Introduction
Aplastic anemia (AA) is a serious disorder
characterized by pancytopenia and hypocellular bone marrow. Severe
AA (SAA) is diagnosed when 2 of 3 blood parameters meet the
following criteria: Absolute neutrophil count, <500/ml; absolute
reticulocyte count, <60,000/ml; and platelet count,
<20,000/ml (1). AA is a disease
that may present at any age, and SAA is always fatal if untreated.
Adolescent and young adult patients (≤30 years old) with SAA may
exhibit marrow failure, in contrast to older adult patients.
Matched sibling hematopoietic stem cell transplantation (HSCT) is
considered to be the primary treatment for patients with SAA
(2). If a matched sibling donor is
not available, it is necessary to screen unrelated histocompatible
donors from a bone marrow library. Umbilical cord blood (UCB) is an
alternative hematopoietic stem cell source for patients with SAA
(3); however, low cell dose, higher
risk of rejection and delayed immune recovery limit its
application. In clinical practice, immunosuppressive treatment
(IST) has been used as the primary procedure for patients without a
compatible donor; however, the relapse rate reported in previous
studies is high (4). In a previous
study (5), the present authors
reported that the patients that received enhanced IST plus one
regime of unrelated UCB transfusion exhibited higher efficiency and
a reduced rate of relapse. In the present report, two patients with
SAA were successfully treated with enhanced immunosuppressive
treatment combined with unrelated UCB. Unexpectedly, the patients
exhibited mixed chimerism following therapy. Chimerism studies were
conducted on multiple occasions following the treatment. By
analyzing short tandem repeat regions, chimerism was identified as
5.2% on day 16, which indicated low levels of donor T cell
engraftment, and increasing to 74.6% on day 74. At 6 months after
therapy, the chimerism level reached 75.2% and remained at 97.75%
at 375 days after therapy.
Case report
Ethical approval and patient
consent
The therapy protocols of the present study were
approved by the Ethics Committee of the General Hospital of Jinan
Military (Jinan, China). Written consent was obtained from the
patient or patient's legal guardian. The patients were screened for
Fanconi anemia and paroxysmal nocturnal hemoglobinuria.
Case 1
A 10-year-old boy was diagnosed with SAA in
September 2012 at the General Hospital of Jinan Military. The
patient had no matched related donor for HSCT. The treatment
regimen consisted of 3 mg/kg rabbit anti-thymocyte globulin (ATG)
from day −5 to day −1, and 50 mg/kg/day cyclosphosphamide (CTX)
from day −3 to day −2. Cyclosporine A (CSA) was administered by
intravenous infusion of 3 mg/kg/day (oral administration from day
−1) to maintain a range of 150–250 ng/ml. Unrelated UCB was
transfused on day 0, and a total of 4.2×107/kg nucleated
cells and 0.9×105/kg CD34+ cells were
transfused. If the neutrophil count was <0.5×109/l,
then granulocyte-colony stimulating factor (G-CSF; 5 mg/kg) was
administered; however, the dose of G-CSF was gradually reduced if
the neutrophil count was >1.5×109/l. No significant
toxicity was observed. Neutrophils reached 0.5×109/l on
day 12 and platelet counts reached 30×109/l on day 27.
