 42.8±7.5 | 0.598 |
|
Post-immunotherapy | 37.5±6.3 | 37.9±6.2 | 0.866 |
| D-value
of pre and post-immunotherapy | −3.7±7.2 | −4.9±5.2 | 0.632 |
| AST (µ/l) |
|
|
|
|
Pre-immunotherapy | 46.1±7.2 | 46.4±7.9 | 0.252 |
|
Post-immunotherapy | 35.8±6.5 | 37.7±5.3 | 0.268 |
| D-value
of pre and post-immunotherapy | −10.7±8.8 | −8.7±5.6 | 0.556 |
| Albumin (g/l) |
|
|
|
|
Pre-immunotherapy | 39.27±4.89 | 41.31±3.24 | 0.200 |
|
Post-immunotherapy | 38.43±5.98 | 44.39±3.87 | 0.004 |
| D-value
of pre and post-immunotherapy | −0.84±4.63 | 3.07±2.29 | 0.008 |
| ChE (µ/l) |
|
|
|
|
Pre-immunotherapy |
5503.49±1246.45 |
6043.28±1785.90 | 0.351 |
|
Post-immunotherapy |
5701.98±1303.10 |
6679.91±1480.46 | 0.069 |
| D-value
of pre and post-immunotherapy | 198.49±562.76 | 636.63±510.47 | 0.037 |
Immunotherapy increased the
proliferation of immune cells
The proliferative activity of immune cells was
compared after 1 and 3 courses of immunotherapy (Table V). The peripheral blood mononuclear
cell (PBMC) count after the first course of immunotherapy
(8.93±4.07×107 cells/l) did not differ significantly from the PBMC
count after the third course of immunotherapy (7.32±2.96×107
cells/l; P=0.996). Furthermore, following in vitro culture, the
absolute number of MDCs and CIK cells did not differ significantly
between samples collected after the first (10.74±5.26 and
2.65±2.29, respectively) or third course of immunotherapy
(16.5±9.16 and 3.23±1.46, respectively). However, the fraction of
CD83+, CD86+ and
CD3+CD8+cells was significantly increased
between the first (50.60±28.65, 62.69±29.50 and 68.70±15.73,
respectively) and the third courses of immunotherapy (74.87±20.87,
86.71±12.77 and 81.80±9.26, respectively; P<0.05).
