Introduction
Anxiety disorders are a group of disorders or
syndromes including generalized anxiety disorder (1). The central characteristic of these
disorders is persistent, maladaptively triggered anxiety (2). Anxiety disorders most commonly coexist
with insomnia, which refers to sleeplessness of any nature
(2). Notably, 70–90% patients with
anxiety disorders exhibit insomnia (3,4).
Compared with normal insomnia in the absence of anxiety disorders,
the sleep efficiency and duration and the amount of slow-wave sleep
are significantly reduced in comorbid insomnia and anxiety
disorders (5). Sleep disturbance in
patients with anxiety disorders is a common complaint that can have
a profound effect on the course of the anxiety disorder (6); therefore, the study of insomnia that is
comorbid with anxiety disorders is particularly important. At
present, little is known about the prevention of this type of
insomnia.
Current medications for the treatment of insomnia
include benzodiazepine-receptor agonists, melatonin and melatonin
variants, antidepressants, antipsychotics and antihistamines
(7). Among these medications,
antidepressants, antipsychotics and antihistamines are usually
preferred due to concerns about the tolerance and dependence
associated with the other medications (7). Citalopram, as an antidepressant
medication, is a selective serotonin reuptake inhibitor (SSRI)
(8). It has been noted that
citalopram is associated with daytime sedation in patients and
could cause an improvement in sleep quality in depressed patients
(9). Citalopram results in a
decrease in the proportion of rapid eye movement sleep and an
increase in the proportion of non-rapid eye movement sleep in
depressed patients (10). Doxepin,
another antidepressant medication, is a tricyclic drug associated
with significant sedative effects in the treatment of insomnia
(11). Doxepin has marked inhibitory
effects on neurotransmitter receptors, including the histamine,
serotonin, α1 adrenergic and muscarinic acetylcholine receptors
(12), and has been shown to
strongly promote the initiation and maintenance of sleep and to
improve sleep quality (11,12). These findings from previous studies
suggest the potential effects of citalopram and doxepin on sleep
disorders; however, to the best of our knowledge, a comparison
between the effects of citalopram and doxepin on sleep quality in
patients with comorbid insomnia and anxiety disorders has yet to be
conducted. The aim of the present study, therefore, was to compare
the effects of citalopram with those of low-dose doxepin on sleep
quality in patients with comorbid insomnia and anxiety disorders
and to assess the safety of these drugs in clinical
application.
Patients and methods
Participants and demographic
characteristics
Between September 2009 and January 2013, 78 patients
with a diagnosis of anxiety disorders and insomnia, according to
the Hamilton Anxiety Rating Scale (HAMA) (score, ≥14) and
Pittsburgh Sleep Quality Index (PSQI; score, ≥7) criteria were
selected from The Jinshan Hospital of Fudan University (Shanghai,
China). The inclusion criteria included patients between 45 and 64
years of age that had not received any psychotropic drugs for ≥2
weeks or hormonal agents and immunomodulators in the 6 months prior
to the initiation of the study. The exclusion criteria were severe
medical conditions (cancer, cardiovascular and cerebrovascular
diseases, thyroid disorders), pregnancy and lactation and mental
retardation disorders. All patients provided signed, informed
consent. The study was approved by the Ethics Committee of the
Faculty of Pharmacy at The Jinshan Hospital of Fudan
University.
Study design
The study followed a randomized and controlled
design. The baseline HAMA and PSQI of each patient were assessed.
The patients were then randomly assigned to receive either
citalopram (20 mg/day, taken after breakfast) or doxepin (12.5
mg/day, taken 30 min before sleep at night) for 12 weeks (n=39 per
group). Sleep quality and the severity of anxiety were measured at
weeks 4, 8 and 12 during treatment using the PSQI and HAMA,
respectively. Citalopram (Citalop™) was obtained from RPG Life
Sciences Ltd. (Mumbai, India), and doxepin (Spectra™) was from
obtained from Ranbaxy Ltd. (Gurgaon, India).