The patient achieved complete response on day 74 and the cell
phenotype of red blood cells was completely changed to the donor
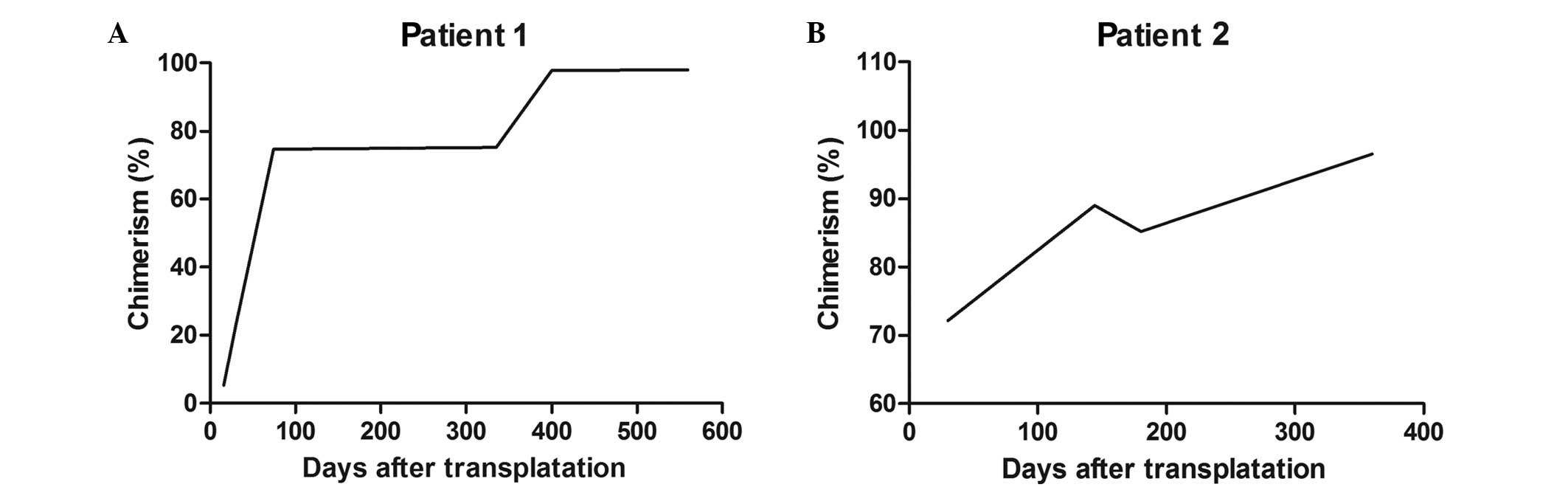
type at 4 months. Chimerism (through the analysis of short tandem
repeat regions) was 5.2% on day 16, increasing to 74.6% on day 74.
At 6 months after therapy, the chimerism reached 75.2% and remained
at 97.75% at 375 days after therapy (Fig. 1). The latest follow-up occurred in
May 2015. There was no indication of graft-versus-host disease
(GVHD) or relapse, and chimerism was 100%.
Case 2
A 26-year-old female was diagnosed with SAA in June
2013 and treated following a similar protocol as for case 1. The
patient was the only child in the family and had no matched related
donor for HSCT. For unrelated UCB transfusion, a total of
1.7×107/kg nucleated cells and 0.36×105/kg
CD34+ cells were transfused. Neutrophils reached
0.5×109/l on day 20 and the platelet count reached
30×109/l on day 32. The patient achieved complete
response on day 87. The cell phenotype of red blood cells was
completely changed to the donor type at 5 months. In addition,
chimerism was 72% on day 30, increasing to 89% on day 144. Due to
Epstein-Barr viremia, the patient discontinued the CSA therapy on
day 150, and the chimerism reduced to 85.2%. Chimerism reached 100%
at 361 days after the therapy (Fig.
1B). During a 2-year follow-up period, the donor chimerism
remained complete implant and normal blood cell counts were
detected, while there was no indication of GVHD and no relapse.
Discussion
Five decades ago, effective therapy for SAA was
limited; however, advances in HSCT and IST have improved the
survival rate of patients with SAA from 10–20% in the 1960s to
80–90% at present (6). Human
leukocyte antigen (HLA)-matched HSCT is recommended as the primary
therapy for young patients with SAA (7); however, the lack of matched donors,
graft rejection, GVHD and poor immune reconstitution limit the
success of HSCT. UCB is an alternative hematopoietic stem cell
source for transplantation. Hemopoietic progenitor cells (HPCs) in
UCB possess extensive proliferative capacity, and the quantity of
HPCs from a single UCB collection is associated with the success of
bone marrow transplantation (BMT) (8). Related and unrelated UCB
transplantation (UCBT) have been successfully used in the treatment
of pediatric patients with various malignant or non-malignant
diseases (9). Compared with BMT,
UCBT is able to evidently reduce acute and chronic GVHD. However,
graft rejection and poor immune reconstitution continue to limit
the success rate of UCBT. A higher number of nucleated graft cells,
certain conditioning regimens and the degree of mismatch between
the graft and recipient are crucial for achieving improved
engraftment following UCBT in patients with SAA, particularly in
adult patients (3,10). In order to overcome the high
engraftment/alloreactivity barrier in SAA, myeloablative
chemotherapy or radiation therapy have been used to induce
sufficient immunosuppression or clearance of reactive recipient T
cell populations. Total lymphoid irradiation or total body
irradiation are typically used in the regimen (11–13);
however, the irradiation has been reported to affect the
neuroendocrine system in children and their subsequent growth and
development (14), in addition to
increasing the risk of malignancies.