 | Table V.T cell count and different subgroup
ratio after culture between third and first immunotherapy. |
Table V.
T cell count and different subgroup
ratio after culture between third and first immunotherapy.
| Parameter | After first
immunotherapy (n=14) | After third
immunotherapy (n=14) | P-value |
|---|
| Cell count
pre-culture |
|
|
|
| PBMC
(x107/l) | 8.93±4.07 | 7.32±2.96 | 0.996 |
| Cell count
post-culture |
|
|
|
| MDC
(x107/l) | 10.74±5.26 | 16.5±9.16 | 0.052 |
| CIK
(x1010/l) | 2.65±2.29 | 3.23±1.46 | 0.424 |
| T cell subgroup
ratio post-culture (%) |
|
|
|
|
CD83+ | 50.60±28.65 | 74.87±20.87 | 0.004 |
|
CD86+ | 62.69±29.50 | 86.71±12.77 | 0.011 |
|
CD3+CD8+ | 68.70±15.73 | 81.80±9.26 | 0.000 |
|
CD3+CD56+ | 29.59±11.42 | 35.82±8.92 | 0.058 |
Rate of recurrence and disease-free
survival (DFS)
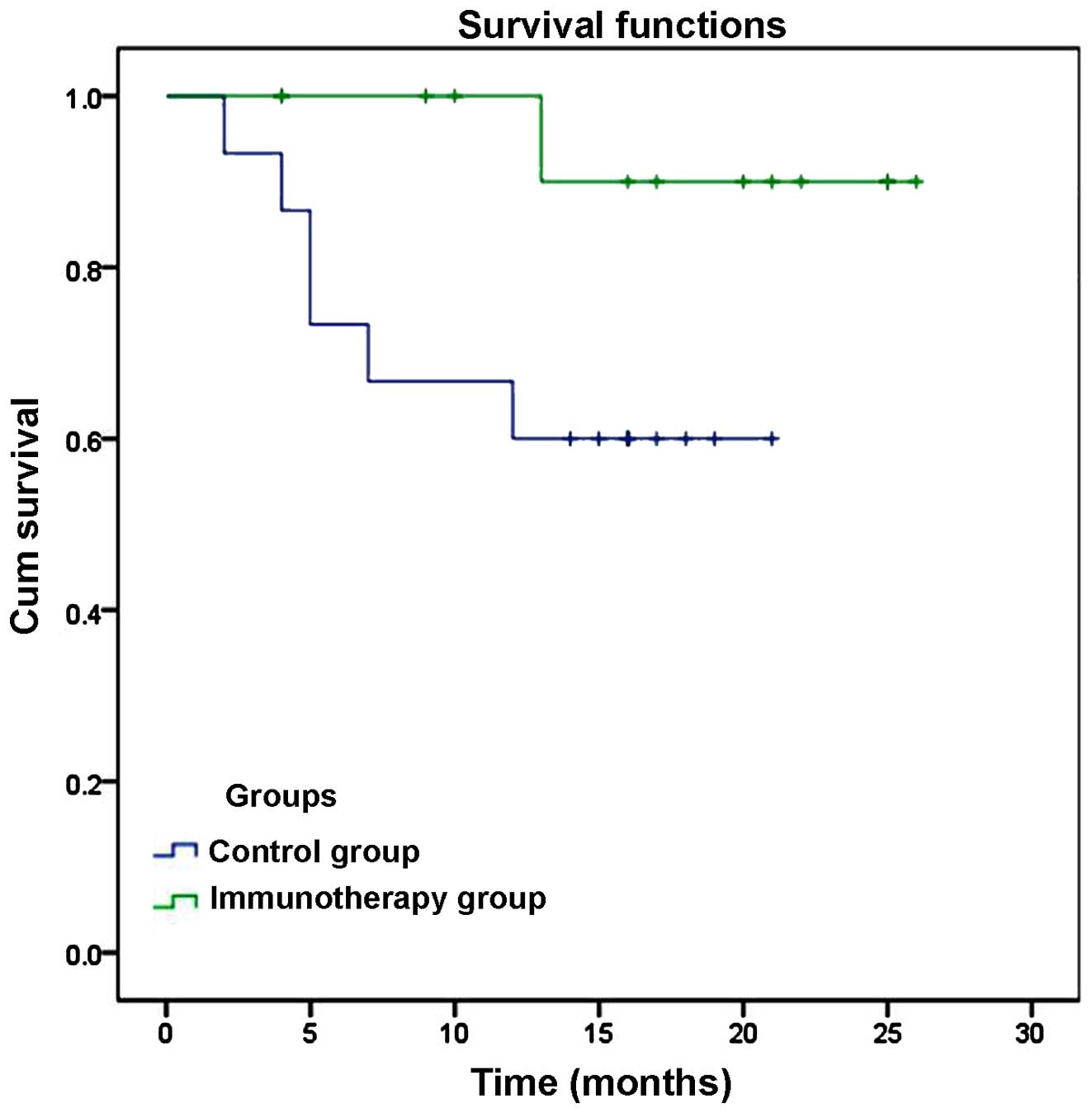
Intrahepatic recurrence was detected in 1 case
(7.1%) at 13 months after ablation, but no extrahepatic recurrences
or fatalities were detected in the immunotherapy group. In the
control group, intrahepatic recurrence was identified in 5 patients
(33.3%) within 1–19 months of ablation, and extrahepatic recurrence
occurred in 1 patient (6.7%). In addition, 3 patients in the
control group succumbed to various causes at 7, 10 and 12 months
after ablation (20%). The cause of mortality was upper
gastrointestinal bleeding in 1 case, liver failure in 1 case and
tumor progression in 1 case. However, due to the low rate of
recurrence and mortality, the rate of DFS and overall survival (OS)
was not significantly lower in the immunotherapy group compared
with the control group (DFS, 92.86 vs. 66.67%, P=0.076; OS, 100%
vs. 80%, P=0.126), as indicated by a Kaplan-Meier plot (Fig. 3).