PSQI assessment
The PSQI is a self-reported questionnaire tool for
the subjective assessment of sleep in adults. It measures seven
components of sleep, including latency, quality, duration,
disturbances, efficiency, the use of sleep medications and daytime
dysfunction. For each component, scores can be assigned from 0 to 3
(13). The global PSQI is the sum of
the scores for each component. Patients with higher scores in each
component or in the global PSQI are more severely affected.
HAMA assessment
The HAMA is widely used in clinical and research
settings to measure the severity of anxiety symptoms. The scale
consists of 14 items defined by a series of symptoms. Each item is
scored from 0 (not present) to 4 (severe), with a total score range
from 0 to 56. A score of <17 indicates mild severity, 18–24
indicates mild to moderate severity and 25–30 indicates a moderate
to severe condition (14).
Adverse reactions and missing
participants
During drug treatment, the following adverse effects
were measured: Headache, aggravated insomnia, blood pressure
increase, hyperexcitability, nausea and vomiting, dizziness,
palpitations, frequent urination, somnolence and numbness. During
the study, 1 patient in the citalopram group and 1 patient in the
doxepin group cease to participate due to unknown reasons.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
The significance of intra-group comparisons was determined using
two-way (treatment × time-course) analysis of variance, while the
significance of inter-group comparisons was determined using the
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
≥3 times, and data are expressed as the mean ± standard error of
the mean.
Results
Demographic and clinical
characteristics
A total of 78 patients met the inclusion criteria
and were randomly assigned to take citalopram or doxepin. Three
patients in the citalopram group and 2 patients in the doxepin
group did not complete the study due to adverse drug reactions. In
total, 35 participants in the citalopram group and 36 participants
in the doxepin group completed the study. Two-way, independent
samples t- and χ2 test analyses revealed no
significant differences between the two groups with respect to age
(P=0.210), gender (P=0.129), initial PSQI (P=0.140) and HAMA