IST is used to treat patients that lack a
histocompatible donor or elderly patients, and ATG combined with
cyclosporine remains the standard procedure for IST (6). Hematological responses to transfusion
independence appear in ~2/3 of patients; however, disease relapse
ultimately occurs in 30–40% of patients (1,4). A
10-year follow-up study revealed that the response rate of SAA is
71% and the actual event-free survival is 58% in 44 treatment-naïve
patients with SAA that received high-dose cyclophosphamide
(15). The success of IST
application is significantly limited by poor response, high relapse
and clonal evolution. In a previous study, the present authors
treated patients with enhanced immunosuppressive treatment (100
mg/kg CTX) and UCB transfusion as an adjuvant therapy (5). Patients rapidly achieved reconstitution
of hematopoiesis, while the efficacy rate was 88% and the 3-year
overall survival rate was 92% (5).
However, there were no signs of implant, so the therapy was named
intensive IST combined with UCB transfusion, rather than UBCT
(16). Furthermore, in a previous
study, CTX (200 mg/kg) and rabbit ATG (7.5 mg/kg/day for 4 days)
were used as a conditioning regimen in a pediatric patient with SAA
that was receiving UBCT (17). The
patient's neutrophil count reached 0.5×109/l on day 37,
while red blood cell and platelet transfusion independence were
reached on days 50 and 52, respectively. The patient demonstrated
stable mixed chimerism at 18 months and full donor chimerism at 20
months after UCBT (17). The doses
of CTX and ATG employed in the present cases were half of the
previous dosages (18), and the
adult patient received a reduced number of total nucleated cells
and CD34+ cells compared with a previous study (18). However, the neutrophils and platelet
engraftment recovered faster compared with those in the previous
study. Furthermore, the appearance of full donor chimerism occurred
earlier and without relapse during the 2-year follow-up period.
Though the treatment may induce a higher risk of rejection due to
the unrelated and mismatched UBC, the engraftment in two patients
in the present study was successful.
In conclusion, the modified protocol used in the
present study, which involved no irradiation, resulted in mixed
chimerism and rapidly achieved complete reconstitution of
hemopoiesis. The present report suggested the feasibility and
efficacy of enhanced IST combined with UCB for the treatment of
patients with SAA. Thus, the improved therapy may be a viable
therapeutic option for patients that lack a suitable HLA-matched
donor and may enhance the response rate of IST.
Acknowledgements
The authors would like to thank Dr Qing-Qing Yu and
Dr Xiao-Chen Song of the General Hospital of Jinan Military (Jinan,
China) for their assistance.
References
|
1
|
Rosenfeld S, Follmann D, Nunez O and Young
NS: Antithymocyte globulin and cyclosporine for severe aplastic
anemia: Association between hematologic response and long-term
outcome. JAMA. 289:1130–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheinberg P and Young NS: How I treat
acquired aplastic anemia. Blood. 120:1185–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peffault de Latour R, Purtill D, Ruggeri
A, Sanz G, Michel G, Gandemer V, Maury S, Kurtzberg J, Bonfim C,
Aljurf M, et al: Influence of nucleated cell dose on overall
survival of unrelated cord blood transplantation for patients with
severe acquired aplastic anemia: A study by eurocord and the
aplastic anemia working party of the European group for blood and
marrow transplantation. Biol Blood Marrow Transplant. 17:78–85.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frickhofen N, Heimpel H, Kaltwasser JP and
Schrezenmeier H: German Aplastic Anemia Study Group: Antithymocyte
globulin with or without cyclosporin A: 11-year follow-up of a
randomized trial comparing treatments of aplastic anemia. Blood.