Discussion
Immunotherapy has been demonstrated to increase the
rate of recurrence-free survival following resection or ablation in
patients with HCC (6–9,11). The
aim of the present study was to assess the efficacy of an expanded
immunotherapy protocol and to evaluate the precise differences in
immune and liver function in order to clarify the impact of
immunotherapy. In a previous study, the present authors reported
the outcome of a phase I clinical study of immunotherapy, which
included immature DCs, CIK cells and CTL following MWA of HCC
(12). In the present study, a
prolonged course of immunotherapy was administered to patients and
an expanded range of observational indices were detected.
The absolute lymphocyte count in the immunotherapy
group exceeded that in the control group after 3 courses of
immunotherapy. Furthermore, certain cytotoxic subsets
(CD3+/CD8+, CD8+CD28+
and CD3+CD16+CD56+) were
over-represented, while negative regulatory or helper subsets
(CD4+CD8+, CD4+ and
CD4+CD25+) were under-represented in the
immunotherapy group between 1 and 12 months after immunotherapy.
After 2 courses of immunotherapy the proliferation rate of MDCs and
T lymphocytes, including CD3+/CD8+ cells, was
significantly increased. In addition, the level of albumin in the
immunotherapy group was increased compared with the control group
after 3 courses of immunotherapy, indicating the accelerated
recovery of liver function. However, the rate of disease free
survival and overall survival within 16 months of MWA did not
differ significantly between the two groups.
The present results indicate that the immunotherapy
protocol induced a wide spectrum of changes in the immune system. A
number of studies have previously reported that an elevated ratio
of lymphocyte to monocytes pre- or postoperatively is positively
correlated with the outcomes of patients with various malignancies
(21,22). Increased lymphocyte counts have been
observed following immunotherapy, indicating elevated antitumor
activity (23). The proliferative
capacity of patient T lymphocytes was elevated after 2 courses of
immunotherapy. The MDC, CD8﹢ and
CD3+CD16+CD56+ T cell subsets
appeared to be particularly enhanced (24,25).
Enhanced levels of cytotoxic subsets
(CD3+/CD8+, CD8+CD28+
and CD3+CD16+CD56+) were detected
in the peripheral blood, within 12 months of treatment, indicating
an enhanced antitumor immune response (26,27).
Immune regulatory or suppressive subsets have been reported to be
elevated in the peripheral blood at 3–6 months after ablation,
relieving the immune response directed towards HCC (28). For the suppressive subset
CD4﹢CD25﹢, the levels were reduced between 3
and 6 months after ablation, which partly mitigated the
immunosuppressive response to HCC (28). In addition, reduced levels of the
suppressive and immunoregulatory subsets
(CD4+CD8+, CD4+ and
CD4+CD25+) were observed between 1 and 12
months after MWA. However, this difference may be attributed to the
increase in the ratio in cytotoxic subsets.
In the current study, immunotherapy appeared to
accelerate the recovery of circulating albumin levels, which is an
indicator of liver function. However, no statistically significant
differences were detected in other measures of liver function
(including ChE, AST and ALT) between patients that received
immunotherapy and those in the control group. The observed recovery
of albumin levels in the present study demonstrates the improvement
of liver cell status and indicates the improvement of the liver
microenvironment.