(P=0.630). The demographic characteristics of the patients are
shown in Table I.
 | Table I.Demographic and clinical
characteristics of the patients. |
Table I.
Demographic and clinical
characteristics of the patients.
| Variable | Citalopram, n=39 | Doxepin, n=39 |
|---|
| Age range
(years) | 45–64 | 45–64 |
| Female gender
(%) | 64.1 | 79.5 |
| Alcohol- or
drug-abuse history | No | No |
| Psychotropic drug
history (in 2 weeks) | No | No |
| Hormonal agent and
immunomodulator history | No | No |
| PSQI before
treatment | ≥7 | ≥7 |
| HAMA before
treatment | ≥14 | ≥14 |
Comparison of the effects of
citalopram and doxepin on sleep quality
To compare the effects of citalopram and doxepin on
sleep quality, the seven components of the PSQI were measured at
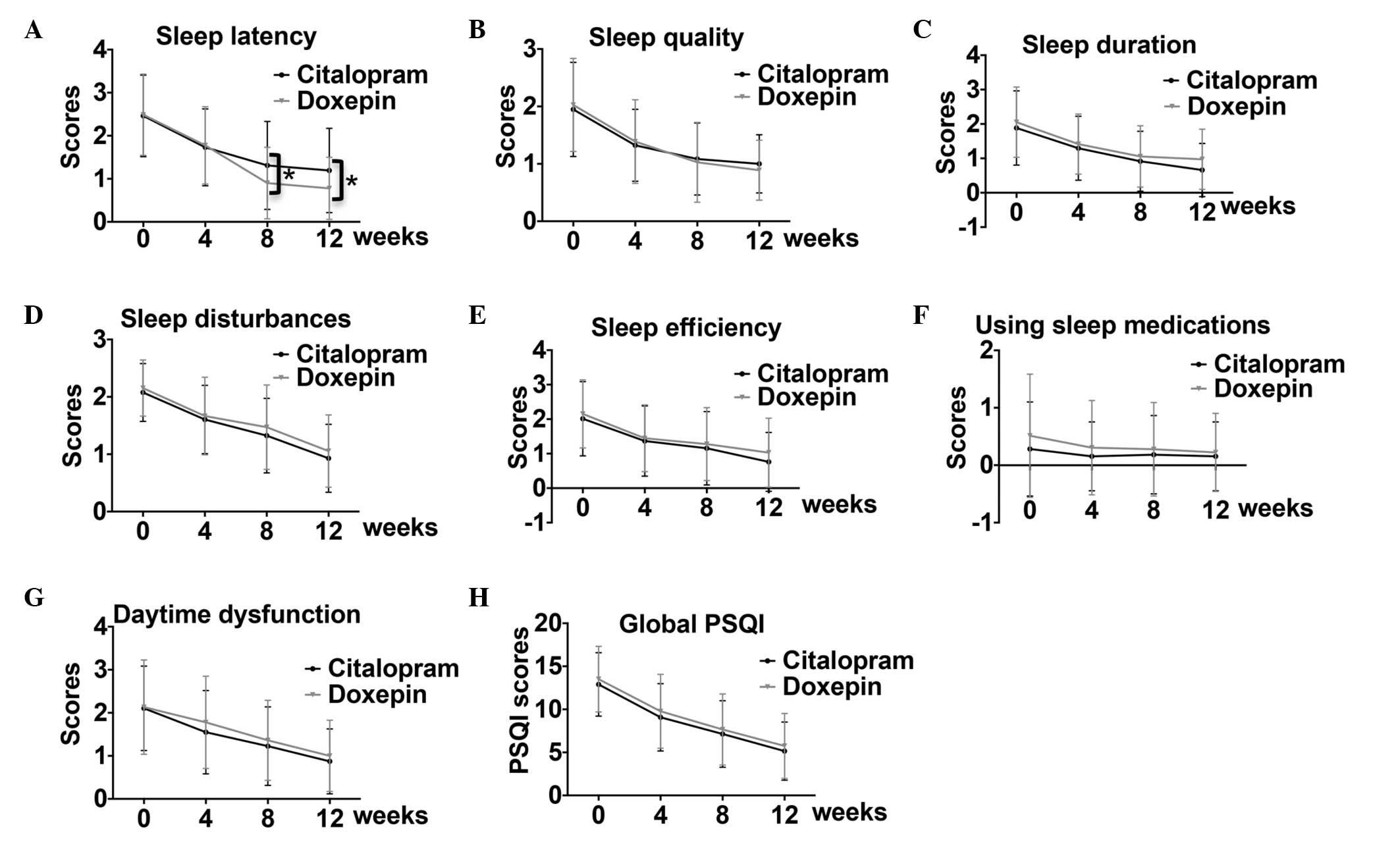
weeks 0, 4, 8 and 12. The data showed that both citalopram and
doxepin treatment gradually improved sleep latency, quality,
duration, disturbances and efficiency, as well as daytime
dysfunction and global PSQI, but not the use of sleep medications,
across the treatment period (Fig.
1). Statistical analysis indicated that, from weeks 0 to 12,
citalopram administration led to significant improvements in sleep
latency and disturbances, as well as daytime dysfunction and global
PSQI (Table II). In addition,
citalopram significantly improved sleep quality and duration from
weeks 0 to 8 and improved sleep efficiency from weeks 0 to 4 and 8
to 12 (Table II). For patients
treated with doxepin, sleep latency, but not daytime dysfunction,
were significantly improved from weeks 0 to 12. The sleep quality
of patients treated with doxepin showed improvements from weeks 0
to 8. In addition, the sleep disturbance was improved from weeks 0
to 4 and 8 to 12, while sleep efficiency and duration was only
improved from weeks 0 to 4 (Table
II). Notably, doxepin administration had a significantly
greater effect on sleep latency, but not on the other components,
at weeks 8 and 12 as compared with citalopram (Fig. 1A). These data suggest that citalopram
widely affected the different components of the PSQI over a
long-term treatment period as compared with doxepin; however,
doxepin had a greater effect on sleep latency than citalopram.
 | Table II.Effects of citalopram and doxepin on
PSQI components across time. |
Table II.