101:1236–1242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou F, Ge L, Yu Z, Fang Y and Kong F:
Clinical observations on intensive immunosuppressive therapy
combined with umbilical cord blood support for the treatment of
severe aplastic anemia. J Hematol Oncol. 4:272011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scheinberg P: Aplastic anemia. Therapeutic
updates in immunosuppression and transplantation. Hematology Am Soc
Hematol Educ Program. 2012:292–300. 2012.PubMed/NCBI
|
|
7
|
Marsh J: Making therapeutic decisions in
adults with aplastic anemia. Hematology Am Soc Hematol Educ
Program. 78–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Broxmeyer HE, Douglas GW, Hangoc G, Cooper
S, Bard J, English D, Arny M, Thomas L and Boyse EA: Human
umbilical cord blood as a potential source of transplantable
hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA.
86:3828–3832. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gluckman E, Rocha V, Boyer-Chammard A,
Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira
E, Laporte JP, et al: Outcome of cord-blood transplantation from
related and unrelated donors. Eurocord transplant group and the
european blood and marrow transplantation group. N Engl J Med.
337:373–381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
MacMillan ML, Walters MC and Gluckman E:
Transplant outcomes in bone marrow failure syndromes and
hemoglobinopathies. Semin Hematol. 47:37–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JW, Kang HJ, Kim EK, Shin HY and Ahn
HS: Successful salvage unrelated umbilical cord blood
transplantation with two units after engraftment failure with
single unit in severe aplastic anemia. J Korean Med Sci.
24:744–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshimi A, Kojima S, Taniguchi S, Hara J,
Matsui T, Takahashi Y, Azuma H, Kato K, Nagamura-Inoue T, Kai S and
Kato S: Japan Cord Blood Bank Network: Unrelated cord blood
transplantation for severe aplastic anemia. Biol Blood Marrow
Transplant. 14:1057–1063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto H, Kato D, Uchida N, Ishiwata K,
Araoka H, Takagi S, Nakano N, Tsuji M, Asano-Mori Y, Matsuno N, et
al: Successful sustained engraftment after reduced-intensity
umbilical cord blood transplantation for adult patients with severe
aplastic anemia. Blood. 117:3240–3242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanders JE: Growth and development after
hematopoietic cell transplant in children. Bone Marrow Transplant.
41:223–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brodsky RA, Chen AR, Dorr D, Fuchs EJ,
Huff CA, Luznik L, Smith BD, Matsui WH, Goodman SN, Ambinder RF and
Jones RJ: High-dose cyclophosphamide for severe aplastic anemia:
Long-term follow-up. Blood. 115:2136–2141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie LN, Fang Y, Yu Z, Song NX, Kong FS,
Liu XM and Zhou F: Increased immunosuppressive treatment combined
with unrelated umbilical cord blood infusion in children with
severe aplastic anemia. Cell Immunol. 289:150–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boiron JM, Cotterêt S, Cony-Makhoul P,
Merel P, Micheau M, Perel Y, Belloc F, Bernard P and Reiffers J:
Stable mixed chimerism without relapse after related allogeneic
umbilical cord blood transplantation in a child with severe
aplastic anemia. Bone Marrow Transplant. 22:819–821. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laughlin MJ, Barker J, Bambach B, Koc ON,
Rizzieri DA, Wagner JE, Gerson SL, Lazarus HM, Cairo M, Stevens CE,
et al: Hematopoietic engraftment and survival in adult recipients
of umbilical-cord blood from unrelated donors. N Engl J Med.
344:1815–1822. 2001. View Article : Google Scholar : PubMed/NCBI
|