The disease free survival and overall survival rate
were not significantly higher in the immunotherapy group compared
with the control group. However, it is possible that a larger study
in which more patients are enrolled and followed-up for a longer
time period (>19 months) may reveal the impact of immunotherapy
of outcomes to be significant. Previous studies including longer
follow-up periods (up to 5 years post-ablation) (6,7), or
enrolling larger numbers of patients (6–8) have
reported an impressive impact of immunotherapy on recurrence rates
and outcome. Therefore, future studies involving more patients for
a longer follow-up period are required. Furthermore, studies should
investigate the cytotoxic capacity of immune cells ex vivo,
in order to determine whether immunotherapy may exert the observed
beneficial effects by enhancing the capacity of immune cells to
destroy remaining or recurring HCC cells.
In conclusion, in the present study, an
immunotherapy protocol employing multiple types of immune cells,
administered after MWA, was found to improve the immune status and
liver function in patients with HCC.
Acknowledgements
The study was supported by grants from the National
Scientific Foundation Committee of China (no. 81127006), Science
Technology Support (no. 2013BAI01B01) and International Cooperation
Project (no. 2012DFG32070).
References
|
1
|
El Serag HB: Hepatocellular carcinoma:
recent trends in the United States. Gastroenterology 127 (5 Suppl
1). 27–34. 2004.
|
|
2
|
Bosetti C, Levi F, Boffetta P, Lucchini F,
Negri E and La Vecchia C: Trends in mortality from hepatocellular
carcinoma in Europe, 1980–2004. Hepatology. 48:137–145. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaibori M, Ishizaki M, Matsui K and Kwon
AH: Predictors of microvascular invasion before hepatectomy for
hepatocellular carcinoma. J Surg Oncol. 102:462–468. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Funaki NO, Tanaka J, Seto SI, Kasamatsu T,
Kaido T and Imamura M: Hematogenous spreading of hepatocellular
carcinoma cells: Possible participation in recurrence in the liver.
Hepatology. 25:564–568. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J, Sherman M, Llovet JM, et al:
Clinical management of hepatocellular carcinoma. Conclusions of the
Barcelona-2000 EASL conference. European association for the study
of the liver. J Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takayama T, Sekine T, Makuuchi M, et al:
Adoptive immunotherapy to lower postsurgical recurrence rates of
hepatocellular carcinoma: A randomised trial. Lancet. 356:802–807.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hui D, Qiang L, Jian W, Ti Z and Da-Lu K:
A randomized, controlled trial of postoperative adjuvant
cytokine-induced killer cells immunotherapy after radical resection
of hepatocellular carcinoma. Dig Liver Dis. 41:36–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weng DS, Zhou J, Zhou QM, et al: Minimally
invasive treatment combined with cytokine-induced killer cells
therapy lower the short-term recurrence rates of hepatocellular
carcinomas. J Immunother. 31:63–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou WP, Wu MC and Chen H: The effects of
combined hepatectomy and immuno-chemotherapy on postoperative
recurrence rate of primary liver cancer. Zhong Hua Wai Ke Za Zhi.
33:35–37. 1995.(In Chinese).
|
|
10
|
Zhong JH, Ma L, Wu LC, et al: Adoptive
immunotherapy for postoperative hepatocellular carcinoma: A
systematic review. Int J Clin Pract. 66:21–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma H, Zhang Y, Wang Q, et al: Therapeutic
safety and effects of adjuvant autologous RetroNectin activated
killer cell immunotherapy for patients with primary hepatocellular
carcinoma after radiofrequency ablation. Cancer Biol Ther.
9:903–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou P, Liang P, Dong B, Yu X, Han Z and
Xu Y: Phase clinical study of combination therapy with microwave
ablation and cellular immunotherapy in hepatocellular carcinoma.