Effects of citalopram and doxepin on
PSQI components across time.
| PSQI components | Comparison
(weeks) | Citalopram
(P-value) | Doxepin
(P-value) |
|---|
| Sleep latency | 0–4 |
3.62×10−6 |
1.36×10−3 |
|
| 4–8 |
9.72×10−3 |
1.04×10−2 |
|
| 8–12 |
9.97×10−3 |
4.37×10−2 |
| Sleep quality | 0–4 |
7.05×10−7 |
6.41×10−4 |
|
| 4–8 |
2.44×10−2 |
3.50×10−2 |
|
| 8–12 |
3.79×10−1 |
3.42×10−1 |
| Sleep duration | 0–4 |
5.27×10−4 |
5.31×10−3 |
|
| 4–8 |
1.33×10−2 |
8.73×10−2 |
|
| 8–12 |
6.94×10−2 |
6.91×10−1 |
| Sleep
disturbance | 0–4 |
5.93×10−7 |
5.84×10−4 |
|
| 4–8 |
8.02×10−3 |
2.47×10−1 |
|
| 8–12 |
2.34×10−4 |
1.20×10−2 |
| Sleep efficiency | 0–4 |
2.43×10−4 |
2.47×10−3 |
|
| 4–8 |
2.29×10−1 |
4.88×10−1 |
|
| 8–12 |
1.61×10−2 |
3.06×10−1 |
| Use of sleep
medications | 0–4 |
2.86×10−1 |
3.54×10−1 |
|
| 4–8 |
7.95×10−1 |
8.86×10−1 |
|
|
8–12 |
7.95×10−1 |
7.54×10−1 |
| Daytime
dysfunction | 0–4 |
7.14×10−4 |
1.65×10−1 |
|
| 4–8 |
4.21×10−2 |
8.26×10−2 |
|
|
8–12 |
1.34×10−2 |
8.64×10−2 |
| Global PSQI | 0–4 |
7.07×10−9 |
1.54×10−4 |
|
| 4–8 |
3.67×10−3 |
3.70×10−2 |
|
|
8–12 |
1.39×10−3 |
4.37×10−2 |
Patients treated with citalopram or
doxepin exhibit improvements in anxiety
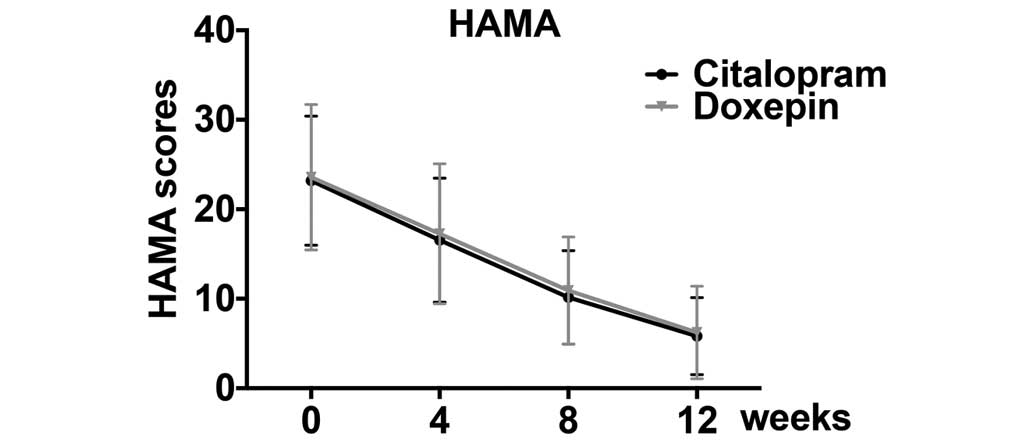
To assess the effects of citalopram and doxepin on
the severity of anxiety, the HAMA was used. Both citalopram and
doxepin decreased the HAMA scores, suggesting their efficacy in
reducing anxiety severity (Fig. 2);
however, no significant difference was found between the citalopram
and doxepin groups (Table III).
These data suggest that citalopram and doxepin are similarly
efficacious at decreasing anxiety severity.
 | Table III.Effects of citalopram and doxepin on
the HAMA scores across time. |
Table III.
Effects of citalopram and doxepin on
the HAMA scores across time.
| Index | Comparison
(weeks) | Citalopram
(P-value) | Doxepin
(P-value) |
|---|
| HAMA score | 0–4 |
5.92×10−8 |
1.62×10−7 |
|
| 4–8 |
5.44×10−9 |
3.82×10−10 |
|
|
8–12 |
3.01×10−7 |
1.06×10−8 |
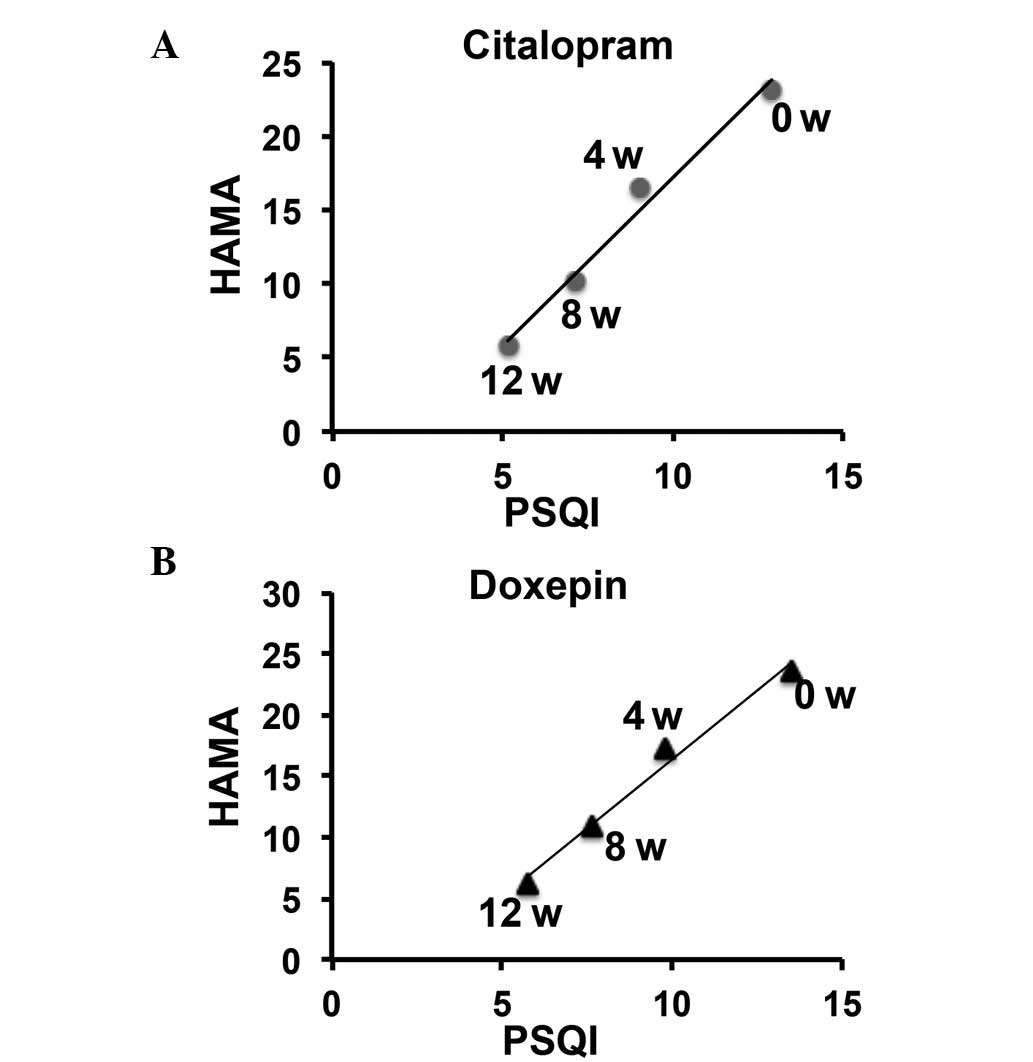
Positive correlation between sleep
quality and anxiety improvement in citalopram- and doxepin-treated
patients
The correlation between sleep quality improvement
and anxiety severity in the citalopram and doxepin groups was next
examined. A significant and positive correlation between the
improvements in sleep quality and anxiety was observed in the
citalopram group (P<0.01, r=0.990) and in the doxepin group
(P<0.01, r=0.992) (Fig. 3 and
Table IV). No significant
difference was found between the citalopram and doxepin groups.
 | Table IV.Effects of citalopram and doxepin on
the sleep quality of patients. |
Table IV.