Cancer Biol Ther. 11:450–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Ye L, Zhang J, Lin M, He S, Mao
X, Zhou X and Zhi F: MELD scores and Child-Pugh classifications
predict the outcomes of ERCP in cirrhotic patients with
choledocholithiasis: A retrospective cohort study. Medicine
(Baltimore). 94:e4332015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SR, Liang P, Yu XL, Cheng ZG, Han ZY
and Yu J: Percutaneous microwave ablation for liver tumours
adjacent to the marginal angle. Int J Hyperthermia. 30:306–311.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Usuda S, Yoshizawa K, Yabu K and Kiyosawa
K: Immunological responses against an autologous human
hepatocellular carcinoma cell line. J Gastroenterol Hepatol.
8:517–523. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong L, Yao SK, Liu JX and Wang N: The
prognostic value of cellular immunity function in patients with
hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi.
13:194–197. 2005.PubMed/NCBI
|
|
17
|
Deguchi Y, Yoshimatsu K and Endo S:
Natural killer-like T cell lymphoma of the small intestine: Report
of a case. Surg Today. 36:474–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu HR and Li WM: Treg-specific
demethylated region activity in isolated regulatory t lymphocytes
is a surrogate for disease severity in hepatocellular carcinoma.
IUBMB Life. 67:355–360. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Passalacqua G, BaenaCagnani CE, Bousquet
J, Canonica GW, Casale TB, Cox L, Durham SR, LarenasLinnemann D,
Ledford D, Pawankar R, et al: Grading local side effects of
sublingual immunotherapy for respiratory allergy: Speaking the same
language. J Allergy Clin Immunol. 132:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cox L, LarenasLinnemann D, Lockey RF and
Passalacqua G: Speaking the same language: The World Allergy
Organization subcutaneous immunotherapy systemic reaction grading
system. J Allergy Clin Immunol. 25:569–574. 2010. View Article : Google Scholar
|
|
21
|
Song R, Ikeguchi M, Zhou G and Kuo MT:
Identification and characterization of a hepatoma cell-specific
enhancer in the mouse multidrug resistance mdr1b promoter. J Biol
Chem. 270:25468–25474. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen TM, Lin CC, Huang PT and Wen CF:
Neutrophil-to-lymphocyte ratio associated with mortality in early
hepatocellular carcinoma patients after radiofrequency ablation. J
Gastroenterol Hepatol. 27:553–561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koh YW, Kang HJ, Park C, et al: The ratio
of the absolute lymphocyte count to the absolute monocyte count is
associated with prognosis in Hodgkin's lymphoma: correlation with
tumor-associated macrophages. Oncologist. 17:871–880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee CS: Lymphocytes and their subsets in
the peripheral blood of hepatocellular carcinoma patients. J Formos
Med Assoc. 90:626–630. 1991.PubMed/NCBI
|
|
25
|
Wang JX, Liu GH, Fan YZ, et al: Effects of
cytotoxic T lymphocytes on hepatoma cell line SMMC-7721 induced by
different subsets of dendritic cells in vitro. Hepatobiliary
Pancreat Dis Int. 5:422–427. 2006.PubMed/NCBI
|
|
26
|
Olioso P, Giancola R, Di Riti M, Contento
A, Accorsi P and Iacone A: Immunotherapy with cytokine induced
killer cells in solid and hematopoietic tumours: A pilot clinical
trial. Hematol Oncol. 27:130–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu YR, Yang CL, Chen LB and Wang Q:
Analysis of CD8 (+) and CD8 (+) CD28 (-) cell subsets in patients
with hepatocellular carcinoma. Di Yi Jun Yi Da Xue Xue Bao.
22:72–73. 2002.(In Chinese). PubMed/NCBI
|
|
28
|
Shibolet O, Alper R, Zlotogarov L, et al:
Suppression of hepatocellular carcinoma growth via oral immune
regulation towards tumor-associated antigens is associated with
increased NKT and CD8+lymphocytes. Oncology. 66:323–330.
2004. View Article : Google Scholar : PubMed/NCBI
|