Effects of citalopram and doxepin on
the sleep quality of patients.
| Medications | Rehabilitation, n
(%) | Significantly
improved, n (%) | Improved, n
(%) | No effects, n
(%) | Discontinued, n
(%) | Total, n |
|---|
| Citalopram | 8
(21.0) | 23 (60.5) | 1 (2.6) | 3 (7.9) | 3 (7.9) | 38 |
| Doxepin | 12 (31.6) | 14 (36.8) | 5
(13.2) | 5
(13.2) | 2 (5.2) | 38 |
Efficacy assessment and adverse
reactions
The efficacy of citalopram and doxepin treatment was
assessed according to the following criteria: i) Rehabilitation
(HAMA and PSQI scores improved by >75%); ii) significantly
improved (HAMA and PSQI scores improved by >50% but <75%);
iii) improved (HAMA and PSQI scores improved by >25% but
<50%); and iv) no effects (HAMA and PSQI scores improved by
<25%). The efficacy of citalopram and doxepin treatment is
presented in Table IV. The data
showed that citalopram and doxepin exhibited different efficacies
in the treatment of the patients (Table
IV). Citalopram promoted rehabilitation in 21.0% of patients
and a significant improvement in 60.5% of patients. By contrast,
doxepin promoted rehabilitation in 31.6% of patients and a
significant improvement in 36.8% of patients. In addition,
citalopram and doxepin administration was associated with different
adverse drug reaction, as shown in Table
V. During this study, 5 patients in the citalopram group and 9
patients in the doxepin group exhibited an adverse reaction,
including headache and aggravated insomnia. Overall, citalopram and
doxepin showed notable efficacy, with few adverse drug reactions,
in the treatment of insomnia in patients with anxiety disorders.
Compared with doxepin, citalopram was more effective at inducing a
significant improvement and less effective at inducing the
rehabilitation of the patients.
 | Table V.Adverse reactions in citalopram- and
doxepin-treated patients. |
Table V.
Adverse reactions in citalopram- and
doxepin-treated patients.
| Medications | Headache (n) | Aggravated insomnia
(n) | Increase in BP
(n) | Hyperexcitability
(n) | Nausea and vomiting
(n) | Dizziness (n) | Palpitations
(n) | Frequent urination
(n) | Somnolence (n) | Numbness (n) |
|---|
| Citalopram | 2 | 2 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| Doxepin | 3 | 3 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 |
Discussion
Insomnia is a common clinical mental illness that
seriously impacts the daily lives and work quality of affected
individuals (15,16). Psychiatric disorders, particularly
anxiety and mood disorders, have been proposed as a frequent cause
of insomniac symptoms (17).
Clinical experience shows that the majority of patients with
anxiety disorders suffer from sleep disturbances (5,6);
however, most of the previous studies that have been conducted
focused on primary insomnia, and little investigation into comorbid
insomnia and anxiety disorders has been instigated.
Citalopram, a commonly used SSRI, is applied for the
clinical treatment of depression and insomnia (10); however, controversy surrounds the
role of SSRIs in the treatment of insomnia in patients with anxiety
disorders. Several previous studies have shown that SSRI treatment
can lead to anxiety and insomnia (18,19). By
contrast, another study has suggested that SSRI treatment in
patients with insomnia and anxiety disorders causes poorer sleep
quality at the beginning but results in greater improvements in
sleep time a few weeks later (20).
In the present study, the results demonstrated that low-dose
citalopram (20 mg/day) markedly improved total sleep duration,
sleep quality and daytime dysfunction in patients with anxiety
disorders (Fig. 1 and Table II). In addition, citalopram
administration resulted in notable improvements in anxiety in the
patients (Fig. 2 and Table III). The current data therefore
support that the theory that low-dose citalopram used in the
treatment of insomnia with anxiety disorders is beneficial.
Doxepin is another antidepressant that is used for
the therapy of anxiety and depression. High doses of doxepin cause
anti-cholinergic effects and can lead to side effects including
increased appetite and weight and decreased blood pressure
(11,21). Low-dose doxepin, however, exhibits a
more specific effect against H1-receptor antagonists and does not
show significant anti-cholinergic effects or result in severe
adverse reactions (22,23). In the present study, low-dose doxepin
(12.5 mg/day) was used to treat comorbid insomnia with anxiety
disorders, and it was found that doxepin significantly improved
sleep latency in patients from weeks 0 to 12 (Fig. 1 and Table
II). Furthermore, only 2 of the participants (5.3%) exhibited
sleepiness as an adverse reaction (Table
V). This is consistent with a previous study, in which it was
found that low-dose doxepin could improve the sleep quality of
insomniac patients (24), thereby
supporting a potential application of doxepin in the treatment of
comorbid insomnia and anxiety disorders. It has been reported that
histamine plays an essential role in the awakening process
(25). Considering the specific role
of low-dose doxepin against H-receptor antagonists, but not other
neurotransmitters, it is reasonable to propose that low-dose
doxepin improves sleep quality by delaying the awakening process
without affecting appetite and the noradrenaline pathway; however,
the detailed mechanisms underlying the action of doxepin require
clarification in future studies.
Although the role of citalopram and doxepin in the
treatment of insomnia has been investigated in previous studies
(10–12,18,21–24,26), the
effects of these drugs in patients with comorbid insomnia and
anxiety disorders are unclear. In the present study, a
comprehensive evaluation of the dose, treatment duration, treatment
efficacy and safety of the clinical application of citalopram and
doxepin in patients with comorbid insomnia and anxiety disorders
was performed. Citalopram and doxepin significantly improved sleep
quality during treatment (Fig. 1 and
Table II), while doxepin
administration resulted in a significantly greater improvement in
sleep latency in patients as compared with citalopram (Fig. 1A). In addition, both citalopram and
doxepin showed a significant and positive correlation between
improvements in sleep quality and anxiety (Fig. 3). The present study therefore
suggested that citalopram and doxepin could have a beneficial
effect in the therapy of patients with comorbid insomnia and
anxiety disorders. Drug resistance, relapse of insomnia, abstinence
and drug dependence during treatment were not assessed in the
present study; therefore, the possibility that long-term treatment
with these drugs could lead to the above effects cannot be
excluded. In conclusion, both citalopram and doxepin can improve
sleep quality and anxiety in patients with comorbid insomnia and
anxiety disorders. Compared with doxepin, citalopram showed a
greater efficacy at inducing a significant improvement in patients
and less efficacy at inducing rehabilitation during treatment.
References
|
1
|
Watson D: Rethinking the mood and anxiety
disorders: A quantitative hierarchical model for DSM-V. J Abnorm
Psychol. 114:522–536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barlow DH: Unraveling the mysteries of
anxiety and its disorders from the perspective of emotion theory.
Am Psychol. 55:1247–1263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Mill JG, Hoogendijk WJ, Vogelzangs N,
van Dyck R and Penninx BW: Insomnia and sleep duration in a large
cohort of patients with major depressive disorder and anxiety
disorders. J Clin Psychiatry. 71:239–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uhde TW, Cortese BM and Vedeniapin A:
Anxiety and sleep problems: Emerging concepts and theoretical
treatment implications. Curr Psychiatry Rep. 11:269–276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papadimitriou GN and Linkowski P: Sleep
disturbance in anxiety disorders. Int Rev Psychiatry. 17:229–236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marcks BA, Weisberg RB, Edelen MO and
Keller MB: The relationship between sleep disturbance and the
course of anxiety disorders in primary care patients. Psychiatry
Res. 178:487–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunnington D, Junge MF and Fernando AT:
Insomnia: Prevalence, consequences and effective treatment. Med J
Aust. 199:S36–S40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hyttel J: Citalopram-pharmacological
profile of a specific serotonin uptake inhibitor with
antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry.
6:277–295. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jindal RD and Thase ME: Treatment of
insomnia associated with clinical depression. Sleep Med Rev.
8:19–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
ShahsavandAnanloo E, Berenji F,
Sadeghniiat K, Alimadadi A, Zahiroddin AR, Tabatabaee M, AbbasiAsl
M and Ghaeli P: Comparing effects of citalopram with fluoxetine on
sleep quality in patients with major depressive disorder. Eur Rev
Med Pharmacol Sci. 17:1155–1161. 2013.PubMed/NCBI
|
|
11
|
Hajak G, Rodenbeck A, Voderholzer U,
Riemann D, Cohrs S, Hohagen F, Berger M and Rüther E: Doxepin in
the treatment of primary insomnia: A placebo-controlled,
double-blind, polysomnographic study. J Clin Psychiatry.
62:453–463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber J, Siddiqui MA, Wagstaff AJ and
McCormack PL: Low-dose doxepin: In the treatment of insomnia. CNS
Drugs. 24:713–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buysse DJ, Reynolds CF III, Monk TH,
Berman SR and Kupfer DJ: The Pittsburgh sleep quality index: A new
instrument for psychiatric practice and research. Psychiatry Res.
28:193–213. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamilton M: The assessment of anxiety
states by rating. Br J Med Psychol. 32:50–55. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sivertsen B, Krokstad S, Øverland S and
Mykletun A: The epidemiology of insomnia: Associations with
physical and mental health. The HUNT-2 study. J Psychosom Res.
67:109–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taylor DJ, Lichstein KL, Durrence HH,
Reidel BW and Bush AJ: Epidemiology of insomnia, depression and
anxiety. Sleep. 28:1457–1464. 2005.PubMed/NCBI
|
|
17
|
Harvey AG: A cognitive model of insomnia.
Behav Res Ther. 40:869–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monaca C, Boutrel B, Hen R, Hamon M and
Adrien J: 5-HT 1A/1B receptor-mediated effects of the selective
serotonin reuptake inhibitor, citalopram, on sleep: Studies in 5-HT
1A and 5-HT 1B knockout mice. Neuropsychopharmacology. 28:850–856.
2003.PubMed/NCBI
|
|
19
|
VazquezPalacios G, Hernández-González M,
Guevara Pérez MA and Bonilla-Jaime H: Nicotine and fluoxetine
induce arousing effects on sleep-wake cycle in antidepressive
doses: A possible mechanism of antidepressant-like effects of
nicotine. Pharmacol Biochem Behav. 94:503–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fava M, McCall WV, Krystal A, Wessel T,
Rubens R, Caron J, Amato D and Roth T: Eszopiclone co-administered
with fluoxetine in patients with insomnia coexisting with major
depressive disorder. Biol Psychiatry. 59:1052–1060. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roth T, Zorick F, Wittig R, McLenaghan A
and Roehrs T: The effects of doxepin HCl on sleep and depression. J
Clin Psychiatry. 43:366–368. 1982.PubMed/NCBI
|
|
22
|
Krystal AD, Durrence HH, Scharf M,
Jochelson P, Rogowski R, Ludington E and Roth T: Efficacy and
safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and
outpatient trial of elderly subjects with chronic primary insomnia.
Sleep. 33:1553–1561. 2010.PubMed/NCBI
|
|
23
|
Roth T, Heith Durrence H, Jochelson P,
Peterson G, Ludington E, Rogowski R, Scharf M and Lankford A:
Efficacy and safety of doxepin 6 mg in a model of transient
insomnia. Sleep Med. 11:843–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krystal AD, Lankford A, Durrence HH,
Ludington E, Jochelson P, Rogowski R and Roth T: Efficacy and
safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in
adults with chronic primary insomnia. Sleep. 34:1433–1442.
2011.PubMed/NCBI
|
|
25
|
Haas HL, Sergeeva OA and Selbach O:
Histamine in the nervous system. Physiol Rev. 88:1183–1241. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goforth HW: Low-dose doxepin for the
treatment of insomnia: Emerging data. Expert Opin Pharmacother.
10:1649–1655. 2009. View Article : Google Scholar : PubMed/NCBI
|